Abstract
Clinical electrophysiology has made the traditional classification of rapid atrial rhythms into flutter and tachycardia of little clinical use. Electrophysiological studies have defined multiple mechanisms of tachycardia, both re-entrant and focal, with varying ECG morphologies and rates, authenticated by the results of catheter ablation of the focal triggers or critical isthmuses of re-entry circuits. In patients without a history of heart disease, cardiac surgery or catheter ablation, typical flutter ECG remains predictive of a right atrial re-entry circuit dependent on the inferior vena cava-tricuspid isthmus that can be very effectively treated by ablation, although late incidence of atrial fibrillation remains a problem. Secondary prevention, based on the treatment of associated atrial fibrillation risk factors, is emerging as a therapeutic option. In patients subjected to cardiac surgery or catheter ablation for the treatment of atrial fibrillation or showing atypical ECG patterns, macro-re-entrant and focal tachycardia mechanisms can be very complex and electrophysiological studies are necessary to guide ablation treatment in poorly tolerated cases.
Keywords: Typical atrial flutter, atypical atrial flutter, macro-re-entrant atrial tachycardia, flutter ablation, classification of atrial tachycardias
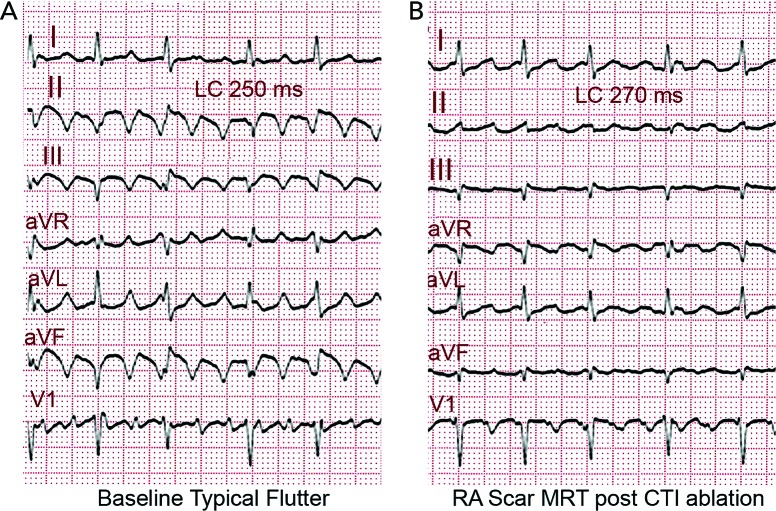
The term ‘flutter’ was coined to designate the visual and tactile rapid, regular atrial contraction induced by faradic stimulation in animal hearts, in contrast with irregular, vermiform contraction in atrial fibrillation (AF).[1,2] On the ECG, flutter was a regular continuous undulation between QRS complexes at a cycle length (CL) of ≤250 ms (≥240 bpm). Slower tachycardias displaying discrete P waves, separated by isoelectric baselines, were called ‘atrial tachycardia’. Early studies suggested that flutter had a re-entrant mechanism[3–5] but others attributed flutter to focal discharge.[6,7] Later human studies left the door open for a focal mechanism.[8] This was not a significant consideration when digitalis and very few antiarrhythmic drugs (AADs) were the only therapeutic armamentarium, but determining the mechanism involved in flutter has become crucial for the design and application of catheter and surgical ablation techniques. Modern electrophysiology (EP) has confirmed the re-entrant mechanism of typical flutter, and has opened wide the spectrum of mechanisms of macro-re-entrant tachycardias (MRTs), prompting a new, more open view of clinical ECG-based classification (see Figure 1A and 1B).[9]
Figure 1: The ECG Pattern May Not Reflect the Mechanism.
(A) ECG fulfilling classical flutter criteria (rate and lack of isoelectric baseline) in a case of focal tachycardia originating in the right superior pulmonary vein. Note the irregular ventricular rate in the face of regular atrial rate. (B) ECG of a case of scar macro-reentrant tachycardia of the right atrium fulfilling the classical ‘atrial tachycardia’ criteria. Discrete P waves separated by stable baselines are recorded between QRS complexes with 2:1 atrioventricular conduction.
Typical Atrial Flutter
The Re-entrant Mechanism
Typical flutter is the type of MRT most frequently found in the clinical setting. The mechanism is a large re-entrant circuit contained in the right atrium (RA) with passive activation of the left atrium (LA).[10] Activation courses superoinferiorly in the anterior and lateral RA and inferosuperiorly in the septal RA, with a critical inferior turning point between the tricuspid ring and inferior vena cava (IVC) known as the cavotricuspid isthmus (CTI) (see Figure 2). An area of transverse conduction block in the posterior RA related to anisotropic conduction at the terminal crest[11–14] and other structures[15] forces activation toward the high RA so that the upper turning point can be at the RA roof or high in the posterior RA, depending of the size of the area of block.[16–18] In either case, the CTI remains an obligatory passage for activation in the inferior RA.
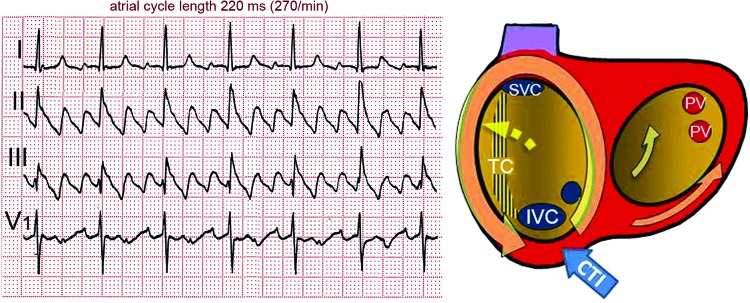
Figure 2: ECG of a Typical Atrial Flutter.
Atrial activity in leads II and III is a continuous undulation with a sharp negative deflection (‘saw-tooth pattern’). There is a biphasic deflection in V1. The schema on the right displays the atria in a left anterior oblique view. Mitral and tricuspid rings are enlarged in order to show the posterior walls. The terminal crest (TC) is shown as a vertically-dashed area reaching from the superior vena cava (SVC) to the inferior vena cava (IVC). The circular arrow shows typical counter clockwise re-entrant activation. CS = coronary sinus ostium; CTI = cavotricuspid isthmus; PV = left pulmonary veins ostia. For further explanation see text.
Either spontaneously or after programmed stimulation, re-entry may occur in the opposite (clockwise) direction – i.e. superoinferior in the septal wall and inferosuperior in the anterolateral wall – with the same zones of block in the posterior RA and obligatory passage through the CTI (see Figure 3).[19] This reverse typical flutter is much less common clinically than the counter clockwise form, but the clinical manifestations are indistinguishable.
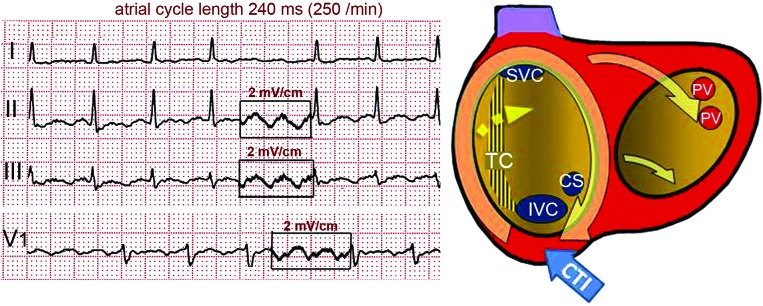
Figure 3: Reverse (Clockwise) Typical Flutter.
Note the dominant positive deflections in the flutter waves and the W-shaped deflection in lead V1. For further explanation and abbreviations, see text and Figure 2.
The ECG Patterns
Typical (counter clockwise) flutter is associated with the ‘common’ flutter pattern[20,21] (see Figure 2): a regular continuous undulation with dominant negative deflections in inferior leads II, III and aVF, often described also as a ‘saw tooth pattern’, and flat atrial deflections in leads I and aVL. Atrial deflections in V1 can be positive, biphasic or negative. The CL is usually 250–170 ms (rates 240–350/min). Reverse typical flutter (see Figure 3) usually shows rounded or bimodal positive deflections in inferior leads II, III and aVF, and a very characteristic bimodal negative wave in the shape of a W is seen in lead V1.[21–22]
One frequent presentation of flutter is in patients treated with class IC AADs for AF. Flutter rate may be slowed by the AAD to ≤200/min, facilitating 1:1 atrioventricular (AV) conduction that due to the effect of the ADD results in aberrant intraventricular conduction and a wide QRS complex tachycardia (see Figure 4).
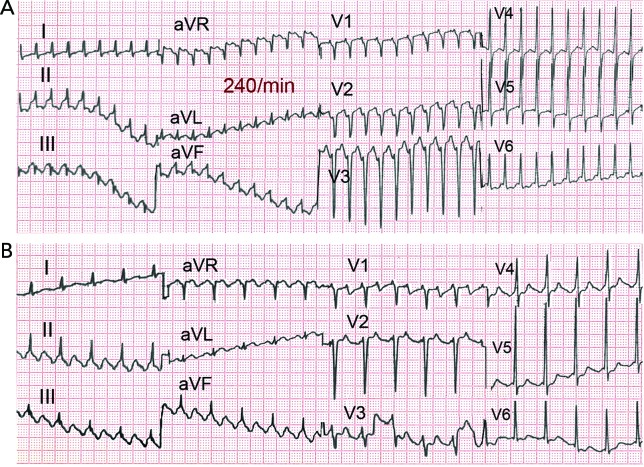
Figure 4: One to One AV Conduction in Flutter Slowed by Flecainide.
(A) Slow flutter (170 BPM) with 1:1 atrioventricular conduction and wide QRS complex (right bundle branch block and superior axis) in a patient treated with flecainide for paroxysmal atrial fibrillation. (B) A 2:1 atrioventricular block with slower ventricular rate and narrow QRS allows recognition of slow but typical flutter waves.
CTI-dependent MRT is also frequent in patients with previous surgical atriotomies or atrial baffle procedures, or after LA ablation for the treatment of AF.[23,24] In these cases ECG patterns are often atypical. Conversely, a typical flutter ECG may be generated by atypical re-entry circuits, independent of the CTI, including LA circuits.[25] Flutter wave morphology can be determined by activation outside the re-entry circuit, which would explain the often-difficult correlation between mechanism and ECG pattern.[26,27]
It should be emphasised that ECG diagnosis is based on atrial deflections and not on ventricular (QRS) rate and rhythm. An irregular ventricular rhythm may be caused by changing degrees of AV nodal block (see Figures 1A and 3), including Wenckebach cycles. In doubtful cases it is essential to document atrial activity dissociated from ventricular activity by increasing AV block by vagal manoeuvres or intravenous adenosine. However adenosine can produce a rebound increase in AV conduction to 1:1 [28,29] and in some cases it can precipitate AF,[30] therefore it should only be used if necessary for diagnosis and resuscitation equipment should be readily available.
Pathogenesis of Typical Flutter
About 80 % of flutter patients are male,[31,32] otherwise flutter occurs in clinical contexts very much like those observed in AF (in old age, hypertension, diabetes, chronic obstructive lung disease, excessive alcohol consumption[33] or during endurance sports practice).[34] In many cases flutter episodes alternate with fibrillation episodes.[32,35] Of those initially presenting with flutter as the only arrhythmia, 50 % develop fibrillation during long-term follow-up.[36] This figure is not far from the proportion of patients developing fibrillation in the long term after CTI ablation for the treatment of typical flutter.[37,38]
The thickness of the terminal crest[39,40] and its capacity to block transverse conduction[41–44] are increased in cases of flutter compared to AF. EP studies have shown areas of low-voltage electrograms[45] and slow conduction in the RA – particularly at the CTI[46–48] – to be a sign of arrhythmogenic myocardial remodelling. LA dilatation and abnormalities in its reservoir function have been described as predictors of the incidence of atrial flutter or fibrillation.[49]
Clinical Presentation
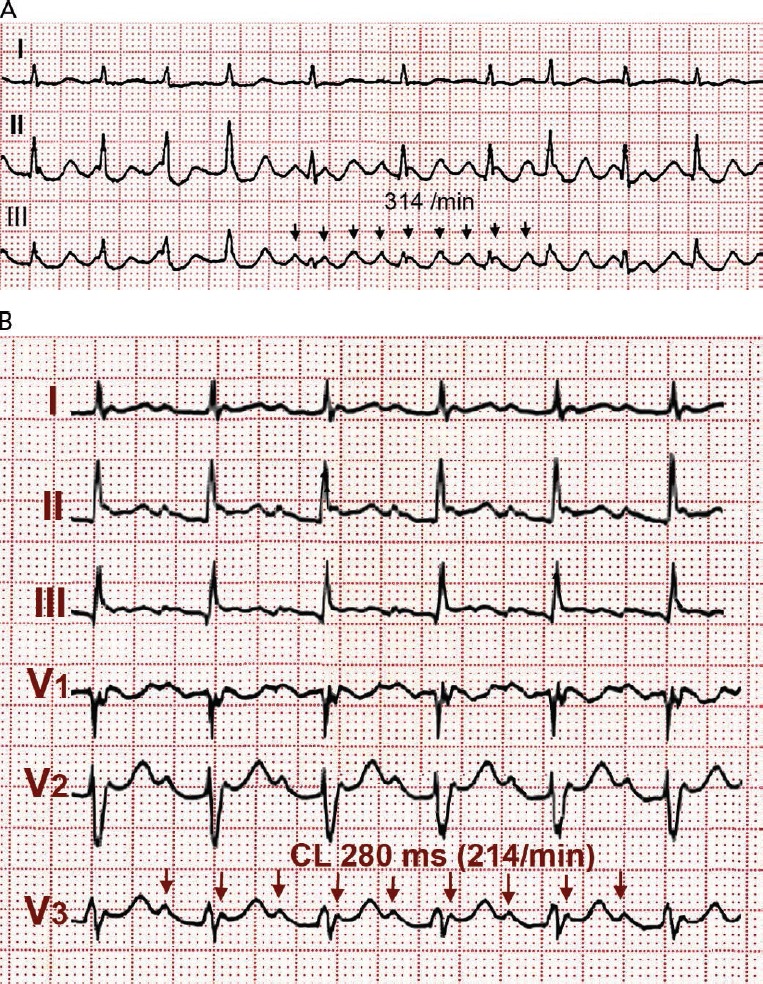
Flutter can be paroxysmal or persistent. Clinical presentation will depend in large part on the ventricular rate, which is most often around 120–150 due to 2:1 AV conduction, but in some cases 1:1 AV conduction leads to extremely high rates with poor clinical tolerance often requiring immediate intervention (see Figure 5). As in AF, loss of effective atrial contraction synchronised to ventricular contraction and rapid ventricular rates may result in hypotension, angina, heart failure, syncope or a feeling of palpitation making the patient seek medical attention.[50] Occasionally flutter can be asymptomatic for weeks or months and the sustained tachycardia can lead to systolic ventricular dysfunction and heart failure (tachycardiomyopathy).[51,52] Ventricular function and atrial dilatation may recover after return to sinus rhythm,[53] but arrhythmia recurrence can again precipitate dysfunction with a risk of sudden death.[54]
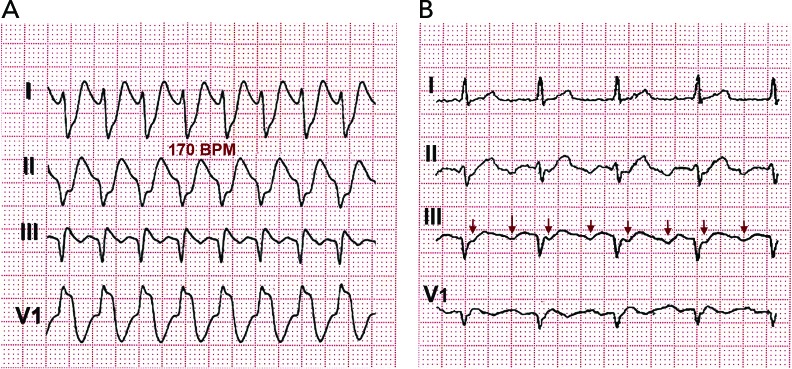
Figure 5: Spontaneous 1:1 AV Conduction in Typical Flutter.
(A) Typical flutter with 1:1 atrioventricular conduction at 240 BPM spontaneously evolving to 2:1 atrioventricular conduction. (B) Typical flutter waves can be easily identified.
LA appendage thrombi, spontaneous echo contrast and low appendage emptying velocities have been detected in cases of flutter submitted to cardioversion,[55] although to a lesser extent than in AF,[56] and normalisation can occur days after return to sinus rhythm.[57] The frequency of systemic embolism in flutter is about one-third that in AF,[36,58,59] but this difference disappears when both flutter and fibrillation occur in the same patient.
Management of Atrial Flutter
Rate control should be the first treatment step in symptomatic patients with a rapid ventricular rate. This is often a difficult goal in flutter, and even associations of the AV node blocking drugs (digoxin, beta-blockers and calcium antagonists) may fail, making cardioversion to sinus rhythm necessary. Dofetilide and ibutilide, pure class III AADs, are effective for interrupting flutter with a small risk of QT prolongation and torsade de pointes. Class IA and IC AADs are relatively ineffective or have no effect[60–65] and can be problematic if they cause a slow atrial flutter rate ≤200/min with 1:1 AV conduction and QRS widening that mimics ventricular tachycardia (see Figure 4).[66,67] Amiodarone may not be very effective at re-establishing sinus rhythm in the acute setting but it does help control ventricular rate.[68,69]
Rhythm Control: Cardioversion
The poor results of rhythm control strategies in AF may not apply in flutter because of a lower recurrence rate after cardioversion in flutter, making a strategy of repeated cardioversions supported with AADs a clinically applicable option.[70,71] Transthoracic direct-current cardioversion, under short-lasting sedation, is the quickest and most effective method to recover sinus rhythm in patients with flutter, with a lower energy delivery and higher success rate than in AF.[72,73]
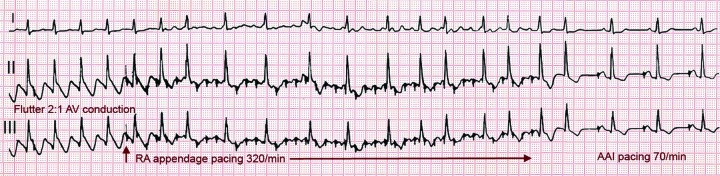
In 50–80 % of cases flutter interruption can be achieved by atrial pacing above the flutter rate through a transvenous catheter, through epicardial electrodes placed during cardiac surgery[8] or by programming fast atrial rates in patients with atrial or dual-chamber pacemakers.[74] Pacing runs of 20–30 s are started at a rate 10 bpm higher than flutter, increasing in 10 bpm steps up to 400 bpm or until flutter is interrupted and sinus (or paced) atrial rhythm is established (see Figure 6). Pacing may induce AF or a faster flutter (type II flutter),[75] probably as an expression of functional re-entry[76] that tends to return to baseline flutter or change to AF. AF induced by pacing usually results in a lower ventricular rate and, not infrequently, terminates spontaneously into sinus rhythm.
Figure 6: Flutter Cardioversion by Rapid Stimulation.
ECG leads I, II and III in a patient with an implanted AAI pacemaker. Typical flutter is present at the onset. Rapid atrial asynchronous (A00) pacing abolishes negative deflections in II and III and a positive P wave appears in I. When pacing stops flutter is no longer present and atrial demand pacing resumes.
Flutter cardioversion by pacing is painless and can be done without sedation or anaesthesia.[77] It may be more effective in postoperative flutter[78] and in younger patients without structural heart disease or heart failure[79] and it may be facilitated by class I AADs.[80,81] Atrial pacing can be applied from the oesophagus[82] but the higher output stimulation necessary may be painful[83] and can occasionally induce ventricular arrhythmias.[84]
The patient should be anticoagulated if cardioversion is planned, be it by direct current shock or pacing, whenever the duration of flutter is >100 h. Patients with flutter of longer duration should be anticoagulated for 3–4 weeks before cardioversion or LA thrombi should be ruled out by transoesophageal echocardiography. Following cardioversion, anticoagulation should be maintained for a minimum of 3–4 weeks in patients with low embolic risk. If high embolic risk is present, anticoagulation should be continued indefinitely, unless prolonged follow-up monitoring demonstrates an absence of recurrence.[85]
Catheter Ablation
Radiofrequency catheter ablation of the CTI has become a standard treatment for typical flutter.[86] The full thickness of the CTI must be ablated along a line reaching from the tricuspid ring (TR) to the IVC. Radiofrequency can be applied point-by-point, keeping the catheter tip stable for 45–60 s at each site or by dragging the catheter tip slowly from the TR to IVC during continuous radiofrequency delivery.[87] The endpoint of the procedure is complete, bidirectional CTI conduction block that has to be checked by recordings along the ablation line[88,89] and differential pacing manoeuvres.[90] CTI block can be transient, so an observation period of 20–30 min is necessary to confirm success.[91,92] Only when this endpoint is reached is flutter recurrence post ablation reduced to ≤10 %. At mid-term (months) conduction may still resume in 15 % of cases, even in the absence of flutter recurrence.[93]
For radiofrequency ablation large tipped (8 mm electrode length)[94] or irrigated tip catheters[95] are more effective than standard tip (4 mm electrode length) catheters. Supporting sheaths may be used to obtain good contact force on the CTI. Radiofrequency application to the CTI can be quite painful and moderate sedation is often needed during the procedure. Cryoablation can also be effective for CTI ablation and has the advantage of being painless.[96] Resumption of CTI conduction at mid-term is more common after cryoablation than after radiofrequency ablation.[93]
Complications are infrequent (around 1 %)[97] and are usually limited to vascular access; however, extension of ablation to the septal RA can result in AV block when using radiofrequency[98,99] and cryoablation.[96] Damage to the right coronary artery is rare but can result in myocardial infarction in some cases with pre-existing coronary atherosclerotic lesions.[100,101] Cardiac perforation secondary to tissue disruption by boiling with audible ‘pops’ can occur when high energy is delivered with large-tip catheter-electrodes.[102,103] A one in 1,000 incidence of cerebrovascular accidents is reported.[97]
The recurrence rate of typical flutter is ≤10 % after successful CTI ablation and definitive flutter suppression can be attained by a second procedure in recurrent cases. The main problem is the incidence of AF after ablation, which can be 30–50 % in the long term (>3 years).[38,104–106] More recent reports have reported even higher incidences of AF.[107] AF is more likely in patients who have had AF episodes before flutter ablation and in those with dilated LA.
The efficacies of CTI ablation and AADs have been compared for the treatment of typical flutter in two randomised studies.[71,108] CTI ablation proved to be advantageous in terms of better quality of life, less hospitalisation and lower flutter recurrence, but the incidence of AF did not improve in both studies. In patients with flutter appearing during AAD treatment of AF, CTI ablation may help stabilise sinus rhythm[109] at the same time allowing the use of class IC AADs without the risk of slow flutter with 1:1 AV conduction. Direct AF ablation has been proposed by some groups as a complement to CTI ablation in patients with both arrhythmias,[110] and even in those with only flutter,[111] to reduce the later incidence of AF. CTI ablation of typical flutter is associated with a favourable prognosis; however, given the higher incidence of severe complications in AF ablation, patients should be carefully selected for this strategy.[112]
There have been no randomised studies published on the risk–benefit ratio of anticoagulation after successful ablation of typical flutter with no associated AF. Prolonged monitoring of atrial rhythm under anticoagulation would appear to be indicated in patients with a high embolic risk score before anticoagulation is discontinued.
Summary: Long-term Strategy
A first and well-tolerated episode of flutter terminated spontaneously or by electrical cardioversion or AAD can be followed clinically with or without AAD coverage. A recurrence rate of around 50 % can be expected in these patients. Amiodarone, dronedarone or sotalol are indicated to prevent recurrences after cardioversion, while class IC AADs should be used cautiously or avoided. Catheter ablation is more effective for the prevention of recurrence and is a better alternative than maintenance AAD, especially in patients with depressed systolic ventricular function. A rate control strategy could be adequate for asymptomatic elderly patients with no deterioration of systolic ventricular function; however, cardioversion in active patients without apparent functional limitation will often improve a patient’s well-being and functional capacity. Chronic anticoagulation should be considered on the bases of embolic and haemorrhagic risk scores, along the same lines as for AF.[85]
Progression to AF after successful CTI ablation for typical flutter underlines the presence of an atrial arrhythmogenic substrate that can evolve in many cases, even in the absence of flutter recurrence. The diagnosis of flutter should thus be complemented with a clinical profile of AF risk factors that could guide ‘upstream therapy’. Recent reports have shown that physical fitness programmes and vigorous treatment of obesity, metabolic syndrome and sleep apnoea can result in a significant reduction in AF recurrence in patients whether or not they undergo AF ablation,[113–116] and this may be applicable to flutter given the very similar risk factor profiles.
Atrial or AV pacing may be necessary in patients in whom conversion to sinus rhythm reveals sick sinus syndrome. In these cases, a device capable of overdrive atrial pacing should be implanted.
In the absence of direct evidence of the risk–benefit ratio of chronic anticoagulation in flutter, present recommendations for anticoagulation are the same as for AF, carefully balanced against bleeding risk scores.[85]
Atypical Flutter/Macro-re-entrant Tachycardia
The term atypical has been applied to rapid atrial tachycardias with ECG patterns differing from the typical and reverse typical flutter described above, and also to re-entrant tachycardias with circuit configuration different from the typical RA flutter circuit, even if they have an ECG pattern similar to typical flutter. ECG waveform can be determined by activation of the atrial myocardium outside the re-entry circuit[26,27] and the precise mechanism generating atypical ECG flutter patterns can only be determined by mapping and pacing EP studies.[117] Atypical flutter is often associated with structural heart disease, especially in patients that have undergone cardiac surgery or extensive catheter ablation for the treatment of AF. In these cases focal (centrifugal) mechanisms can coexist with MRT with indistinguishable ECG patterns, making EP study the only way to unveil the mechanisms causing the arrhythmia and plan ablation when clinically indicated.[118,119]
Right Atrial Macro-re-entrant Tachycardias
MRT circuits turning around the superior vena cava and part of the terminal crest, not involving the CTI, can occasionally be found in patients without surgical atriotomy (see Figure 7). In patients with RA surgical atriotomy, the scar can become the centre of the MRT, but the small incisions used to cannulate the superior vena cava and IVC are rarely arrhythmogenic by themselves. Lateral wall, superoinferior atriotomy is a frequent cause of atypical (non-CTI-dependent) MRT. ECG pattern may or may not be atypical (see Figures 1B and 8) and it is not unusual for two or more ECG patterns to alternate, as CTI-dependent typical flutter often coexists with the scar MRT (see Figure 8).[23] A patch closing an interatrial septal defect can also become the centre of a MRT circuit (see Figure 7). Atypical flutter or MRT related to surgery often occurs years after the procedure, suggesting that an atrial remodelling process is necessary to make re-entry stable around the surgical obstacles in many cases.
Figure 7: Atypical Right Atrial Macro-reentrant Circuits.
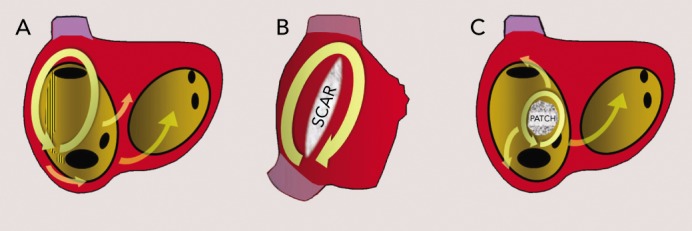
A) Upper loop reentry (left anterior oblique view). Activation rotates around the superior vena cava (SVC) and the terminal crest (TC) but the cavotricuspid isthmus (CTI) is not part of the circuit. B) Right lateral view of the right atrium showing reentry around a surgical scar. C) Reentry around an atrial septal defect repair patch. Modified from Cosio et al., 2003.[117]
Figure 8: Two Flutter Mechanisms, Typical and Atypical, in the Same Patient.
(A) ECG of typical flutter and (B) right atrium macro-re-entrant tachycardia in the same patient. The scar macro-re-entrant tachycardia was induced after cavotricuspid isthmus ablation. Note the cycle length prolongation and atrial wave morphology resembling clockwise flutter in (B).
In patients not subjected to cardiac surgery or AF ablation, unexcitable areas of low voltage, most often located in the lateral RA,[120,121] can become the central obstacle sustaining atypical MRT. These areas are probably related to chronic atrial overload or cardiomyopathy and they are often considered to be fibrotic myocardium but there is no direct evidence of their histology. Low-voltage areas are most prevalent in the RA after a Fontan procedure,[119] leading to the difficult management of recurrent MRTs in these cases.
Left Atrial Macro-re-entrant Tachycardias
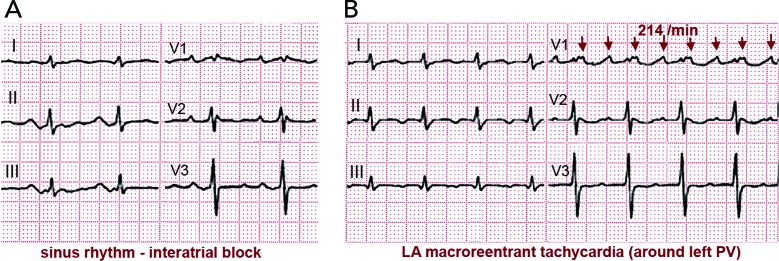
Surgical atriotomy scars are a well-known cause of MRT of the LA[122–124] often combined with re-entry around low-voltage, inexcitable areas not related to atriotomy. In recent years the incidence of atypical flutter/MRT has become epidemic, with a wide variety of re-entry circuits following extensive LA ‘substrate ablation’ for the treatment of persistent AF (see Figure 9). A ‘maturation’ process appears to be necessary to make a MRT circuit stable, as tachycardia inducibility at the end of an AF ablation procedure does not predict later clinical occurrence.[125,126] Recovery of slow conduction across ablation lines in the mid-term appears to be the arrhythmogenic mechanism in most cases.[127,128] An ECG pattern of interatrial (Bachmann) block is often associated with atypical flutter/MRT based in the LA.[129,130] This ECG pattern may be associated too with inexcitable, low-voltage areas in the LA (see Figure 10).[131]
Figure 9: Schematic Representation of Macro-re-entrant Tachycardia Mechanisms in the Left Atrium.
ECGs of a 63-year-old woman with mild mitral stenosis post-commissurotomy. (A) In the sinus rhythm note a very wide P wave with terminal negative deflection in II and III, diagnostic of interatrial (Bachmann) block. (B) ECG during macro-re-entrant tachycardia with very-low-voltage P waves in limb leads and late positive deflection in V1. Activation is rotated around the left pulmonary veins, supported by a wide area of low voltage in the posterior and superior left atrium.
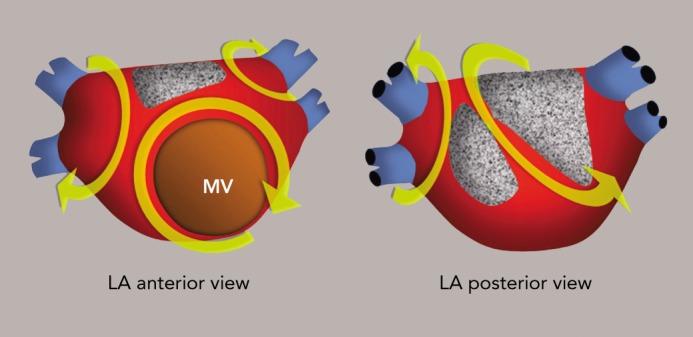
Figure 10: Left Atrial Macro-reentrant Tachycardia in a Patient with Interatrial Block.
Stippled, grey areas represent low-voltage, unexcitable ‘scars’. Yellow curved arrows indicate multiple possible re-entry pathways. The pulmonary veins are represented in blue. MV = mitral valve.
EP studies with RA and LA activation mapping and the response to pacing are necessary to reveal the mechanism in order to guide catheter or surgical ablation. MRT involving the interatrial septum is particularly difficult to treat and success rates are lower than in MRT based on the free atrial walls.[24,132] Ablation of all inducible tachycardias is the accepted objective; however, the significance of non-clinically-inducible tachycardias is not well known. Long-term recurrences can occur despite repeat ablation.[133,134] Some authors describe better results by targeting areas of focal activity as possible triggers than with ablation of re-entry circuits.[135]
MRT can occur after surgical ‘maze’ procedures for the treatment of AF on the basis of re-established slow conduction across suture lines.[136,137] Heart transplantation with atrial-to-atrial suture is practically an experimental model of flutter.[5,138] Knowledge acquired by mapping and ablation-scar-related MRT should help the surgeons and electrophysiologists function as a team to devise non-arrhythmogenic incisions, avoiding surgical approaches that have proved arrhythmogenic, such as the superior transeptal approach to the LA.[139]
Management of Atypical Flutter/Macro-re-entrant Tachycardia
Management of atypical flutter does not differ from that of typical flutter, but the more frequent association with structural heart disease and the multiple possible mechanisms causing an atypical ECG pattern are important factors to consider before making therapeutic decisions. There is very little specific evidence regarding indications for anticoagulation in patients with atypical flutter/MRT and the same indications as in AF are generally recommended.[85]
When atypical flutter/MRT is poorly tolerated and is not controlled with AADs, catheter ablation should be considered. There is no set rule for catheter ablation of atypical MRT tachycardia circuits. Mapping and entrainment studies are necessary to define the focal (centrifugal spread) or MRT mechanism and localise the focal sources or the target isthmus or isthmuses. These procedures may be complicated by the induction of multiple MRT circuits that are not clinically documented. Ablation success is lower than in typical flutter and the recurrence rate is higher, especially in circuits located in the paraseptal areas.[24,132–134] On the other hand, CTI-dependent flutter is a frequent finding in patients with atrial tachycardia and surgical or ablation scars.[24] In cases with multiple MRT circuits, CTI ablation may make ablation success easier by stabilising the atypical MRT circuit and thus making mapping and ablation possible. In cases of atypical MRT of the RA, CTI ablation could be considered even if typical flutter is not documented in order to prevent the later appearance of typical flutter.
Prognosis in these complex cases is difficult to predict[24,128,132–135] but long remissions of tachycardias can be attained in many cases of free wall RA and LA scar. Indications for ablation should be established, taking into account the underlying pathology, quality of life and limitations in functional capacity.
Postoperative Atrial Flutter
The incidence of atrial arrhythmias in the early postoperative period (days) after cardiac surgery is 20–30 %.[140] This high incidence is related to inflammatory changes in the atrial myocardium,[141] not unlike the experimental pericarditis animal models,[142] and it may be prevented by anti-inflammatory corticosteroid treatment.[143,144] AF is the most commonly reported arrhythmia but flutter can also occur in this setting,[8,35,78] although its frequency in relation to AF is not clear. There are very few data on the long-term follow-up of this postoperative flutter, but the incidence of AF in such cases is reported to be around 30 %.[145] If this incidence is extrapolated to flutter, it would appear reasonable to consider that in the early postoperative period after cardiac surgery flutter is an acute, one-time event in the majority of patients and ablation treatment should not be contemplated unless recurrences are documented.
References
- 1.MacWilliam JA. Fibrillar contraction of the heart. JPhysiol. 1887;8:296–310. doi: 10.1113/jphysiol.1887.sp000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolly WA, Ritchie WT Auricular flutter and fibrillation. Heart. 1911;2:177–86. [Google Scholar]
- 3.Lewis T, Feil S, Stroud WD. Observations upon flutter and fibrillation. II. The nature of auricular flutter. Heart. 1920;7:191–233. [Google Scholar]
- 4.Lewis T, Drury AN, Illuescu CC. A demonstration of circus movement in clinical flutter of the auricles. Heart. 1921;8:341–59. [Google Scholar]
- 5.Rosenblueth A, García Ramos J. Estudios sobre el flútter y la fibrilación. II. La influencia de los obstáculos artificiales en el flútter auricular experimental. Arch Inst Cardiol Mex. 1947;17:1–19. [PubMed] [Google Scholar]
- 6.Scherf D, Romano F J, Terranova R. Experimental studies on auricular flutter and auricular fibrillation. Am Heart J. 1948;36:241–51. doi: 10.1016/0002-8703(48)90403-7. [DOI] [PubMed] [Google Scholar]
- 7.Prinzmetal M, Corday E, Oblath RW et al. Auricular flutter. Am J Med. 1951;11:410–30. doi: 10.1016/0002-9343(51)90177-5. [DOI] [PubMed] [Google Scholar]
- 8.Waldo AL, McLean wah, Karp RB et al. Entrainment and interruption of atrial flutter with atrial pacing. Studies in man following open heart surgery. Circulation. 1977;56:737–45. doi: 10.1161/01.cir.56.5.737. [DOI] [PubMed] [Google Scholar]
- 9.Saoudi N, Cosío F, Waldo A et al. Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases; a Statement from a Joint Expert Group from The Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 2001;22:1162–82. doi: 10.1053/euhj.2001.2658. [DOI] [PubMed] [Google Scholar]
- 10.Cosio FG, Arribas F, López Gil M et al. Atrial flutter mapping and ablation. I. Studying atrial flutter mechanisms by mapping and entrainment. PACE. 1996;19:841–53. doi: 10.1111/j.1540-8159.1996.tb03368.x. [DOI] [PubMed] [Google Scholar]
- 11.Olgin JE, Kalman JM, Fitzpatrick AP et al. Role of right atrial endocardial structures as barriers to conduction during human type I atrial flutter. Activation and entrainment mapping guided by intracardiac echocardiography. Circulation. 1995;92:1839–48. doi: 10.1161/01.cir.92.7.1839. [DOI] [PubMed] [Google Scholar]
- 12.Tai C-T, Chen S-A, Chen Y-J et al. Conduction properties of the crista terminalis in patients with typical atrial flutter: basis for a line of block in the reentrant circuit. J Cardiovasc Electrophysiol. 1998;9:811–9. doi: 10.1111/j.1540-8167.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 13.Shumacher B, Jung W, Schmidt H et al. Transverse conduction capabilities of the crista terminalis in patients with atrial flutter and atrial fibrillation. J Am Coll Cardiol. 1999;34:363–73. doi: 10.1016/s0735-1097(99)00211-9. [DOI] [PubMed] [Google Scholar]
- 14.Arenal A, Almendral J, Alday JM et al. Rate-dependent conduction block of the crista terminalis in patients with typical atrial flutter: influence on evaluation of cavotricuspic isthmus conduction block. Circulation. 1999;99:2771–9. doi: 10.1161/01.cir.99.21.2771. [DOI] [PubMed] [Google Scholar]
- 15.Friedman PA, Luria D, Fenton AM et al. Global right atrial mapping of human atrial flutter: the presence of posteromedial (sinus venosa region) functional block and double potentials: a study in biplane fluoroscopy and intracardiac echocardiography. Circulation. 2000;101:1568–77. doi: 10.1161/01.cir.101.13.1568. [DOI] [PubMed] [Google Scholar]
- 16.Santucci PA, Varma N, Cytron J et al. Electroanatomic mapping of postpacing intervals clarifies the complete active circuit and variants in atrial flutter. Heart Rhythm. 2009;6:1586–95. doi: 10.1016/j.hrthm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Dixit S, Lavi N, Robinson M et al. Noncontact electroanatomic mapping to characterize typical atrial flutter: participation of right atrial posterior wall in the reentrant circuit. J Cardiovasc Electrophysiol. 2011;22:422–30. doi: 10.1111/j.1540-8167.2010.01917.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng J, Cabeen WR Jr, Scheinman MM. Right atrial flutter due to lower loop reentry: mechanism and anatomic substrates. Circulation. 1999;99:1700–5. doi: 10.1161/01.cir.99.13.1700. [DOI] [PubMed] [Google Scholar]
- 19.Cosío FG, Goicolea A, López-Gil M et al. Atrial endocardial mapping in the rare form of atrial flutter. Am J Cardiol. 1991;66:715–20.. doi: 10.1016/0002-9149(90)91136-t. [DOI] [PubMed] [Google Scholar]
- 20.Puech P. L’activité électrique auriculaire normale et pathologique. Montpellier: Reliure Inconnue. 1956. pp. 214–40.
- 21.Milliez P, Richardson AW, Obioha-Ngwu O et al. Variable electrocardiographic characteristics of isthmus-dependent atrial flutter. J Am Coll Cardiol. 2002;40:1125–32. doi: 10.1016/s0735-1097(02)02070-3. [DOI] [PubMed] [Google Scholar]
- 22.Kalman JM, Olgin JE, Saxon LA et al. Electrocardiographic and electrophysiologic characterization of atypical atrial flutter in man: use of activation and entrainment mapping and implications for catheter ablation. J Cardiovasc Electrophysiol. 1996;8:121–44. doi: 10.1111/j.1540-8167.1997.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 23.Lukac P, Pedersen AK, Mortensen PT et al. Ablation of atrial tachycardia after surgery for congenital and acquired heart disease using an electroanatomic mapping system: Which circuits to expect in which substrate? Heart Rhythm. 2005;2:64–72. doi: 10.1016/j.hrthm.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Coffey JO, d’Avila A, Dukkipati S et al. Catheter ablation of scar-related atypical atrial flutter. Europace. 2013;15:414–9. doi: 10.1093/europace/eus312. [DOI] [PubMed] [Google Scholar]
- 25.Bochoeyer A, Yang Y, Cheng J et al. Surface electrocardiographic characteristics of right and left atrial flutter. Circulation. 2003;108:60–6. doi: 10.1161/01.CIR.0000079140.35025.1E. [DOI] [PubMed] [Google Scholar]
- 26.Okumura K, Plumb VJ, Pagé PL et al. Atrial activation sequence during atrial flutter in the canine pericarditis model and its effects on the polarity of the flutter wave in the electrocardiogram. J Am Coll Cardiol. 1991;17:509–18. doi: 10.1016/s0735-1097(10)80124-x. [DOI] [PubMed] [Google Scholar]
- 27.Schoels W, Offner B, Brachmann J et al. Circus movement atrial flutter in the canine sterile pericarditis model. Relation of characteristics of the surface electrocardiogram and conduction properties of the reentrant pathway. J Am Coll Cardiol. 1994;23:799–808. doi: 10.1016/0735-1097(94)90771-4. [DOI] [PubMed] [Google Scholar]
- 28.Slade AK1, Garratt CJ. Proarrhythmic effect of adenosine in a patient with atrial flutter. Br Heart J. 1993;70:91–2. doi: 10.1136/hrt.70.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brodsky MA, Allen BJ, Grimes JA et al. Enhanced atrioventricular conduction during atrial flutter after intravenous adenosine. N Engl J Med. 1994;330:288–9. doi: 10.1056/NEJM199401273300413. [DOI] [PubMed] [Google Scholar]
- 30.Strickberger SA, Man KC, Daoud EG et al. Adenosine-induced atrial arrhythmia: a prospective analysis. Ann intern Med. 1997;127:417–22. doi: 10.7326/0003-4819-127-6-199709150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Granada J, Uribe W, Chyou PH et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36:2242–6. doi: 10.1016/s0735-1097(00)00982-7. [DOI] [PubMed] [Google Scholar]
- 32.Elesber AA, Rosales AG, Herges RM et al. Relapse and mortality following cardioversion of new-onset vs. recurrent atrial fibrillation and atrial flutter in the elderly. Eur Heart J. 2006;27:854–60. doi: 10.1093/eurheartj/ehi753. D0i:10.1093/eurheartj/ehi753. [DOI] [PubMed] [Google Scholar]
- 33.Marcus GM, Smith LM, Whiteman D et al. Alcohol intake is significantly associated with atrial flutter in patients under 60 years of age and a shorter right atrial effective refractory period. Pacing Clin Electrophysiol. 2008;31:266–72. doi: 10.1111/j.1540-8159.2008.00985.x. [DOI] [PubMed] [Google Scholar]
- 34.Mont L, Elosua R, Brugada J. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. Europace. 2009;11:11–7. doi: 10.1093/europace/eun289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tunick PA, McElhinney L, Mitchell T et al. The alternation between atrial flutter and atrial fibrillation. Chest. 1992;101:34–6. doi: 10.1378/chest.101.1.34. [DOI] [PubMed] [Google Scholar]
- 36.Biblo LA, Yuan Z, Quan KJ et al. Risk of stroke in patients with atrial flutter. Am J Cardiol. 2001;87:346–9. doi: 10.1016/s0002-9149(00)01374-6. [DOI] [PubMed] [Google Scholar]
- 37.Anselme F, Saoudi N, Poty H et al. Radiofrequency catheter ablation of common atrial flutter: significance of palpitations and quality-of-life evaluation in patients with proven isthmus block. Circulation. 1999;99:534–40. doi: 10.1161/01.cir.99.4.534. [DOI] [PubMed] [Google Scholar]
- 38.Luria DM, Hodge DO, Monahan KH et al. Effect of radiofrequency ablation of atrial flutter on the natural history of subsequent atrial arrhythmias. J Cardiovasc Electrophysiol. 2008;19:1145–50. doi: 10.1111/j.1540-8167.2008.01206.x. [DOI] [PubMed] [Google Scholar]
- 39.Ohkubo K, Watanabe I, Okumuray et al. Anatomic and electrophysiologic differences between chronic and paroxysmal atrial flutter: Intracardiac echocardiographic analysis. PACE. 2008;31:432–7. doi: 10.1111/J.1540-8159.2008.01012.x. [DOI] [PubMed] [Google Scholar]
- 40.Morita N, Kobayahi Y, Horie T et al. The undetermined geometrical factors contributing to the transverse conduction block of the crista terminalis. PACE. 2009;32:868–78. doi: 10.1111/j.1540-8159.2009.02402.x. [DOI] [PubMed] [Google Scholar]
- 41.Olgin JE, Kalman JM, Fitzpatrick AP et al. Role of right atrial endocardial structures as barriers to conduction during human type I atrial flutter. Activation and entrainment mapping guided by intracardiac echocardiography. Circulation. 1995;92:1839–48. doi: 10.1161/01.cir.92.7.1839. [DOI] [PubMed] [Google Scholar]
- 42.Tai C-T, Chen S-A, Chen Y-J et al. Conduction properties of the crista terminalis in patients with typical atrial flutter: basis for a line of block in the reentrant circuit. J Cardiovasc Electrophysiol. 1998;9:811–9. doi: 10.1111/j.1540-8167.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 43.Shumacher B, Jung W, Schmidt H et al. Transverse conduction capabilities of the crista terminalis in patients with atrial flutter and atrial fibrillation. J Am Coll Cardiol. 1999;34:363–73. doi: 10.1016/s0735-1097(99)00211-9. [DOI] [PubMed] [Google Scholar]
- 44.Arenal A, Almendral J, Alday JM et al. Rate-dependent conduction block of the crista terminalis in patients with typical atrial flutter: influence on evaluation of cavotricuspid isthmus conduction block. Circulation. 1999;99:2771–9. doi: 10.1161/01.cir.99.21.2771. [DOI] [PubMed] [Google Scholar]
- 45.Medi C, Teh AW, Roberts-Thomson K et al. Right atrial remodeling is more advanced in patients with atrial flutter than with atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:1067–72. doi: 10.1111/j.1540-8167.2012.02364.x. [DOI] [PubMed] [Google Scholar]
- 46.Tai C-T, Chen S-A, Chiang C-E et al. Characterization of low right atrial isthmus as the slow conduction zone and pharmacological target in typical atrial flutter. Circulation. 1997;96:2601–11. doi: 10.1161/01.cir.96.8.2601. [DOI] [PubMed] [Google Scholar]
- 47.Da Costa A, Mourot S, Roméyer-Bouchard C et al. Anatomic and electrophysiological differences between chronic and paroxysmal forms of common atrial flutter and comparison with controls. Pacing Clin Electrophysiol. 2004;27:1202–11. doi: 10.1111/j.1540-8159.2004.00610.x. [DOI] [PubMed] [Google Scholar]
- 48.Huang J-L, Tai C-T, Lin y-j et al. Right atrial substrate properties associated with age in patients with typical atrial flutter. Heart Rhythm. 2008;5:1144–51. doi: 10.1016/j.hrthm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Abhayaratna WP, Fatema K, Barnes ME et al. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. 2008;101:1626–9. doi: 10.1016/j.amjcard.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 50.Alboni P Scarfò S, Fucà G et al. Atrial and ventricular pressures in atrial flutter. Pacing Clin Electrophysiol. 1999;22:600–4. doi: 10.1111/j.1540-8159.1999.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 51.Luchsinger JA, Steinberg JS. Resolution of cardiomyopathy after ablation of atrial flutter. J Am Col Cardiol. 1998;32:205–10. doi: 10.1016/s0735-1097(98)00183-1. [DOI] [PubMed] [Google Scholar]
- 52.Pizzale S, Lemery R, Green MS et al. Frequency and predictors of tachycardia-induced cardiomyopathy in patients with persistent atrial flutter. Can J Cardiol. 2009;25:469–72. doi: 10.1016/s0828-282x(09)70119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Seara J, Gude F. Cabanas-Grandío P et al. Structural and functional inverse cardiac remodeling after cavotricuspid isthmus ablation in patients with typical atrial flutter. RevEsp Cardiol (Engl Ed) 2012;65:1003–9. doi: 10.1016/j.recesp.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 54.Nerheim P, Birger-Botkin S, Piracha L et al. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004;110:247–52. doi: 10.1161/01.CIR.0000135472.28234.CC. [DOI] [PubMed] [Google Scholar]
- 55.Irani WN, Grayburn PA, Afridi I. Prevalence of thrombus, spontaneous echo contrast, and atrial stunning in patients undergoing cardioversion of atrial flutter. A prospective study using transesophageal echocardiography. Circulation. 1997;95:962–6. doi: 10.1161/01.cir.95.4.962. [DOI] [PubMed] [Google Scholar]
- 56.Grimm RA, Stewart WJ, Arheart K et al. Left atrial appendage “stunning” after electrical cardioversion of atrial flutter: an attenuated response compared with atrial fibrillation as the mechanism for lower susceptibility to thromboembolic events. J Am Coll Cardiol. 1997;29:582–9. doi: 10.1016/s0735-1097(96)00551-7. [DOI] [PubMed] [Google Scholar]
- 57.Takami M, Suzuki M, Sugi K et al. Time course for resolution of left atrial appendage stunning after catheter ablation of chronic atrial flutter. J Am Coll Cardiol. 2003;41:2207–11. doi: 10.1016/s0735-1097(03)00496-0. [DOI] [PubMed] [Google Scholar]
- 58.Wood KA, Eisenberg SJ, Kalman JM et al. Risk of thromboembolism in chronic atrial flutter. Am J Cardiol. 1997;79:1043–7. doi: 10.1016/s0002-9149(97)00044-1. [DOI] [PubMed] [Google Scholar]
- 59.Seidl K, Hauer B, Schwick NG et al. Risk of thromboembolic events in patients with atrial flutter. Am J Cardiol. 1998;82:580–3. doi: 10.1016/s0002-9149(98)00419-6. [DOI] [PubMed] [Google Scholar]
- 60.Crijns HJ, Van Gelder IC, Kingma JH et al. Atrial flutter can be terminated by a class III antiarrhythmic drug but not by a class IC drug. Eur Heart J. 1994;15:1403–8. doi: 10.1093/oxfordjournals.eurheartj.a060402. [DOI] [PubMed] [Google Scholar]
- 61.Falk RH, Pollak A, Singh SN et al. Intravenous dofetilide, a class III antiarrhythmic agent, for the termination of sustained atrial fibrillation or flutter. Intravenous Dofetilide Investigators. J Am Coll Cardiol. 1997;29:385–90. doi: 10.1016/s0735-1097(96)00506-2. [DOI] [PubMed] [Google Scholar]
- 62.Stambler BS, Wood MA, Ellenbogen KA. Antiarrhythmic actions of intravenous ibutilide compared with procainamide during human atrial flutter and fibrillation: electrophysiological determinants of enhanced conversion efficacy. Circulation. 1977;96:4298–306. doi: 10.1161/01.cir.96.12.4298. [DOI] [PubMed] [Google Scholar]
- 63.Singh S, Zoble RG, Yellen L et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–90. doi: 10.1161/01.cir.102.19.2385. [DOI] [PubMed] [Google Scholar]
- 64.Tai CT1, Chen SA, Feng AN et al. Electropharmacologic effects of class I and class III antiarrhythmia drugs on typical atrial flutter: insights into the mechanism of termination. Circulation. 1998;97:1935–45. doi: 10.1161/01.cir.97.19.1935. [DOI] [PubMed] [Google Scholar]
- 65.Bianconi L, Castro A, Dinelli M et al. Comparison of intravenously administered dofetilide versus amiodarone in the acute termination of atrial fibrillation and flutter A multicentre, randomized, double-blind, placebo-controlled study. Eur Heart J. 2000;21:1265–73. doi: 10.1053/euhj.1999.2039. [DOI] [PubMed] [Google Scholar]
- 66.Crozier IG, Ikram H, Kenealy M et al. Flecainide acetate for conversion of acute supraventricular tachycardia to sinus rhythm. Am J Cardiol. 1987;59:607–9. doi: 10.1016/0002-9149(87)91178-7. [DOI] [PubMed] [Google Scholar]
- 67.Murdock CJ, Kyles AE, Yeung-Lai-Wah JA et al. Atrial flutter in patients treated for atrial fibrillation with propafenone. Am J Cardiol. 1990;66:755–7. doi: 10.1016/0002-9149(90)91144-u. [DOI] [PubMed] [Google Scholar]
- 68.McAlister HF, Luke RA, Whitlock RM et al. Intravenous amiodarone bolus versus oral quinidine for atrial flutter and fibrillation after cardiac operations. J Thorac Cardiovasc Surg. 1990;99:911–8. [PubMed] [Google Scholar]
- 69.Kafkas NV1, Patsilinakos SP, Mertzanos GA et al. Conversion efficacy of intravenous ibutilide compared with intravenous amiodarone in patients with recent-onset atrial fibrillation and atrial flutter. Int J Cardiol. 2007;118:321–5. doi: 10.1016/j.ijcard.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Crijns HJ, Van Gelder IC, Tieleman RG et al. Long-term outcome of electrical cardioversion in patients with chronic atrial flutter. Heart. 1997;77:56–61. doi: 10.1136/hrt.77.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Da Costa A, Thévenin J, Roche F et al. Loire-Ardèche-Drôme-Isère-Puy-de-Dôme Trial of Atrial Flutter Investigators. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676–81. doi: 10.1161/CIRCULATIONAHA.106.638395. [DOI] [PubMed] [Google Scholar]
- 72.Van Gelder IC, Crijns HJ, Van Gilst WH et al. Prediction of uneventful cardioversion and maintenance of sinus rhythm from direct-current electrical cardioversion of chronic atrial fibrillation and flutter. Am J Cardiol. 1991;68:41–6. doi: 10.1016/0002-9149(91)90707-r. [DOI] [PubMed] [Google Scholar]
- 73.Gallagher MM, Guo xh, Poloniecki JD et al. Initial energy setting, outcome and efficiency in direct current cardioversion of atrial fibrillation and flutter. J Am Coll Cardioi. 2001;38:1498–504. doi: 10.1016/s0735-1097(01)01540-6. [DOI] [PubMed] [Google Scholar]
- 74.Barold SS, Wyndham CR, Kappenberger LL et al. Implanted atrial pacemakers for paroxysmal atrial flutter Long-term efficacy. Ann Intern Med. 1987;107:144–9. doi: 10.7326/0003-4819-107-2-144. [DOI] [PubMed] [Google Scholar]
- 75.Wells JL, MacLean wa, James TN et al. Characterization of atrial flutter. Studies in man after open heart surgery using fixed atrial electrodes. Circulation. 1979;60:665–73. doi: 10.1161/01.cir.60.3.665. [DOI] [PubMed] [Google Scholar]
- 76.Allessie MA, Lammers WJEP Bonke FIM et al. Intraatrial reentry as a mechanism for atrial flutter induced by acetylcholine and rapid pacing in the dog. Circulation. 1984;70:123–35. doi: 10.1161/01.cir.70.1.123. [DOI] [PubMed] [Google Scholar]
- 77.Orlando J, Cassidy J, Aronow WS. High reversion of atrial flutter to sinus rhythm after atrial pacing in patients with pulmonary disease. Chest. 1977. pp. 71–5802. [DOI] [PubMed]
- 78.Peters RW, Shorofsky SR, Pelini M et al. Overdrive atrial pacing for conversion of atrial flutter: comparison of postoperative with nonpostoperative patients. Am Heart J. 1999;137:100–3. doi: 10.1016/s0002-8703(99)70464-3. [DOI] [PubMed] [Google Scholar]
- 79.Greenberg ML, Kelly TA, Lerman BB, DiMarco JP Atrial pacing for conversion of atrial flutter. Am J Cardiol. 1986;58:95–9. doi: 10.1016/0002-9149(86)90249-3. [DOI] [PubMed] [Google Scholar]
- 80.Olshansky B, Okumura K, Hess PG et al. Use of procainamide with rapid atrial pacing for successful conversion of atrial flutter to sinus rhythm. J Am Coll Cardiol. 1988;11:359–64. doi: 10.1016/0735-1097(88)90102-7. [DOI] [PubMed] [Google Scholar]
- 81.Della Bella P Tondo C, Marenzi G et al. Facilitating influence of disopyramide on atrial flutter termination by overdrive pacing. Am J Cardiol. 1988;61:1046–9. doi: 10.1016/0002-9149(88)90123-3. [DOI] [PubMed] [Google Scholar]
- 82.Doni F, Della Bella P Kheir A et al. Atrial flutter termination by overdrive transesophageal pacing and the facilitating effect of oral propafenone. Am J Cardiol. 1995;76:1243–6. doi: 10.1016/s0002-9149(99)80350-6. [DOI] [PubMed] [Google Scholar]
- 83.Gallay P Bertinchant JP, Lehujeur C et al. [Arch Transesophageal stimulation in the treatment of atrial flutter and tachysystole.] Mal Coeur Vaiss. 1985;78:311–6. [in French] [PubMed] [Google Scholar]
- 84.Guaragna RF, Barbato G, Bracchetti D. [Ventricular fibrillation induced by transesophageal stimulation performed for the treatment of atrial flutter]. G. Ital Cardiol. 1988;18:160–2. [in Italian] [PubMed] [Google Scholar]
- 85.Katritsis DG, Boriani G, Cosio FG European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Eur Heart J. 2016. p. ehw455. [DOI] [PubMed]
- 86.Cosio FG, Arribas F, López-Gil M et al. Atrial flutter mapping and ablation II. Radiofrequency ablation of atrial flutter circuits. Pacing Clin Electrophysiol. 1996;19:965–75. doi: 10.1111/j.1540-8159.1996.tb03394.x. [DOI] [PubMed] [Google Scholar]
- 87.Chen SA, Chiang CE, Wu TJ et al. Radiofrequency catheter ablation of common atrial flutter: comparison of electrophysiologically guided focal ablation technique and linear ablation technique. J Am Coll Cardiol. 1996;27:860–8. doi: 10.1016/0735-1097(95)00565-X. [DOI] [PubMed] [Google Scholar]
- 88.Poty H, Saoudi N, Nair M et al. Radiofrequency catheter ablation of atrial flutter: further insights into the various types of isthmus block-application to ablation during sinus rhythm. Circulation. 1996;94:3204–13. doi: 10.1161/01.cir.94.12.3204. [DOI] [PubMed] [Google Scholar]
- 89.Cauchemez B, Haissaguerre M, Fischer B, et al Electrophysiological effects of catheter ablation of inferior vena cava-tricuspid annulus isthmus in common atrial flutter. Circulation. 1996;93:284–94. doi: 10.1161/01.cir.93.2.284. [DOI] [PubMed] [Google Scholar]
- 90.Shah D, Haïssaguerre M, Takahashi A et al. Differential pacing for distinguishing block from persistent conduction through an ablation line. Circulation. 2000;102:1517–22. doi: 10.1161/01.cir.102.13.1517. [DOI] [PubMed] [Google Scholar]
- 91.Schwartzman D, Callans DJ, Gottlieb CD et al. Conduction block in the inferior vena caval-tricuspid valve isthmus: association with outcome of radiofrequency ablation of type I atrial flutter. J Am Coll Cardiol. 1996;28:1519–31. doi: 10.1016/s0735-1097(96)00345-2. [DOI] [PubMed] [Google Scholar]
- 92.Shah DC, Takahashi A, Jaïs P et al. Tracking dynamic conduction recovery across the cavotricuspid isthmus. J Am Coll Cardiol. 2000;35:1478–84. doi: 10.1016/s0735-1097(00)00600-8. [DOI] [PubMed] [Google Scholar]
- 93.Kuniss M, Vogtmann T, Ventura R et al. Prospective randomized comparison of durability of bidirectional conduction block in the cavotricuspid isthmus in patients after ablation of common atrial flutter using cryothermy and radiofrequency energy: the CRYOTIP study. Heart Rhythm. 2009;6:1699–705. doi: 10.1016/j.hrthm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 94.Tsai CF, Tai CT, Yu WC et al. Is 8-mm more effective than 4-mm tip electrode catheter for ablation of typical atrial flutter? Circulation. 1999;100:768–71. doi: 10.1161/01.cir.100.7.768. [DOI] [PubMed] [Google Scholar]
- 95.Jaïs P Shah DC, Haïssaguerre M et al. Prospective randomized comparison of irrigated-tip versus conventional-tip catheters for ablation of common flutter. Circulation. 2000;101:772–6. doi: 10.1016/j.ehj.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 96.Feld GK, Daubert JP, Weiss R et al. Cryoablation Atrial Flutter Efficacy Trial Investigators. Acute and long-term efficacy and safety of catheter cryoablation of the cavotricuspid isthmus for treatment of type 1 atrial flutter. Heart Rhythm. 2008;5:1009–14. doi: 10.1016/j.hrthm.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 97.Gil-Ortega I, Pedrote-Martínez A, Fontenla-Cerezuela A. Spanish Catheter Ablation Registry Collaborators. Spanish Catheter Ablation Registry 14th Official Report of the Spanish Society of Cardiology Working Group on Electrophysiology and Arrhythmias (2014). Rev Esp Cardiol (Engl Ed) 2015; 68: 1127–37. doi: 10.1016/j.rec.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 98.Anselme F, Klug D, Scanu P et al. Randomized comparison of two targets in typical atrial flutter ablation. Am J Cardioi. 2000;85:1302–7. doi: 10.1016/s0002-9149(00)00760-8. [DOI] [PubMed] [Google Scholar]
- 99.Passman RS, Kadish AH, Dibs SR et al. Radiofrequency ablation of atrial flutter: a randomized controlled study of two anatomic approaches. Pacing Clln Electrophysiol. 2004;27: 83–8. doi: 10.1111/j.1540-8159.2004.00390.. [DOI] [PubMed] [Google Scholar]
- 100.Weiss C, Becker J, Hoffmann M et al. Can radiofrequency current isthmus ablation damage the right coronary artery? Histopathological findings following the use of a long (8 mm) tip electrode. Pacing Clln Electroptiysiol. 2002;25:860–2. doi: 10.1046/j.1460-9592.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- 101.Sassone B, Leone O, Martinelli GN et al. Acute myocardial infarction after radiofrequency catheter ablation of typical atrial flutter: histopathological findings and etiopathogenetic hypothesis. Ital Heart J. 2004;5:403–7. doi: 10.3346/jkms.2014.29.2.292. [DOI] [PubMed] [Google Scholar]
- 102.Hillock RJ, Melton IC, Crozier IG. Radiofrequency ablation for common atrial flutter using an 8-mm tip catheter and up to 150 W. Europace. 2005;7:409–12. doi: 10.1016/j.eupc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 103.Hsu LF, Jaïs P Hocini M et al. Incidence and prevention of cardiac tamponade complicating ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2005; 28:S106–9. doi: 10.1111/j.1540-8159.2005.00062.x. [DOI] [PubMed] [Google Scholar]
- 104.Paydak H, Kall JG, Burke MC et al. Atrial fibrillation after radiofrequency ablation of type I atrial flutter: time to onset, determinants, and clinical course. Circulation. 1998;98:315–22. doi: 10.1161/01.cir.98.4.315. [DOI] [PubMed] [Google Scholar]
- 105.Anselme F, Saoudi N, Poty H et al. Radiofrequency catheter ablation of common atrial flutter: significance of palpitations and quality-of-life evaluation in patients with proven isthmus block. Circulation. 1999;99:534–40. doi: 10.1161/01.cir.99.4.534. [DOI] [PubMed] [Google Scholar]
- 106.Schmieder S, Ndrepepa G, Dong J et al. Acute and long-term results of radiofrequency ablation of common atrial flutter and the influence of the right atrial isthmus ablation on the occurrence of atrial fibrillation. Eur Heart J. 2003;24:956–63. doi: 10.1016/s0195-668x(02)00846-1. [DOI] [PubMed] [Google Scholar]
- 107.Ellis K, Wazni O, Marrouche N et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophi/siol. 2007;18:799–802. doi: 10.1111/j.1540-8167.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 108.Natale A, Newby KH, Pisanó E et al. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am Coil Cardiol. 2000;35:1898–904. doi: 10.1016/s0735-1097(00)00635-5. [DOI] [PubMed] [Google Scholar]
- 109.Bottoni N, Donateo P Quartieri F et al. Outcome after cavotricuspid isthmus ablation in patients with recurrent atrial fibrillation and drug-related typical atrial flutter. Am J Cardiol. 2004;94:504–7. doi: 10.1016/j.amjcard.2004.04.069. [DOI] [PubMed] [Google Scholar]
- 110.Mohanty S, Mohanty P Di Biase L et al. Results from a single-blind, randomized study comparing the impact of different ablation approaches on long-term procedure outcome in coexistent atrial fibrillation and flutter (APPROVAL). Circulation. 2013;127:1853–60. doi: 10.1161/CIRCULATIONAHA.113.001855. [DOI] [PubMed] [Google Scholar]
- 111.Mohanty S, Natale A, Mohanty P et al. Pulmonary Vein Isolation to Reduce Future Risk of Atrial Fibrillation in Patients Undergoing Typical Flutter Ablation: Results from a Randomized Pilot Study (REDUCE AF). J Cardiovasc Electrophysiol. 2015;26:819–25. doi: 10.1111/jce.12688. [DOI] [PubMed] [Google Scholar]
- 112.Clementy N, Desprets L, Pierre B et al. Outcomes After Ablation for Typical Atrial Flutter (from the Loire Valley Atrial Fibrillation Project). Am J Cardiol. 2014;114:1361–7. doi: 10.1016/j.amjcard.2014.07.066. [DOI] [PubMed] [Google Scholar]
- 113.Pathak RK, Middeldorp ME, Lau DH et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–31. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 114.Mohanty S, Mohanty P Di Biase L et al. Long-term outcome of catheter ablation in atrial fibrillation patients with coexistent metabolic syndrome and obstructive sleep apnea: Impact of repeat procedures versus lifestyle changes. J Cardiovasc Electrophysiol. 2014;25:930–8. doi: 10.1111/jce.12468. [DOI] [PubMed] [Google Scholar]
- 115.Pathak RK, Middeldorp ME, Meredith M et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol. 2015;65:2159–69. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 116.Pathak RK, Elliott A, Middeldorp ME et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J Am Cull Cardiul. 2015;66:985–96. doi: 10.1016/j.jacc.2015.06.488. D0I: 10.1016/j.jacc.2015.06.488. [DOI] [PubMed] [Google Scholar]
- 117.Cosío FG, Martín-Peñato A, Pastor A et al. Atypical flutter: a review. PACE. 2003;26:2157–69. doi: 10.1046/j.1460-9592.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 118.Gerstenfeld EP, Callans DJ, Dixit S et al. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation. 2004;110:1351–7. doi: 10.1161/01.CIR.0000141369.50476.D3. [DOI] [PubMed] [Google Scholar]
- 119.Nakagawa H, Shaha N, Matsudaira K et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair o congenital heart disease: isolated channels between scars allow “focal” ablation. Circulation. 2001;103:669–709. doi: 10.1053/euhj.2002.328. [DOI] [PubMed] [Google Scholar]
- 120.Kall JG, Rubenstein DS, Kopp DE et al. Atypical atrial flutter originating in the right atrial free wall. Circulation. 2000;101: 270–9. doi: 10.1161/01.cir.101.3.270. [DOI] [PubMed] [Google Scholar]
- 121.Stevenson IH, Kistler PM, Spence SJ et al. Scar-related right atrial macroreentrant tachycardia in patients without prior atrial surgery: electroanatomic characterization and ablation outcome. Heart Rhythm. 2005;2:594–601. doi: 10.1016/j.hrthm.2005.02.1038. [DOI] [PubMed] [Google Scholar]
- 122.Jaïs P1, Shah DC, Haïssaguerre M, Hocini M et al. Mapping and ablation of left atrial flutters. Circulation. 2000;101:2928–34. doi: 10.1161/01.CIR.101.25.2928. [DOI] [PubMed] [Google Scholar]
- 123.Ouyang F1, Ernst S, Vogtmann T et al. Characterization of reentrant circuits in left atrial macroreentrant tachycardia: critical isthmus block can prevent atrial tachycardia recurrence. Circulation. 2002;105:1934–42. doi: 10.1161/01.cir.0000015077.12680.2e. [DOI] [PubMed] [Google Scholar]
- 124.Markowitz SM, Brodman RF, Stein KM et al. Lesional tachycardias related to mitral valve surgery. J Am Coll Cardiol. 2002;39:1973–83. doi: 10.1016/s0735-1097(02)01905-8. [DOI] [PubMed] [Google Scholar]
- 125.Chugh A, Oral H, Lemola K et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005;2:464–71. doi: 10.1016/j.hrthm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 126.Daoud EG, Weiss R, Augostini R et al. Proarrhythmia of circumferential left atrial lesions for management of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:157–65. doi: 10.im/j.1540-8167.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 127.Chae S, Oral H, Good E et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coil Cardiol. 2007;50:1781–7. doi: 10.1016/j.jacc.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 128.Matsuo S, Wright M, Knecht S et al. Peri-mitral atrial flutter in patients with atrial fibrillation ablation. Heart Rhythm, 2009;7:2–8. doi: 10.1016/j.hrthm.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 129.Bayés de Luna A, Cladellas M, Oter R et al. Interatrial conduction block and retrograde activation of the LA and paroxysmal supraventricular tachyarrhythmia. Eur Heart J. 1988;9:1112–8. doi: 10.1093/oxfordjournals.eurheartj.a062407. [DOI] [PubMed] [Google Scholar]
- 130.Daubert C, Gras D, Berder V et al. Resynchronisation atriale permanente par la stimulacion biatriale synchrone pour le tratement préventif du flutter auriculaire associé à un block interauriculaire de haut degré. Arch Mai Coeur Vaiss. 1994;87:1535–46. [PubMed] [Google Scholar]
- 131.Hinojar R, Pastor A, Cosio FG. Bachmann block pattern resulting from inexcitable areas peripheral to the Bachmann’s bundle: controversial name or concept? Europace. 2013;15:1272. doi: 10.1093/europace/eut142. [DOI] [PubMed] [Google Scholar]
- 132.Marrouche NF, Natale A, Wazni OM et al. Left septal atrial flutter: electrophysiology, anatomy, and results of ablation. Circulation. 2004;109:2440–7. doi: 10.1161/01.CIR.0000129439.03836.96. [DOI] [PubMed] [Google Scholar]
- 133.Miyazaki S, Shah AJ, Hocini M et al. Recurrent spontaneous clinical perimitral atrial tachycardia in the context of atrial fibrillation ablation. Heart Rhythm. 2015;12:104–10. doi: 10.1016/j.hrthm.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 134.Aktas MK, Khan MN, Di Biase L et al. Higher rate of recurrent atrial flutter and atrial fibrillation following atrial flutter ablation after cardiac surgery. J Cardiovasc Electrophysiol. 2010;21:760–5. doi: 10.1111/j.1540-8167.2009.01709.x. [DOI] [PubMed] [Google Scholar]
- 135.Bai R, Di Biase L, Mohanty P et al. Ablation of perimitral flutter following catheter ablation of atrial fibrillation: impact on outcomes from a randomized study (PROPOSE) J Cardiovasc Eiectrophysioi. 2012;23:137–44. doi: 10.1111/j.1540-8167.2011.02182.x. [DOI] [PubMed] [Google Scholar]
- 136.Akar JG, Al-Chekakie MO, Hai A et al. Surface electrocardiographic patterns and electrophysiologic characteristics of atrial flutter following modified radiofrequency MAZE procedures. J Cardiovasc Electrnphysiol. 2007;18:349–55. doi: 10.1111/j.1540-8167.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 137.McElderry HT, McGiffin DC, Plumb VJ et al. Proarrhythmic aspects of atrial fibrillation surgery: mechanisms of postoperative macroreentrant tachycardias. Circulation. 2008;117:155–62. doi: 10.1161/CIRCULATIONAHA.107.688358. [DOI] [PubMed] [Google Scholar]
- 138.Nof E, Stevenson WG, Epstein LM et al. Catheter ablation of atrial arrhythmias after cardiac transplantation: findings at EP study utility of 3-D mapping and outcomes. J Cardiovasc Eledruptiysiul. 2013;24:498–502. doi: 10.1111/jce.12078. [DOI] [PubMed] [Google Scholar]
- 139.Lukac P, Hjortdal V, Pedersen AK et al. The superior transseptal surgical approach to mitral valve creates slow conduction. Pacing Clin Electrophysiol. 2006;29:719–26. doi: 10.1111/j.1540-8159.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 140.Frost L, Mølgaard H, Christiansen EH et al. Atrial fibrillation and flutter after coronary artery bypass surgery: epidemiology, risk factors and preventive trials. Int J Cardiol. 1992;36:253–61. doi: 10.1016/0167-5273(92)90293-c. [DOI] [PubMed] [Google Scholar]
- 141.Ishii Y, Schuessler RB, Gaynor SL et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–8. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 142.Pagé PL, Plumb VJ, Okumura K et al. A new animal model of atrial flutter. J Am Cull Cardiul. 1986;8:872–9. doi: 10.1016/s0735-1097(86)80429-6. [DOI] [PubMed] [Google Scholar]
- 143.Ho KM, Tan JA. Benefits and risks of corticosteroid prophylaxis in adult cardiac surgery: a dose-response meta-analysis. Circulation. 2009;119:1853–66. doi: 10.1161/CIRCULATIONAHA.108.848218. [DOI] [PubMed] [Google Scholar]
- 144.Goldstein RN, Ryu K, Khrestian C, van Wagoner DR et al. Prednisone prevents inducible atrial flutter in the canine sterile pericarditis model. J Cardiovasc Electrnphysiol. 2008;19:74–81. doi: 10.1111/j.1540-8167.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 145.Lee SH, Kang DR, Uhm JS et al. New-onset atrial fibrillation predicts long-term newly developed atrial fibrillation after coronary artery bypass graft. Am Heart J. 2014;167:593–600. doi: 10.1016/j.ahj.2013.12.010. [DOI] [PubMed] [Google Scholar]