INTRODUCTION
South Asian Indians (SAIs) are one of fastest growing groups in the United States. The U.S. 2010 Census reported a 69.8% increase in SAIs from the year 2000 to 2010, approximately 3.2 million people [1]. SAIs live throughout the United States and currently represent the largest Asian group in 23 U.S. states.
The leading cause of non-communicable disease deaths among SAIs in India are cardiovascular disease (CVD) and diabetes, representing approximately 26% mortality overall [2]. Major CVD risk factors include physical inactivity, elevated blood pressure, blood glucose, hyperlipidemia, adiposity [3], and tobacco smoking among males [2]. SAIs have greater insulin resistance (a potent CVD risk factor) when compared to Whites, Hispanics, and Blacks with comparable or lower body mass index (BMI: weight in kg/height in m2) [4]. Diet is an important contributor to CVD risk. SAIs have reported high dietary intake of carbohydrates, saturated fatty acids, and omega-6 polyunsaturated fatty acids (PUFAs), and a decreased intake of monounsaturated fatty acids (MUFAs) and fiber compared to recommended nutrient guidelines [5].
SAIs who immigrate to the United States continue to have high rates of CVD and diabetes [6]. The prevalence of metabolic syndrome among SAI immigrants in the United States has been reported as high as 32% and type 2 diabetes (T2DM) as 18% [6]. Increasing age and greater length of U.S. residency have been found to be significant predictors of CVD risk factors in this group [6].
CVD and diabetes dietary risk factors begin to emerge in youth and track into adulthood [7]. Little is known about dietary behaviors among first-generation urban SAI adolescents who are acculturating to a new way of life as well as adjusting to growth and developmental milestones. Adolescence is a critical life stage for the development of healthy dietary patterns. Urban SAI adolescents living in India are known to ingest high-fat, high-carbohydrate, low-fiber diets that are associated with CVD and T2DM [8]. Dietary patterns of first-generation urban SAI adolescents who have immigrated to the United States have not been well studied. It is important to examine dietary profiles of first-generation urban SAI adolescents who have immigrated to the
United States to identify whether they are meeting nutrient guidelines to develop preventive strategies to promote healthy dietary patterns in this population.
Theoretical/Conceptual Framework
The social ecological framework [9] is based on an ecological approach to health promotion and emphasizes the multidimensional nature of health behavior that is considered within the context of the individual and the environment. In this model, it is posited that the development of disease is shaped by multilevel factors that can have incremental impacts on risk of disease. From an ecological health promotion perspective, it is important to examine how both biobehavioral and cultural determinants impact risk for T2DM and CVD. We used this model to take the first step toward better understanding factors that underlie dietary choices which can influence risk and ultimately disease.
Study Aim
The aim of this study was to describe macronutrient, vitamin, mineral, and beverage intake, and health characteristics among first-generation urban SAI adolescents living in the United States and to identify patterns of dietary deficiencies compared to U.S. dietary recommendations.
METHODS
Design and Participants
Through a cross-sectional descriptive design, we examined demographic characteristics, dietary profiles, body composition, and length of residency in the United States among first-generation urban adolescent SAIs (aged 13–21 years). Adolescents were eligible if they self-identified as SAI (originating from India, Pakistan, Nepal, Bangladesh, and Sri Lanka) and were the first generation to be born in the United States or to emigrate to the United States, and were able to read and write in English. Following institutional review board approval, subjects were recruited through four community centers in an urban Midwestern metropolitan area.
Data Collection
SAI adolescents were recruited from four urban Midwestern health centers that serve that population. The four participating centers provide comprehensive and integrated social services to immigrant populations from South Asian countries residing within a large metropolitan area. The primary coordinating center was located within an urban community of South Asian immigrants, many of whom had recently immigrated to the United States. The participants from the urban center were low-income and followed traditional lifestyles. The remaining participants from the other three centers located in suburban areas were from middle-class families and followed lifestyles that blended traditional and westernized cultures. Adolescent informed assent and parental consent were obtained for those less than 18 years, and informed consent for those 18 to 21 years. Participants completed questionnaires for demographics, birthplace, length of U.S. residency, and dietary patterns. All data collection, including body composition, blood pressure, and fasting glucose, were gathered by trained personnel.
Measures
Usual dietary, beverage, and vitamin-supplement intake over the past 12 months was obtained using a validated FFQ (Block Brief 2000®). This 95-item self-report questionnaire provides the frequency (never to every day) and portion size (using pictures) of a broad range of foods and typical eating patterns over the past year. The range of foods includes an extensive list of individual food types (e.g., beans, rice, peas, tomatoes, peppers, eggs, oranges, bananas, apples, grapes) and cooking methods (e.g. boiled, baked, stewed, fried). The food list for this questionnaire was developed from the NHANES III dietary recall data. Usual daily nutrient intakes were then calculated using a computer software program (NutriQuest©). Macronutrient intakes, carbohydrates, proteins, and fats were calculated in both grams and percent of total calorie intake. Fat intake was further categorized into grams of saturated, poly-, and mono-unsaturated fatty acid sources. Vitamin and mineral intakes were calculated from food and vitamin sources and reported in the appropriate units for each nutrient (e.g., mg, mcg, IU). Numbers were provided of daily servings of food groups: fruits, vegetables, breads/cereals, meat/fish/beans/eggs, dairy, and fats/sweets. Frequency and quantity of sugar-sweetened beverage (SSB) intake were also obtained.
Height was measured to the nearest cm using a portable stadiometer, and body weight to the nearest one-tenth kg using a digital scale. BMI (kg/m2) was calculated for all subjects. Subjects were categorized into body weight groups using Centers for Disease Control (CDC) BMI percentiles for those 13–20 years [10] and BMI for those 21 years of age [11]. Waist circumference was measured to the nearest cm using established procedures [12]. An index of central obesity (ICO), the ratio of waist circumference to height, was calculated (waist circumference [cm]/height [cm]) as a second index of adiposity that takes into account height [13].
Blood pressure was measured in a rested seated position using a sphygmomanometer according to National High Blood Pressure Education Program Working Group (NHBPEP) guidelines. Elevated BP was defined as a blood pressure percentile > 90% (for gender, age, and height) for those aged 13–17 years [14]. Adult blood pressure classifications were used for those aged 18–21 years [15]. Fasting capillary blood glucose was obtained with a fingerstick (One Touch Ultra®, Lifescan, Inc., Milpitas, CA). American Diabetes Association (ADA) standards were used to categorize glucose values: normal < 100 mg/dl; prediabetes 100–125 mg/dl; diabetes > 126 mg/dl [16].
Analysis
Descriptive statistics were used to examine calorie, macronutrient, fiber, vitamin, mineral, and beverage intakes, and numbers of servings of food groups. Dietary intakes were compared to the USDA dietary reference intakes (DRI) for established age groups (9–13; 14–18; and 19–30 years) and by gender [17]. The DRIs included recommended dietary allowances (RDA) and Acceptable Macronutrient Distribution Ranges (AMDR) [18]. Adolescents whose calorie intakes were below 500 or greater than 5,000 calories per day were eliminated from the analysis, as they may reflect under- or over-reporting of food intake [19]. Total vitamin and mineral intakes were calculated from the sum of food and vitamin supplements. The frequency of beverage intake was calculated for juice, milk, and sugar-sweetened beverages (SSB). Nutrient intake, health parameters (BMI, waist circumference, ICO, blood pressure, and capillary blood glucose), and length of time in United States were compared using the Pearson correlation coefficient. MANOVA was used to examine for differences in nutrient consumption and health parameters by age and gender.
RESULTS
Sixty-two adolescents completed the study. Six subjects were eliminated from the analysis due to insufficiently reported caloric intakes (below 500 calories per day). Of the remaining 56 adolescents that were included in the analysis, 58.9% were female. The ages ranged 13–21 years (M = 15.9 ± 2.5). Most were born in the United States (n = 33). Of the 23 adolescents who immigrated to the United States, nearly half (n = 11) had lived in the United States greater than 10 years. The predominant religious preferences reported were Hindu (58.9%) and Muslim (30.4%; Table 1). The BMI ranged from 15.2 to 36.2 (M = 22.17 ± 4.6). Using CDC weight categories, 3.6% were underweight, 66.1% healthy weight, 17.9% overweight, and 7% obese (Table 1). More girls were overweight or obese (36.3%) than boys (21.7%). Blood pressures were elevated in 9% of the sample (above the 90th percentile for adolescents up to age 17 or in the pre-hypertensive range for those 18–21 years). Fasting capillary blood glucose was above normal (prediabetic range) for 51% of the sample; none were in the diabetic range (Table 1).
Table 1.
Demographic and health information (N = 56)
| N | (%) | |
|---|---|---|
| Male | 23 | (41.1) |
| Female | 33 | (58.9) |
| Age | ||
| 13 years | 15 | (26.8) |
| 14–18 years | 30 | (53.6) |
| 19–21 years | 11 | (19.6) |
| Born in the U.S. | ||
| Yes | 33 | (58.9) |
| No | 23 | (41.1) |
| Duration of stay in U.S. for those born outside of U.S. | ||
| < 1 year | 3 | (13) |
| 1–5 years | 3 | (13) |
| 6–10 years | 6 | (26.1) |
| > 10 years | 11 | 47.8) |
| Religious Preference | ||
| Muslim | 17 | (30.4) |
| Hindu | 33 | (58.9) |
| Christian | 1 | (1.8) |
| Jain | 2 | (3.6) |
| Sikh | 1 | (1.8) |
| Other | 2 | (3.6) |
| BMI | ||
| Underweight | 2 | (3.6) |
| Healthy weight | 37 | (66.1) |
| Overweight | 10 | (17.9) |
| Obese | 7 | (7.0) |
| Blood pressure | ||
| Normal | 51 | (91) |
| Above normal | 5 | (9) |
| Fasting Capillary Blood Glucose | ||
| Normal | 26 | (49) |
| Prediabetes | 27 | (51) |
Mean Macronutrient and Fiber Intake
Total calorie intake ranged from 574 to 4,644 calories. The mean caloric intake for males was 1,906 ± 972 and for females 1,417 ± 699. Mean calorie intake increased with increasing age. Protein and carbohydrate intakes were within the recommended percentages of the AMDR. Fat intake exceeded the recommended percentages for males in the 13-to-18-year range and for females in the age 13 group (Table 2). Saturated fat exceeded USDA recommendations of < 10% in all ages and both genders. Fiber intake was below USDA recommendations for all ages and both genders.
Table 2.
Macronutrient intake (DRI) by gender and age (Mean, SD by USDA age groups)
| Nutrient | RDA | % Calories (AMDR) | Gender | 13 yrs (N = 15) |
14–18 yrs (N = 30) | 19–21 yrs (N = 11) |
|---|---|---|---|---|---|---|
| Calories | M | 1370 ±841 | 2056 ±989 | 2054 ±1063 | ||
| F | 1304 ±489 | 1421 ±837 | 1571 ±670 | |||
| Calories per kg | M | 27 ± 19 | 36 ± 22 | 31 ± 18 | ||
| F | 27 ± 13 | 25 ± 11 | 27 ± 14 | |||
| Protein (g) | 13 (34); 14–18 (52); 19–21 (56) | M | 44 ± 24 | 65 ± 34 | 69 ± 43 | |
| 13 (34); 14–21 (46) | F | 36 ± 10 | 40 ± 17 | 54 ± 33 | ||
| Protein (%) | 13–18 (10–30); 19–21 (10–35) | M | 13 ± 1 | 13 ± 3 | 13 ± 2 | |
| 13–18 (10–30); 19–21 (10–35) | F | 12 ± 3 | 12 ± 3 | 13 ± 4 | ||
| Total Fat (g) | M | 55 ± 30 | 83 ± 41 | 79 ± 47 | ||
| F | 54 ± 24 | 53 ± 43 | 55 ± 35 | |||
| Total Fat (% of kcal) | 13–18 (25–35); 19–21 (20–35) | M | 38 ± 5 | 36 ± 5 | 34 ± 3 | |
| 13–18 (25–35); 19–21 (20–35) | F | 36 ± 6 | 33 ± 11 | 30 ± 9 | ||
| Total Fat (kcal) | M | 499 ± 274 | 745 ±367 | 715 ±419 | ||
| F | 482 ±215 | 479 ±386 | 494 ±314 | |||
| Carbohydrate (g) | M | 180 ±120 | 272 ±131 | 274 ±120 | ||
| F | 177 ±67 | 207 ±120 | 222 ± 87 | |||
| Carbohydrate (% of kcal) | All ages (45–65) | M | 51 ± 5 | 53 ± 7 | 55 ± 4 | |
| All ages (45–65) | F | 55 ± 6 | 58 ± 13 | 59 ± 13 | ||
| Carbohydrate (kcal) | M | 720 ±481 | 1086 ±524 | 1095 ±479 | ||
| F | 708 ±268 | 826 ±482 | 889 ±348 | |||
| Saturated Fats (g) | All ages (< 10%) | M | 19 ± 8 | 31 ± 16 | 28 ± 18 | |
| All ages (< 10%) | F | 19 ± 9 | 18 ± 13 | 18 ± 8 | ||
| Monounsaturated Fats (g) | M | 21 ± 10 | 31 ± 17 | 31 ± 19 | ||
| F | 21 ± 10 | 20 ± 19 | 24 ±19 | |||
| Polyunsaturated Fats (g) | M | 12 ± 11 | 15 ± 8 | 14 ± 8 | ||
| F | 11 ± 5 | 11 ± 10 | 10 ± 6 | |||
| Cholesterol (mg) | All ages (< 300) | M | 123 ±67 | 179± 98 | 275 ±141 | |
| All ages (< 300) | F | 95 ± 42 | 90 ± 43 | 128 ± 95 | ||
| Total Fiber (g) | 13 (25); 14–18 (31); 19–21 (34) | M | 12 ± 6 | 16 ± 8 | 15 ± 6 | |
| 13 (22); 14–18 (25); 19–21 (28) | F | 12 ± 3 | 15 ± 12 | 14 ± 7 |
M = male; F = female. RDA = level of nutrient likely to meet the needs of 97.5% of the population. AMDR = range for an energy source associated with reduced risk of chronic disease (source: National Research Council, IOM).
Mean Mineral and Vitamin Intakes
Sodium intake exceeded recommendations in males 14–21 years. Potassium and magnesium intakes were below recommended levels for all ages and both sexes. Calcium intake was below recommended levels for all ages in females and in the 13-to-18-year age group in males. Vitamin D intake was deficient in all ages and both genders (Tables 3 and 4).
Table 3.
Mineral intake (DRI) by gender and age (Mean, SD by USDA age groups)
| Nutrient | RDA | Gender | 13 yrs | 14–18 yrs | 19–21 yrs |
|---|---|---|---|---|---|
| Sodium (mg) | 13 (<2,200); 14–21 (<2,300) | M | 1649 ± 1191 | 2312 ± 1215 | 2419 ± 1777 |
| 13 (<2,200); 14–21 (<2,300) | F | 1384 ± 474 | 1404 ± 888 | 1606 ± 933 | |
| Potassium (mg) | 13 (4500); 14–21 (4700) | M | 2033 ± 889 | 2932 ± 1074 | 3158 ± 1042 |
| 13 (4500); 14–21 (4700) | F | 1939 ± 618 | 2495 ± 1491 | 2572 ± 1201 | |
| Phosphorus (mg) | 13–18 (1250); 19–21 (700) | M | 927 ± 437 | 1300 ± 539 | 1260 ± 697 |
| 13–18 (1250); 19–21 (700) | F | 807 ± 234 | 863 ± 367 | 1022 ± 538 | |
| Calcium | 13–18 (1,300); 19–21 (1,000) | M | 826 ± 283 | 1149 ± 449 | 1066 ± 394 |
| 13–18 (1,300); 19–21 (1,000) | F | 737 ± 255 | 853 ± 340 | 898 ± 568 | |
| Magnesium (mg) | 13 (240); 14–18 (410); 19–21 (400) | M | 206 ± 104 | 294 ± 119 | 295 ± 125 |
| 13 (240); 14–18 (360); 19–21 (310) | F | 196 ± 58 | 250 ± 146 | 255 ± 128 | |
| Iron (mg) | 13 (8); 14–18 (11); 19–21 (8) | M | 11± 8 | 21 ± 17 | 19 ± 12 |
| 13 (8); 14–18 (15); 19–21 (18) | F | 17 ± 22 | 14 ± 8 | 12 ± 7 | |
| Zinc (mg) | 13 (8); 14–21 (11) | M | 5.7 ± 3 | 10.4 ± 7 | 9.2 ± 7 |
| 13 (8); 14–18 (9); 19–21 (8) | F | 6.7 ± 5 | 7.3 ± 6 | 8.0 ± 5 | |
| Folate (mcg) | 13 (300); 14–21 (400) | M | 320 ± 229 | 450 ± 196 | 504 ± 203 |
| 13 (300); 14–21 (400) | F | 305 ± 162 | 372 ± 227 | 412 ± 253 | |
| Niacin (mg) | 13 (12); 14–21 (16) | M | 11 ± 8 | 17 ± 10 | 27 ±14 |
| 13 (12); 14–21 (14) | F | 12 ± 8 | 12 ± 8 | 21 ± 19 |
M = male; F = female. RDA = level of nutrient likely to meet the needs of 97.5% of the population (source: National Research Council, IOM).
Table 4.
Vitamin intake (DRI) by gender and age (Mean, SD for USDA age groups)
| Nutrient | RDA | Gender | 13 yrs (N = 15) | 14–18 yrs (N = 30) | 19–21 yrs (N = 11) |
|---|---|---|---|---|---|
| Vitamin A (RE [mcg]) | 13 (600); 14–21 (900) | M | 918 ± 684 | 1267 ± 717 | 1088 ± 483 |
| 13 (600); 14–21 (700) | F | 763 ±457 | 1255 ±940 | 1502 ±1199 | |
| Vitamin C (mg) | 13 (45); 14–18 (75); 19–21 (90) | M | 101 ± 56 | 145 ± 68 | 282 ± 226 |
| 13 (45); 14–18 (65); 19–21 (75) | F | 98 ± 48 | 149 ± 107 | 176 ±141 | |
| Vitamin D (mcg) | All ages (15) | M | 5.8 ± 2.2 | 8.7 ± 4.3 | 7.5 ± 2.3 |
| All ages (15 | F | 4.5 ± 4.2 | 5.7 ± 5 | 3.8 ± 2.1 | |
| Vitamin E | 13 (11); 14–21 (15) | M | 7.7 ± 5.8 | 15 ± 15 | 10 ± 3 |
| 13 (11); 14–21 (15) | F | 8.5 ± 7.3 | 11 ± 12 | 8.8 ± 6 | |
| Vitamin B1/Thiamin (mg) | 13 (0.9); 14–21 (1.2) | M | 1.1 ± 0.7 | 1.7 ± 0.6 | 2.5 ± 1.3 |
| 13 (0.9); 14–18 (1.0); 19–21 (1.1) | F | 1.1 ± 0.6 | 1.3 ± 0.7 | 2.1 ± 1.9 | |
| Vitamin B6 (mg) | 13 (1.0); 14–21 (1.3) | M | 1.2 ± 0.7 | 1.9 ± 0.7 | 2.5 ± 0.8 |
| 13 (1.0); 14–18 (1.2); 19–21 (1.3) | F | 1.3 ± 0.8 | 1.6 ± 1.1 | 1.9 ± 1.3 | |
| Vitamin B12 (mcg) | 13 (1.8); 14–21 (2.4) | M | 3.7 ± 2.7 | 5.7 ± 4.0 | 6.4 ± 3.5 |
| 13 (1.8); 14–21 (2.4) | F | 3.0 ± 1.8 | 3.3 ± 3.5 | 4.4 ± 3.4 | |
| Vitamin B2/Riboflavin (mg) | 13 (0.9); 14–21 (1.3) | M | 1.5 ± 0.6 | 2.2 ± 0.8 | 2.9 ± 1.2 |
| 13 (0.9); 14–18 (1.0); 19–21 (1.1) | F | 1.5 ± 0.7 | 1.7 ± 0.8 | 2.4 ± 2.2 |
M = male; F = female. RDA = level of nutrient likely to meet the needs of 97.5% of the population (source: National Research Council, IOM).
Individual Distribution of Nutrients
Of the males, 83% exceeded the RDA for grams of carbohydrates, 91% for total fat and saturated fatty acids. Of the females, 73% exceeded the RDA for carbohydrates, 64% for fat, and 82% for saturated fatty acids. Most adolescents (96% of males and 94% of females) did not consume the recommended amount of daily fiber.
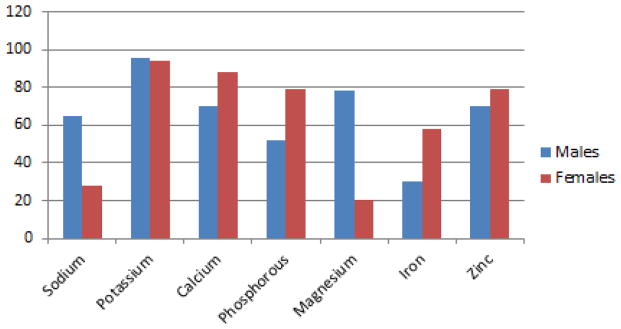
Examination of the mineral intake compared to RDAs for age revealed that 96% of males and 94% of females did not meet the recommended level of potassium intake (Figure 1). More than 50% of males and females did not meet recommended calcium and phosphorous intakes.
Figure 1.
Percentage of boys and girls whose usual daily intake for minerals is below recommended guidelines
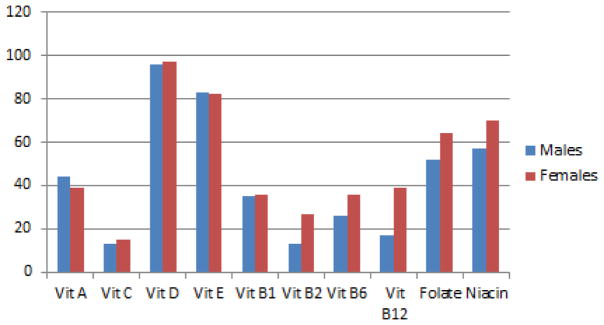
Examination of the vitamin intake compared to RDAs for age and gender revealed that 96% of males and 97% of females did not meet the recommended amount of dietary vitamin D (Figure 2). Over 50% of males and females did not meet recommended vitamin E, niacin, and folate intakes. Most adolescents (> 50%) consumed adequate amounts of vitamin C and the B vitamins (Figure 2). Controlling for BMI, males ingested significantly more calories, protein, fat, sodium, cholesterol, calcium, vitamin D, and vitamin B12 than females.
Figure 2.
Percentage of boys and girls whose usual daily intake is below recommended guidelines for vitamins
Description of Dietary Intake According to Food Groups
Breads and cereals comprised the largest component of participants' diets, and the number of bread and cereal servings increased with advancing age (Table 5). Both males and females did not eat the recommended amounts of fruits and vegetable servings per day. Males ate fewer vegetables than females across all age groups. Meats, fish, beans, and egg servings were low for all ages and both genders. Significant gender differences were noted. Males ate more servings of meat, fish, beans, and eggs (p = 0.007) and more servings of dairy products (p = 0.038).
Table 5.
Number of nutrient group servings by sex and age
| Nutrient group | Sex | 13 years | 14–18 years | 19–21 years |
|---|---|---|---|---|
| Fruits | M | 2 | 1.5 | 1.8 |
| F | 1.6 | 2.0 | 1.3 | |
| Vegetables | M | 0.9 | 2.0 | 1.8 |
| F | 2 | 2.6 | 2.8 | |
| Breads, cereals | M | 3 | 4.9 | 5.4 |
| F | 2.6 | 3.3 | 4.3 | |
| Meat, fish, beans, eggs | M | 0.6 | 1.0 | 1.7 |
| F | 0.3 | 0.3 | 0.5 | |
| Milk, yogurt, cheese | M | 1.8 | 3.5 | 2.5 |
| F | 1.4 | 1.5 | 2.0 | |
| Fat, oils, sweets | M | 0.9 | 3.0 | 1.6 |
| F | 2.9 | 2.2 | 2.3 |
Vegetarian Dietary Pattern
Slightly more than half of the sample (59%) reported that they did not eat chicken, meat, or fish. Of this group, an additional 11 (20%) did not consume eggs. There were no gender differences between those who ate meat and those who did not. Those that did not eat chicken, meat, or fish had significantly lower cholesterol intake (t = 4.153, df = 53, p = .001) and higher niacin intake (t = 2.076, df = 53, p = .043). There were no other significant differences in macronutrients, fiber, vitamins, and minerals intake.
Beverage Consumption
Beverage intake was comprised primarily of milk, with 60% consuming milk daily. Only 9% consumed low-fat (1% milk fat) or non-fat milk; whereas 38% consumed whole milk, and 45% consumed reduced-fat (2%) milk. SSBs were consumed less frequently than milk: 7% consumed a SSB daily, and 14% consumed an SSB 5–6 times weekly. Juice was the most infrequently consumed beverage: 5% consumed juice daily, with the majority (23%) consuming juice 2–3 times per month.
Length of U.S. Residency, Health, and Dietary Patterns
Length of time living in the United States was associated with lower percent of calories consumed from sweets (r = −0.285, p = .033). BMI was positively associated with length of residency in males (r = 0.478, p = .021) but not females. There were no significant differences in dietary nutrient intake based on BMI, waist circumference, blood pressure, or fasting capillary blood glucose levels.
DISCUSSION
The purpose of this study was to describe the macronutrient, vitamin, mineral, and beverage intake, and the health characteristics among first-generation urban SAI adolescents in the U.S., to identify dietary deficiencies compared to the U.S. dietary recommendations. The adolescents in this sample reported several dietary patterns that place them at risk for future negative health consequences. The mean daily percentages of total calories consumed from carbohydrates and proteins were within U.S. dietary guidelines. However, upon examining individual dietary intakes, more than half of the adolescents consumed more than the RDA for carbohydrates. This may be explained by a dietary pattern higher in consumption of bread and cereal. Slightly over half of the sample did not consume meat, which may have contributed to a higher carbohydrate intake by substituting carbohydrates for proteins.
Mean fiber, potassium, and magnesium intakes were below recommended levels for all ages and both males and females; this may reflect low fruit, vegetable, and whole grain consumption in this sample. Elevated refined carbohydrate and low fiber intake have been reported among SAIs in India as well [5]. High carbohydrate intake is a potential target for nutritional intervention because it has been associated with insulin resistance, a potent cardiovascular risk factor and antecedent to T2DM [20, 21].
The proportion of total fat intake exceeded recommended amounts for males 13 to 18 years old and 13-year-old females, and saturated fat exceeded the recommended 10% of calories for all age groups and sexes. Saturated fat intake by urban SAI adolescents living in India has been reported to be high as well, ranging from 9.4% [22] to 11% of total calories consumed [8]. The predominance of whole milk consumption over low-fat and non-fat milk options may have contributed to the high saturated fat intake in this group. Changing to lower-fat dairy products is one easy modification to lower fat intake.
The low potassium (94–96%), low magnesium (78–79%), and high sodium intakes (35% in males) may have serious future implications for cardiovascular health. Adequate amounts of potassium and magnesium and controlled amounts of sodium as recommended by the DASH dietary pattern are associated with lower blood pressure [23]. Nine percent of this sample had elevated blood pressure, and, although there were no significant associations with the dietary profiles, this is a population at risk for hypertension later in life [24]. Low magnesium intake has also been associated with impaired glucose metabolism and insulin resistance [25], which may be clinically important because over half of this sample (51%) had blood glucose levels in the prediabetic range. The pattern of low potassium, magnesium, and fiber intakes may be explained by the shared food sources of these three nutrients and is an important area for targeted education.
Nearly all of the adolescents consumed insufficient amounts of vitamin D (96% of males and 97% of females) and calcium (70% of males and 88% of females). Low dietary intake is one factor contributing to vitamin D deficiency [26], which is associated with cardiovascular disease and diabetes [27]. In Northern India, the prevalence of hypovitaminosis D among adolescents is as high as 35.7% [28]. Those of a lower SES had greater hypovitaminosis D than those of higher SES categories, suggesting the influence of dietary factors. In those migrating to the United States, SAIs had lower serum 25-hydroxyvitamin D levels compared to White Caucasians living in the same geographic area [29], suggesting that SAIs are at higher risk for vitamin D deficiency.
At least 60% of the sample reported daily consumption of milk. Although milk consumption was greater than juice and SSBs, 40% were not consuming milk daily. This may explain the low vitamin D and calcium intakes and highlights the need to address approaches to increase the intake of these nutrients. It is plausible that SSBs replaced milk products for some participants, as 14% consumed a SSB 5 to 6 times per week.
There were significant gender differences among many of the nutrients. Males ingested significantly more calories, protein, and fat even when controlling for BMI. Sodium, cholesterol, calcium, vitamin D, and vitamin B12 were also higher among males than females. Further exploration is needed of whether these differences are due to males eating greater quantities vs. different food sources.
In the current study, we found that cholesterol intake and consumption of sweets were lower among those who had lived in the United States longer. This may be explained by the changing tastes and choices of foods in the United States. Consumption of sweets is very popular in India [8], whereas adolescents who have acculturated to a westernized diet may be more likely to choose salty snack options. The role of acculturation and health is complex and has both positive and negative consequences. Although we did not specifically measure acculturation in the current study [30], greater acculturation in one group of SAIs living in California was associated with an increased number of CVD risk factors, but not in a second group [30]. The explanation for the differences between groups may have been related to differences in socioeconomic status. Determinants of positive behaviors associated with acculturation need further exploration so that they can be incorporated into strategies to promote healthy behavior.
In comparison, most adolescents in the U.S. consume less than the recommended number of fruits and vegetables and whole grains, while consuming more sodium than recommended [31, 32]. Thus, the nutrient deficiencies in our sample are similar to those of adolescents living in the United States. Within these broader deficiencies, nutrient consumption has been found to vary by race and ethnicity. Arcan et al. reported that Hispanic adolescents consumed more fruits and vegetables compared to Whites, Somalis, and Hmong [31]. More Somalis consumed energy drinks, fast food, and breakfast than the other groups. Hmong adolescents reported the lowest intake of SSBs. Masters et al. [33] reported that non-Hispanic Whites consumed the greatest amount of salty snacks compared to non-Hispanic Blacks and Hispanics. Dietary intake is also influenced by socioeconomic status (SES). Although some studies have found fewer differences by income among children compared to adults [34], adolescents with higher SES are more likely to have access to fruits and vegetables and low-fat milk [33, 34].
We did not find any association of dietary patterns with BMI, waist circumference, index of central obesity (ICO), glucose, or blood pressure, which may be related to the small sample size. Nearly one-fourth (24.9%) were overweight or obese (females more than males), which is lower than the overall adiposity rates reported for U.S. adolescents [35]. Monitoring for a healthy weight continues to be important for this population. A recent meta-analysis revealed that SAI adults who immigrated to the United States (particularly females) had higher BMI levels than other migrant groups and natives of the U.S. population [36].
Fasting capillary glucose levels were above normal in 51% of this sample, a risk factor for future diabetes. This is of particular concern because SAIs demonstrate increased insulin resistance at lower BMI levels compared to Whites, Blacks, and Hispanics [4]. Early identification of rising blood glucose trends in young people is critical to begin instituting dietary and other lifestyle interventions to reduce the incidence of diabetes in this population.
Limitations of this study were the small convenience sample and that it used self-reported measures to evaluate dietary profiles. The FFQ did not include SAI dishes and may not have captured the breadth of food choices. Additionally, we did not capture family SES levels, which may also impact nutrient choices.
Dietary behavior is a result of a complex interaction of cultural, environmental, and socioeconomic factors. Immigration to a new country with different food availability and cultural norms influences dietary behaviors [37]. Urban adolescents in South Asia report dietary patterns that are high in fat and refined carbohydrates, which promote obesity, insulin resistance, diabetes, and CVD. First-generation urban adolescent SAIs in the United States are confronted with similar dietary trends, despite differing food availability.
New Contribution to the Literature
The first-generation urban SAI adolescents in this study consumed a high-fat diet with excess amounts of saturated fat, while consuming inadequate amounts of potassium, magnesium, calcium, and vitamin D. This trend could be reversed by advocating for increased consumption of low-fat milk and other low-fat dairy products. Converting to whole grain carbohydrate sources and adding more fruits and vegetables would increase the amount of daily fiber, magnesium, potassium, and other important mineral sources.
Table 6.
Health information*
| Male (n = 23) | Female (n = 33) | p value(units/d) | |
|---|---|---|---|
| Body mass index (BMI) | 21.6 ± 4.3 | 22.5 ± 4.9 | 0.484 |
| Waist circumference (cm) | 77.9 ± 13.1 | 71.4± 18.2 | 0.150 |
| Index central obesity (ICO) | 0.46 ± 0.08 | 0.45 ± 0.12 | 0.631 |
| Blood pressure (systolic) | 111 ± 11 | 106 ± 12 | 0.107 |
| Blood pressure (diastolic) | 64 ± 12 | 65 ± 10 | 0.663 |
| Capillary blood glucose (fasting) | 101 ± 8 | 98 ± 13 | 0.448 |
All values are reported as mean ± standard deviation.
Acknowledgments
Supported in part by the University of Chicago: Diabetes Research and Training Center: NIH-NIDDK: P60 DK020595-32S3 and by the UIC College of Nursing Internal Research Support Program (IRSP). The authors report no financial conflict. The authors thank Kevin Grandfield, UIC Department of Biobehavioral Health Science Publication Manager, for editorial assistance.
References
- 1.U.S. Census Bureau. [accessed February 20 2014];The Asian population 2010. 2010 Available from http://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf.
- 2.World Health Organization. [accessed February 20 2014];India population. 2011 Available from http://www.who.int/nmh/countries/ind_en.pdf?ua=1.
- 3.Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. International Journal of Obesity. 2011;35:167–187. doi: 10.1038/ijo.2010.135. [DOI] [PubMed] [Google Scholar]
- 4.Peterson KF, Dufour S, Feng J, Befroy D, Dziura J, Man CD, Cobelli C, Shulman GI. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. PNAS. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra A, Khurana L, Isharwal S, Bhardwaj S. South Asian diets and insulin resistance. The British Journal of Nutrition. 2009;101:465–473. doi: 10.1017/S0007114508073649. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanyam A, Rao S, Misra R, Sekhar RV, Ballantyne CM. Prevalence of metabolic syndrome and associated risk factors in Asian Indians. Journal of Immigrant and Minority Health. 2008;10:313–323. doi: 10.1007/s10903-007-9092-4. [DOI] [PubMed] [Google Scholar]
- 7.Lake AA, Mathers JC, Rugg-Gunn AJ, Adamson AJ. Longitudinal change in food habits between adolescence (11–12 years) and adulthood (32–33 years): the ASH30 Study. Journal of Public Health (Oxford, England) 2006;28:10–16. doi: 10.1093/pubmed/fdi082. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Shah P, Goel K, et al. Imbalanced dietary profile, anthropometry, and lipids in urban Asian Indian adolescents and young adults. Journal of the American College of Nutrition. 2010;29:81–91. doi: 10.1080/07315724.2010.10719820. [DOI] [PubMed] [Google Scholar]
- 9.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. American Journal of Public Health. 2008;98:1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control (CDC) and Prevention. [accessed August 9 2009];About BMI for children and teens. 2009 Available from http://www.cdc.gov/healthyweight/assessing/bmi/childrens_BMI/about_childrens_BMI.html.
- 11.Centers for Disease Control and Prevention. [accessed February 20 2014];Healthy Weight for Adults. 2014 Available from http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/
- 12.World Health Organization. Waist circumference and waist–hip ratio: report of a WHO expert consultation. WHO; Geneva: 2008. [Google Scholar]
- 13.Parikh RM, Joshi SR, Menon PS, Shah NS. Index of central obesity – A novel parameter. Medical Hypotheses. 2007;68:1272–1275. doi: 10.1016/j.mehy.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 14.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes--2015. Diabetes Care. 2015;38(Suppl 1):S8–16. [PubMed] [Google Scholar]
- 17.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. U.S. Government Printing Office; Washington, DC: 2010. [Google Scholar]
- 18.Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. U.S. Government Printing Office; Washington, DC: 2011. [Google Scholar]
- 19.Berkey CS, Willett WC, Tamimi RM, Rosner B, Frazier AL, Colditz GA. Vegetable protein and vegetable fat intakes in pre-adolescent and adolescent girls, and risk for benign breast disease in young women. Breast Cancer Research and Treatment. 2013;141:299–306. doi: 10.1007/s10549-013-2686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballard KD, Quann EE, Kupchak BR, et al. Dietary carbohydrate restriction improves insulin sensitivity, blood pressure, microvascular function, and cellular adhesion markers in individuals taking statins. Nutrition Research. 2013;33:905–912. doi: 10.1016/j.nutres.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Light K, Henderson M, et al. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. The Journal of Nutrition. 2014;144:81–86. doi: 10.3945/jn.113.182519. [DOI] [PubMed] [Google Scholar]
- 22.Isharwal S, Arya S, Misra A, et al. Dietary nutrients and insulin resistance in urban Asian Indian adolescents and young adults. Annals of Nutrition & Metabolism. 2008;52:145–151. doi: 10.1159/000127416. [DOI] [PubMed] [Google Scholar]
- 23.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. The New England Journal of Medicine. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh A. Factor analysis of risk variables associated with metabolic syndrome in Asian Indian adolescents. American Journal of Human Biology. 2007;19:34–40. doi: 10.1002/ajhb.20570. [DOI] [PubMed] [Google Scholar]
- 25.Hruby A, Meigs JB, O'Donnell CJ, Jacques PF, McKeown NM. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged Americans. Diabetes Care. 2014;37:419–427. doi: 10.2337/dc13-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harinarayan CV, Joshi SR. Vitamin D status in India--its implications and remedial measures. The Journal of the Association of Physicians of India. 2009;57:40–48. [PubMed] [Google Scholar]
- 27.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. The Lancet Diabetes & Endocrinology. 2014;2:76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 28.Marwaha RK, Tandon N, Reddy DR, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. The American Journal of Clinical Nutrition. 2005;82:477–482. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- 29.Awumey EM, Mitra DA, Hollis BW, Kumar R, Bell NH. Vitamin D metabolism is altered in Asian Indians in the southern United States: a clinical research center study. Journal of Clinical Endocrinology and Metabolism. 1998;83:169–173. doi: 10.1210/jcem.83.1.4514. [DOI] [PubMed] [Google Scholar]
- 30.Ivey SL, Mehta KM, Fyr CL, Kanaya AM. Prevalence and correlates of cardiovascular risk factors in South Asians: Population-based data from two California surveys. Ethnicity & Disease. 2006;16:886–893. [PubMed] [Google Scholar]
- 31.Arcan C, Larson N, Bauer K, Berge J, Story M, Neumark-Sztainer D. Dietary and weight-related behaviors and body mass index among Hispanic, Hmong, Somali, and White adolescents. Journal of the Academy of Nutrition and Dietetics. 2014;114:375–383. doi: 10.1016/j.jand.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. U.S. Department of Agriculture and U.S. Department of Health and Human Services; Washington, DC: 2010. [Google Scholar]
- 33.Masters MA, Stanek Krogstrand KL, Eskridge KM, Albrecht JA. Race/ethnicity and income in relation to the home food environment in US youth aged 6 to 19 years. Journal of the Academy of Nutrition and Dietetics. 2014;114:1533–1543. doi: 10.1016/j.jand.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Kirkpatrick SI, Dodd KW, Reedy J, Krebs-Smith SM. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. Journal of the Academy of Nutrition and Dietetics. 2012;112:624–635. e626. doi: 10.1016/j.jand.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez R, Miranda C, Everett B. Prevalence of obesity among migrant Asian Indians: a systematic review and meta-analysis. International Journal of Evidence-based Healthcare. 2011;9:420–428. doi: 10.1111/j.1744-1609.2011.00243.x. [DOI] [PubMed] [Google Scholar]
- 37.Misra R, Patel TG, Davies D, Russo T. Health promotion behaviors of Gujurati Asian Indian immigrants in the United States. Journal of Immigrant Health. 2000;2:223–230. doi: 10.1023/A:1009544414050. [DOI] [PubMed] [Google Scholar]