Abstract
Background:
Human toxocariasis is contained in the list of neglected diseases. The infection occurs after ingestion of embryonated eggs in contaminated soil. The present study was carried out to estimate the extent of soil contamination with Toxocara spp. eggs in the public places.
Methods:
Soil samples were collected randomly from 41 public places in various parts in and around of Ardabil, Iran, between March 2013 and March 2014. Data were examined by microscopy following sodium nitrate flotation.
Results:
Of the 200 collected soil samples, 35 (17.5%) were positive for soil parasites. The eggs of Toxocara spp. were found in 14 (7%) soil samples.
Conclusion:
This investigation gives baseline knowledge regarding soil contamination with Toxocara spp. eggs in Ardabil city and provides information for local control of toxocariasis.
Keywords: Toxocara spp., Eggs, Soil, Public places, Iran
Introduction
The Toxocara spp. larval stage, the etiological agent of human toxocariasis, is a significant medical problem worldwide (1). Infected dogs and cats harboring adult worms can spread more than 50000 embryonated eggs/g feces into human environment every day (2, 3). These eggs need a period of development in the soil to become infective (4) and can be viable in the environment for a long time (5). Therefore, contact with contaminated soil is the most widely identified source of human infection (6, 7). In fact, humans are typically infected by ingestion of embryonated eggs from the soil (8). For this reason, more surveys have been conducted in recent years to evaluate the frequency of Toxocara eggs in the soil of public places in various parts of the world (4, 9–11). Several reports of these studies indicated widespread contamination of the soil with the eggs of Toxocara spp. contaminated with the dogs and cats feces (7).
Ardabil City has the high density of stray dogs and cats. These animals roam freely in the environment and produce offspring that may contaminate the soil of public areas with Toxocara spp. eggs. Additionally, the population of pets is increasing in this city. However, soil contamination with Toxocara spp. eggs have been sporadically reported from Iran (12, 13). There is no previous report about soil contamination with Toxocara spp. eggs in Ardabil City.
The present research was designed to estimate the extent of soil contamination with Toxocara spp. eggs in different public places of Ardabil City, Iran.
Materials and Methods
This cross-sectional descriptive study was conducted in Ardabil, Iran, the capital of Ardabil province (North West Iran, 38° 14′ N and 48° 17′ E), between Mar 2013 and Mar 2014. The climate of the city is extremely cold, snowy in winter, and temperate in summer with an average annual rainfall of about 304 mm. The mean yearly humidity is approximately 70%.
Overall, 200 soil samples were collected from 41 public places in various parts (North, South, West, East and Central), 40 samples in each parts, of Ardabil City, including sidewalks, public parks, squares, children playgrounds and rubbish dumps by simple random selection (Fig. 1).
Fig. 1:
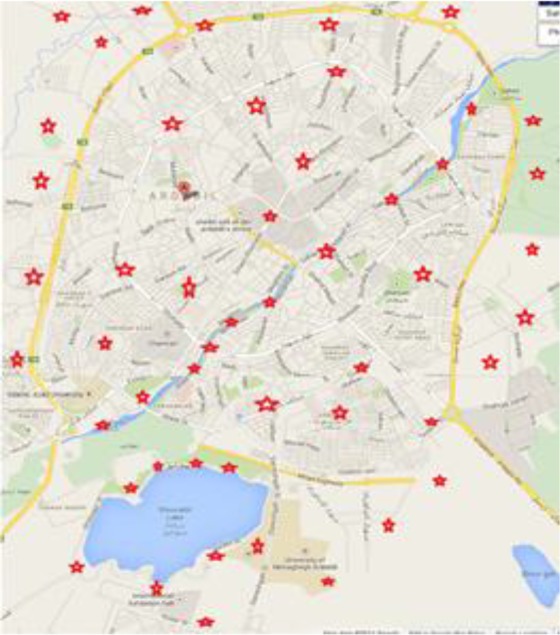
Map showing soil sampling in various parts of public places in Ardabil City, Iran
Sampling was done in all 4 seasons of the year.
To carry out this, at each sites approximately 150 gram of soil sample was taken with the aid of a small shovel from 8 cm ground depth (14). The samples were placed in sealed and labeled plastic bags and transported to the laboratory. Then, they were dried at room temperature for 2–3 days and passed through a 150 μm mesh sieve.
To recover Toxocara spp. eggs, the soil samples were examined by a centrifugal-floatation method with the use of saturated sodium nitrate solution (15). Briefly, 20 gr of the soil sample was placed in a Erlenmeyer’s flask containing 50 ml of 5% NaOH (Merck, Germany) mixed and left for 1h to separate eggs from the soil. The samples were then vortexed for 10min, the suspension was transferred to falcon tube and centrifuged at 1500rpm for 3min. The supernatant was discarded and saturated NaNO3 (Merck, Germany) with specific gravity of 1.30 was added and centrifuged again at 1500rpm for 3min. Finally, the Na-NO3 was added to the tube to form a meniscus and a cover slip was overlaid. After 30 min, the cover slip was transferred onto a microscopic slide.
The preparations were evaluated for presence of Toxocara spp. eggs under the light microscope at 100X and 400X magnification. Based on the size and morphology, the parasites were indicated as Toxocara spp. eggs or others.
Results
Of 200 soil samples collected in 41 public places, 35 (17.5%) were positive for helminths eggs and nematode larvae (Table 1). Six genera of nematode eggs and larvae (Toxocara spp. egg (Fig. 2), Ascaris spp. egg, Trichuris spp. egg, Toxoascaris egg, oxyur spp. egg and nematode larvae) and one genera of trematode egg (Dicrocoelium spp. egg) were recovered (Table 2). Toxocara spp. eggs were the most common helminth egg in the public places of Ardabil City (7%) followed by Ascaris spp. eggs (4%) and others (Table 2).
Table 1:
Contamination rate of soil in the various parts of the Ardabil City, Iran with helminths eggs and nematode larvae
| Zone | Number of sites studied | Number of positive samples | Percent |
|---|---|---|---|
| Central | 8 | 2 | 5 |
| North | 8 | 8 | 20 |
| South | 9 | 19 | 47.5 |
| East | 8 | 3 | 7.5 |
| West | 8 | 3 | 7.5 |
| Total | 41 | 35 | 17.5 |
Fig. 2:
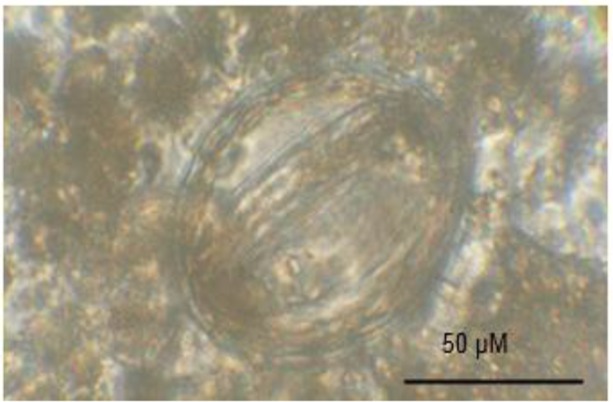
Eggs of Toxocara spp. isolated from the soil of Ardabil public places in floatation method
Table 2:
Type and frequency of helminthes eggs and nematode larvae recovered in various parts of the Ardabil City
| Parasites | Number of positive samples | Frequency |
|---|---|---|
| Toxocara spp. egg | 14 | 7 |
| Ascaris spp. egg | 8 | 4 |
| Trichuris spp egg | 5 | 2.5 |
| Nematode larvae | 4 | 2 |
| Toxascaris egg | 2 | 1 |
| Oxyur egg | 1 | 0.5 |
| Dicrocoelium spp. egg | 1 | 0.5 |
The contamination of different parts of Ardabil public places with Toxocara spp. eggs are presented in Table 3.
Table 3:
Contamination rate of soil in the various parts of the Ardabil City, Iran with Toxocara spp. Eggs
| Zone | Number of sites studied | Number of positive samples | Percent |
|---|---|---|---|
| Central | 8 | 1 | 2.5 |
| North | 8 | 1 | 2.5 |
| South | 9 | 7 | 17.5 |
| East | 8 | 5 | 12.5 |
| West | 8 | 0 | 0 |
The burden of Toxocara spp. eggs and other detected parasites in current research during the whole year showed that in the spring 6 (12%) out of 50 soil samples were positive for Toxocara spp eggs followed by Ascaris spp. Egg 1(2%), Trichuris spp. egg 1(2%) and others. None of the samples was positive for Dicrocoelium spp egg and Nematode larvae.
In the summer Toxocara spp. egg was found in 1 (2%) of the soil samples and 1 (2%) of the samples was positive for Trichuris spp. egg.
In the autumn 13 out of 50 soil samples were positive for parasitic stages, 4 (8%) out of 200 samples contained Ascaris spp. eggs, 4 (8%) of the samples were positive for Nematode larvae, 3 (6%) were positive for Trichuris spp. eggs, 1(2%) for Toxocara spp. egg and 1 (2%) for Dicrocoelium spp. egg.
In the winter 10 out of 50 soil samples contained parasitic stages, Toxocara spp. eggs were found in 6 (12%) of the samples and eggs of Ascaris spp. were found in 3 (6%) and Toxascaris egg was found in 1 (2%) samples (Table 4).
Table 4:
Parasitic burden of parasites in the soil in public places of Ardabil City in the whole year, Mar 2013 to Mar 2014
| Parasites | Spring (%) | Summer (%) | Autumn (%) | Winter (%) |
|---|---|---|---|---|
| Toxocara spp. egg | 6 (12) | 1 (2) | 1 (2) | 6 (12) |
| Ascaris spp. egg | 1 (2) | - | 4 (8) | 3 (6) |
| Trichuris spp. egg | 1 (2) | 1 (2) | 3 (6) | - |
| Nematode larvae | - | - | 4 (8) | - |
| Toxascaris egg | 1 (2) | - | - | 1 (2) |
| Oxyur spp. egg | 1 (2) | - | - | - |
| Dicrocoelium spp. egg | - | - | 1 (2) | - |
Discussion
The ascarids Toxocara canis and Toxocara cati are common nematodes of dogs and cats, respectively. The soil contamination with Toxocara spp. eggs is a significant etiological agent of visceral larva migrans, ocular larva migrans and covert toxocariasis in humans (16). Adult worms of Toxocara have been reported in dogs and cats in Iran. Studies have documented the prevalence of 10%–51.6% (17, 18) and 9.4% – 52.7% (8, 19, 20) in dogs and cats, respectively.
This is the first epidemiological survey on frequency of different parasites in soil samples of public places in Ardabil City using centrifugal-floatation method with an emphasis on Toxocara spp. eggs.
The presence of Toxocara spp. eggs in the soil samples in this survey implied that the stray dogs and cats were infected and defecated in this area. There has been a positive relation between the frequency and concentration of Toxocara spp. eggs in the soil and the possibility of human infection (15). Therefore, this alarmingly frequency of Toxocara spp. eggs in the soil represents a potential hazard for human. There is no data available regarding the human toxocariasis in Ardabil Province.
The data obtained on the soil contamination rate with the Toxocara spp. eggs was as in London/UK (6.3%), Dublin/Ireland (6%), Utrecht/Netherland (7%), Andhra Pradesh/India (6.5%), Pozani/Poland (6.3%), Assam/India (6.12%), Shiraz/Iran (6.3%), different zones/Costa Rica (7%), Urmia/Iran (7.8%) and Qazvin/Iran (5.8%) (9, 21–29).
The rate of contamination in Ardabil City was lower than other studies such as Sao Paulo/Brazil (29%), Kolaczkowwo/Poland (14.5%), Khorram Abad/Iran (22.2%), Erzurum/Turkey (64.28%), Tehran/Iran (10%), Tehran/Iran (38.7%), Bareilly City/India (12.84%), Abadan/Iran (29.2%), Mashhad/Iran (9.2%) and Kermanshah/Iran (18%) (6, 13, 30–37).
The level of soil contamination found in present investigation was higher than reports from Murcia/Spain (1.24%), Melbourne/Australia (1%), Pondicherry/India (2.21%) and Chennai/India (4.75%) (1, 38–40).
The highest frequency of contamination was found in the southern parts of the city. The influence of the location of the area on the environmental contamination is an ongoing controversial issue (41). This fact may be due to the low socioeconomic status of inhabitants and the easy access to dogs and cats in these places.
The frequency of Toxocara spp. eggs during summer, and autumn was lower than during spring and winter. Our results are in agreement with previous studies showing the seasonal discrepancy influencing in the prevalence of Toxocara spp. eggs in the environment. The differences of contamination rate may be due to culture, geographical parameters, climate condition, seasonal change, soil type, type of population of cat and dog, people’s attitudes toward pets, sample collection, methodology of examination and diagnostic techniques. Thus, the comparison of all such surveys is not reasonable.
Conclusion
Although, this investigation gives baseline knowledge regarding soil contamination with Toxocara spp. eggs and will provide significant information about toxocariasis. Data available about infection in human were limited. Further survey should focus on distinguishing toxocariasis in human in order to develop suitable methods for prevention and control of the disease.
Acknowledgments
The authors express their gratitude for the financial support provided by the office of Ardabil University of Medical Sciences. The authors declare that there is no conflict of interests.
References
- 1. Thomas D, Jeyathilakan N. Detection of Toxocara eggs in contaminated soil from various public places of Chennai city and detailed correlation with literature. J Parasit Dis. 2014; 38 (2): 174– 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Despommier D. Toxocariasis: Clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16 (2): 265– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee AC, Schantz PM, Kazacos KR, Montgomery SP, Bowman DD. Epidemiologic and zoonotic aspects of ascarid infections in dogs and cats. Trends Parasitol. 2010; 26 (4): 155– 61. [DOI] [PubMed] [Google Scholar]
- 4. Sadjjadi SM, Khosravi M, Mehrabani D, Orya A. Seroprevalence of Toxocara infection in school children in Shiraz, southern Iran. J Trop Pediatr. 2000; 46 (6): 327– 30. [DOI] [PubMed] [Google Scholar]
- 5. Harbinder S, Bali HS, Arvinder K. Prevalence of Toxocara spp. Eggs in the soil of public and private places in Ludhiana and Kellon area of Punjab, India. Epidemiol Sante Anim. 1997: 31– 2. [Google Scholar]
- 6. Zibaei M, Abdollahpour F, Birjandi M, Firoozeh F. Soil contamination with Toxocara spp. Eggs in the public parks from three areas of Khorramabad, Iran. Nepal Med Coll J. 2010; 12 (2): 63– 5. [PubMed] [Google Scholar]
- 7. Traversa D, Frangipane di Regalbono A, Di Cesare A, La Torre F, Drake J, Pietrobelli M. Environmental contamination by canine geohelminths. Parasit Vectors. 2014; 7: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pezeshki A, Rezaeian M, Zarebavani M. Toxocara cati infection in cats in Tehran and their importance in medicine. Sci J Anim Sci. 2013; 2 (7): 195– 8. [Google Scholar]
- 9. Tavassoli M, Hadian M, Charesaz S, Javadi S. Toxocara spp. Eggs in public parks of Urmia city, west Azerbaijan Province Iran. Iran J Parasitol. 2008; 3 (3): 24– 29. [Google Scholar]
- 10. Azian MY, Sakhone L, Hakim SL, Yusri MY, Nurulsyamzawaty Y, Zuhaizam AH, Rodi IM, Maslawaty MN. Detection of helminth infections in dogs and soil contamination in rural and urban areas. Southeast Asian J Trop Med Public Health. 2008; 39 (2): 205– 12. [PubMed] [Google Scholar]
- 11. Khademvatan S, Abdizadeh R, Tavalla M. Molecular characterization of Toxocara spp. from soil of public areas in Ahvaz southwestern Iran. Acta Trop. 2014; 135: 50– 4. [DOI] [PubMed] [Google Scholar]
- 12. Abdi J, Darabi M, Sayehmiri K. Epidemiological situation of toxocariasis in Iran: Meta-analysis and systematic review. Pak J Biol Sci. 2012; 15 (22): 1052– 5. [DOI] [PubMed] [Google Scholar]
- 13. Maraghi S, Mazhab Jafari K, Sadjjadi SM, Latifi SM, Zibaei M. Study on the contamination of Abadan public parks soil with Toxocara spp. Eggs. J Environ Health Sci Eng. 2014; 12: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uga S, Matsumura T, Aoki N, Kataoka N. Prevalence of Toxocara species eggs in the sandpits of public parks in Hyogo prefecture, Japan. Kiseichugaku Zasshi. 1989; 38 (5): 280– 4. [Google Scholar]
- 15. Mizgajska-Wiktor H. Recommended method for recovery of Toxocara and other geohelminth eggs from soil. Wiad Parazytol. 2005; 51 (1): 21– 2. [PubMed] [Google Scholar]
- 16. Nourian AA, Amiri M, Ataeian A, Haniloo A, Mosavinasab SN, Badali H. Seroepidemiological study for toxocariasis among children in Zanjan-northwest of Iran. Pak J Biol Sci. 2008; 11 (14): 1844– 7. [DOI] [PubMed] [Google Scholar]
- 17. Eslami A. Veterinary helminthology. Iran: University of Tehran Press; 1997. [Google Scholar]
- 18. Fallah M, Azimi A, Taherkhani H. Seroprevalence of toxocariasis in children aged 1–9 years in western Islamic Republic of Iran, 2003. East Mediterr Health J. 2007; 13 (5): 1073– 7. [DOI] [PubMed] [Google Scholar]
- 19. Jamshidi Sh, Meshki B, Toghani M. A study of helminthic infection of gastrointestinal tract in stray cats at urban, areas in Isfahan. J Fac Vet Med Unvi Tehran. 2002; 57 (2): 25– 7. [In Persian] [Google Scholar]
- 20. Meshgi B, Jamshidi Sh, Saadati D, Hooshyar H, Bokaie S. An overview of Toxocara cati infection in stray cats in the metropolitan region of Tehran, Iran, and a comparison of two diagnostic methods. Int J Vet Res. 2010; 4 (1): 53– 56. [Google Scholar]
- 21. Gillespie SH, Pereira M, Ramsay A. The prevalence of Toxocara canis ova in soil samples from parks and gardens in the London area. Public Health. 1991; 105 (4): 335– 9. [DOI] [PubMed] [Google Scholar]
- 22. Holland C, O’connor P, Taylor MR, Hughes G, Anthony Girdwood RW, Smith H. Families, parks, gardens and toxocariasis. Scand J Infect Dis. 1991; 23 (2): 225– 31. [DOI] [PubMed] [Google Scholar]
- 23. Jansen J, Van Knapen F, Schreurs M, Van Wijngaarden T. Toxocara ova in parks and sand-boxes in the city of Utrecht. Tijdschr Diergeneeskd. 1993; 118 (19): 611– 4. [In Dutch] [PubMed] [Google Scholar]
- 24. Kumar BV, Hafeez M. A study on the prevalence of Toxocora spp eggs at public places in Andhra pradesh. J Commun Dis. 1998; 30 (3): 197– 8. [PubMed] [Google Scholar]
- 25. Maśnik E. Relationships between the prevalence of Toxocara eggs in dogs’ faeces and soil. Wiad Parazytol. 2000; 46 (2): 239– 44. [PubMed] [Google Scholar]
- 26. Singh LA, Das SC, Baruah I. Observations on the soil contamination with the zoonotic canine gastrointestinal parasites in selected rural areas of Tezpur, Assam, India. J Parasit Dis. 2004; 28 (2): 121– 3. [Google Scholar]
- 27. Motazedian H, Mehrabani D, Tabatabaee SH, Pakniat A, Tavalali M. Prevalence of helminth ova in soil samples from public places in Shiraz. East Mediterr Health J. 2006; 12 (5): 562– 5. [PubMed] [Google Scholar]
- 28. Paquet-Durand I, Hernández J, Dolz G, Zuniga JJ, Schnieder T, Epe C. Prevalence of Toxocara spp., toxascaris leonina and ancylostomidae in public parks and beaches in different climate zones of Costa rica. Acta Trop. 2007; 104 (1): 30– 7. [DOI] [PubMed] [Google Scholar]
- 29. Saraei M, Zakilo M, Tavazoei Y, Jahanihashemi H, Shahnazi M. Contamination of soil and grass to Toxocara spp. Eggs in public parks of Gazvin, Iran. Asian Pac J Trop Biomed. 2012; 2 (2): 1156– 58. [Google Scholar]
- 30. Santarém VA, Franco Eda C, Kozuki FT, Fini D, Prestes-Carneiro LE. Environmental contamination by Toxocara spp. Eggs in a rural settlement in Brazil. Rev Inst Med Trop Sao Paulo. 2008; 50 (5): 279– 81. [DOI] [PubMed] [Google Scholar]
- 31. Jarosz W, Mizgajska-Wiktor H, Kirwan P, Konarski J, Rychlicki W, Wawrzyniak G. Developmental age, physical fitness and Toxocara seroprevalence amongst lower-secondary students living in rural areas contaminated with Toxocara eggs. Parasitology. 2010; 137 (1): 53– 63. [DOI] [PubMed] [Google Scholar]
- 32. Avcioglu H, Balkaya I. The relationship of public park accessibility to dogs to the presence of Toxocara species ova in the soil. Vector Borne Zoonotic Dis. 2011; 11 (2): 177– 80. [DOI] [PubMed] [Google Scholar]
- 33. Khazan H, Khazaei M, Tabaee SS, Mehrabi A. Prevalence of Toxocara spp. Eggs in public parks in Tehran city, Iran. Iran J Parasitol. 2012; 7 (3): 38– 42. [PMC free article] [PubMed] [Google Scholar]
- 34. Tavalla M, Oormazdi H, Akhlaghi L, Razmjou E, Lakeh MM, Shojaee S, Hadighi R, Meamar AR. Prevalence of parasites in soil samples in Tehran public places. Afr J Biotechnol. 2012; 11 (20): 4575– 78. [Google Scholar]
- 35. Sudhakar NR, Samanta S, Sahu S, Raina OK, Gupta SC, Madhu DN, Kumar A. Prevalence of Toxocara species eggs in soil samples of public health importance in and around bareilly, Uttar pradesh, India. Vet World. 2013; 6 (2): 87– 90. [Google Scholar]
- 36. Berenji F, Movahedi Rudy AG, Fata A, Tavassoli M, Mousavi Bazaz M, Salehi Sangani G. Soil contamination with Toxocara spp. Eggs in public parks of Mashhad and Khaf, northeast of Iran. Iran J Parasitol. 2015; 10 (2): 286– 9. [PMC free article] [PubMed] [Google Scholar]
- 37. Ghashghaei O, Khedri J, Jahangiri-nasr F, Hashemi SH, Nourollahi Fard SR. Contamination of soil samples of public parks with Toxocara spp. Eggs in Kermanshah, Iran. J Fac Vet Med Istanbul Univ. 2016; 42 (1): 47– 50. [Google Scholar]
- 38. Ruiz de Ybáñez MR, Garijo MM, Alonso FD. Prevalence and viability of eggs of Toxocara spp. And Toxascaris leonina in public parks in eastern spain. J Helminthol. 2001; 75 (2): 169– 73. [PubMed] [Google Scholar]
- 39. Carden SM, Meusemann R, Walker J, Stawell RJ, MacKinnon JR, Smith D, Stawell AM, Hall AJ. Toxocara canis: Egg presence in Melbourne parks and disease incidence in Victoria. Clin Exp Ophthalmol. 2003; 31 (2): 143– 6. [DOI] [PubMed] [Google Scholar]
- 40. Das SS, Kumar D, Sreekrishnan R, Ganesan R. Soil contamination of public places, play grounds and residential areas with ova of Toxocara. Indian J Vet Res. 2009; 18 (2): 13– 6. [Google Scholar]
- 41. Sprenger LK, Green KT, Molento MB. Geohelminth contamination of public areas and epidemiological risk factors in Curitiba, Brazil. Rev Bras Parasitol Vet. 2014; 23 (1): 69– 73. [DOI] [PubMed] [Google Scholar]


