Abstract
Previous findings suggest that exercise is a safe and efficacious means of improving physiological and psychosocial outcomes in female breast cancer survivors. To date, most research has focused on post-treatment interventions. However, given that the type and severity of treatment-related adverse effects may be dependent on the type of treatment, and that the effects are substantially more pronounced during treatment, an assessment of the safety and efficacy of exercise during treatment is warranted. In this review, we present and evaluate the results of randomized controlled trials (RCTs) conducted during breast cancer treatment. We conducted literature searches to identify studies examining exercise interventions in breast cancer patients who were undergoing chemotherapy or radiation. Data were extracted on physiological and psychosocial outcomes. Cohen’s d effect sizes were calculated for each outcome. A total of 17 studies involving 1,175 participants undergoing active cancer therapy met the inclusion criteria. Findings revealed that, on average, exercise interventions resulted in moderate to large improvements in muscular strength: resistance exercise (RE, d = 0.86), aerobic exercise (AE, d = 0.55), small to moderate improvements in cardiovascular functioning (RE, d = 0.45; AE, d = 0.17, combination exercise (COMB, d = 0.31) and quality of life (QoL; RE, d = 0.30; AE, d = 0.50; COMB, d = 0.63). The results of this review suggest that exercise is a safe, feasible, and efficacious intervention in breast cancer patients who are undergoing different types of treatment. Additional research addressing the different modes of exercise during each type of treatment is warranted to assess the comparable efficacy of the various exercise modes during established breast cancer treatments.
More than 2 million women in the United States are either undergoing active treatment or have completed treatment for breast cancer. Despite the established efficacy of breast cancer treatments such as chemotherapy and radiation, they have adverse effects on patients’ cardiovascular, metabolic, and quality-of-life (QoL) outcomes. Some adverse effects can be acute, occurring primarily during treatment, whereas others may have a delayed onset and persist for years after the cessation of treatment. Moreover, it seems that the mode of treatment a patient receives may determine the magnitude and variability of these side effects.1 For example, chemotherapy often leads to nausea, vomiting, depression,2 reduced bone-mineral density,3 and cardiac toxicity.4 Cancer-related fatigue has been reported in patients who undergo chemotherapy with or without radiotherapy.2 Radiation has been linked with cardiac toxicity and damage to surrounding organs from unintentional irradiation.4,5 Furthermore, the use of these treatments sequentially may result in more pronounced acute toxicity and fatigue.6
It is becoming more widely recognized that exercise is an important intervention in the multidisciplinary management of breast cancer. Moreover, exercise can play an important role in preserving fitness and function across the survivorship continuum.7 However, if exercise is to be established as a standard of cancer care, there is a need for clarity in the evidence supporting the safety and efficacy of exercise interventions during different types of treatment. To date, most research has focused on post-treatment care and the restoration of function. Indeed, several review articles have summarized the benefits of exercise after treatment in breast cancer patients.8–11 However, given that the adverse effects are substantially more pronounced during treatment, an assessment of the safety and efficacy of exercise during primary treatment is warranted. Furthermore, since the severity of side effects may be dependent on the type of treatment, it is important clinically to evaluate the effects of exercise during different types of breast cancer treatment.
The purpose of this systematic review of the effects of exercise interventions during chemotherapy and radiation in breast cancer patients is to present and evaluate the results of randomized controlled trials (RCTs) conducted in this area to date. In addition, we aim to provide an estimation of the magnitude of the change in outcomes using Cohen’s d effect sizes and to summarize recruitment, retention, adherence, and overall methodological quality of the interventions.
Methods
Inclusion criteria
Studies were included in the review if they examined exercise during chemotherapy or radiation for breast cancer and were published in English. In accordance with previous literature, interventions during therapy were defined as those that began with or after the initiation of treatment and concluded either 1 week after the last radiation treatment, 3 weeks after the last intravenous chemotherapy treatment, or 3 weeks after the cessation of chemotherapy.12,13 Also included were studies that used interventions aimed at improving cardiovascular endurance, or muscular strength and endurance. Interventions that used alternative techniques such as yoga or tai-chi, were also included. For the purposes of this study, only RCTs that allowed for effect sizes to be calculated were included.
Study selection and data abstraction
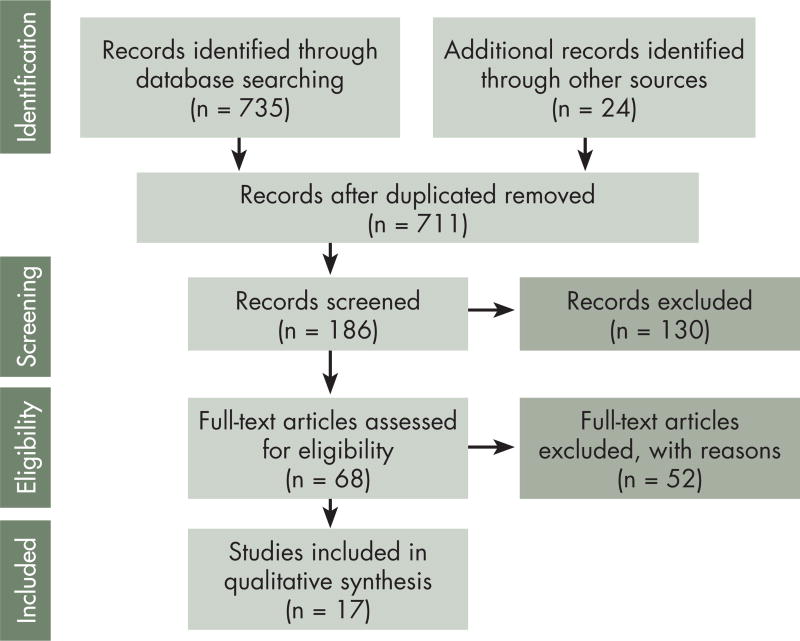
We performed computer and manual searches following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).14 An original search of titles and abstracts in PubMed and MEDLINE databases was conducted in October 2014. A subsequent search was conducted in April 2015 to ensure inclusion of any additional published manuscripts. Search terms including exercise (physical activity, weight training, resistance exercise, cardiovascular training, rehabilitation, aerobic, yoga) and cancer treatment (primary therapy, therapy, cancer treatment, chemotherapy, radiation, hormonal therapy) were entered in different combinations. In accordance with PRISMA guidelines, a summary of the results of the computerized search was outlined (Figure). Manual searches were conducted using reference lists of previous reviews and meta-analyses of the exercise oncology literature.
FIGURE 1.
PRISMA flow diagram showing the results of the computerized search
Data synthesis
Quantitative effect size calculations were used to synthesize the results from the interventions included in the present review. Quantitative synthesis was conducted using Cohen’s d effect sizes. These were either obtained directly from the studies themselves, or calculated using statistical information provided in the study. Cohen’s d effect sizes are classified as: small, 0.20; moderate, 0.50; and large, 0.80. Effect sizes were calculated by taking the difference of the mean values obtained at baseline and follow-up assessments and dividing by the pooled standard deviation. For the purpose of this review, positive effect sizes indicated an improvement in outcomes and negative effect sizes represented unfavorable changes in an outcome. Specific outcomes of interest were QoL, fatigue, and various performance/functional outcomes (aerobic capacity, strength, and so on). Study quality was assessed by 2 independent freviewers (CMF, BCF) using the Delphi list for evaluation of the quality of RCTs.15 Each item was rated Yes or No based on the methods reported in each study. Studies that did not provide methodological information that directly addressed a particular quality indicator were recorded as not having met that indicator in the evaluation. Given that there is presently no validated summary scoring system for the Delphi criteria list, the number of indicators met by each of the studies included in the systematic review was tabulated.
Results
Study characteristics
A total of 17 studies involving 1,175 participants met the inclusion criteria of this review (Table 1). Sample sizes ranged from 20–242 participants. The resistance exercise (RE) intervention characteristics included a range of 1–3 sets of 8–12 repetitions at training loads ranging from 40%–80% 1 repetition maximum (1RM), lasting from 12 weeks to 6 months. The aerobic exercise (AE) intervention characteristics included training intensities ranging from 50%–80% age-predicted maximal heart rate (APM HR), for 10–60 minutes, 3–6 days a week, lasting from 6 weeks to 6 months. Combined interventions included a combination of aerobic and resistance training. Specific details about these interventions were not reported in the studies.
TABLE 1.
Study characteristics and description of the feasibility outcomes from the exercise interventions
| Study | Sample size |
Intervention characteristics (mode, duration, supervision, sets, reps, load) |
Overall findings | Recruitment rate,% |
Retention rate,% |
Adherence rate,% |
Adverse events |
|---|---|---|---|---|---|---|---|
| Battaglini32 | 20 | 15 wk of supervised RE; 2–3 sets of 6–12 reps at 40%–60% 1RM | Improvements in muscular strength and fatigue | na | 100 | na | No events reported |
| Battaglini20 | 20 | 15 wk of supervised RE+AE; 2–3 sets of 6–12 reps at 40%–60% 1 RM, 6–12 min AE | Improvements in lean body mass, body fat, and strength | na | 100 | na | No events reported |
| Cadmus29 | 6 mo of home-based AE; 150 min/wk, 60%–80% AP-MHR | Improvements in depression and happiness | 64 | No events reported | |||
| Chandwani28 | 132 | 6 wk of yoga 3 d/wk during radiation | Improvements in QoL, fatigue, sleep quality, and depression | 55 | 69 | na | No events reported |
| Courneya22 | 242 | RE: Supervised for duration of chemo, 2 sets of 8–1 2 reps at 60%–70% 1RM. AE: Supervised 3 d/wk, 15–45 min, 60%–80% VO2max. | RE yielded improvements in upper- and lower-body strength. Both exercise groups had a higher completion rate of chemotherapy, compared with control group. | 33 | 92.1 | 72 (AE) 68 (RE) | 2 mild/moderate events involving nausea, dizziness and weakness |
| Dolan23 | 242 | RE: Supervised for duration of chemo, 2 sets of 8–1 2 reps at 60%–70% 1RM. AE: Supervised 3 d/wk, 15–45 min, 60%–80% VO2max. | Participants in both groups experienced decline in hemoglobin regardless of intervention. RE and AE interventions maintained VO2max throughout treatment. | 33 | 92 | 72 (AE) 68 (RE) | 2 mild/moderate events involving nausea, dizziness and weakness |
| Hornsby16 | 20 | 12 wk of supervised AE, 3 d/wk, 15–45 min, 60%–100% VO2peak | Improvements in social well-being. Non-significant improvements in VO2peak and QoL. | 73 | 95 | 66 | 1 mild/moderate involving leg pain during AE |
| Husebø25 | 67 | Home-based RE+AE: RE: total body exercises 3 d/wk AE: 30 min/d light-vigorous intensity | Increase in fatigue at completion of chemo, with return to baseline at 6-mo follow-up. Improvement in 6MWT and MET at 6-mo follow-up. | 72 | 76 | 58 | 1 mild/moderate event involving knee discomfort |
| Hwang26 | 40 | 5 wk mixed AE+RE 50 min, 3 d/wk | Improvement in QoL | na | 92.5 | na | No events reported |
| Kim30 | 41 | 8-wk supervised AE, 3 d/wk, 30 min, 60%–70% VO2peak | Improvement in VO2peak and voluntary exercise | na | 55 | 78.3 | No events reported |
| Mock31 | 52 | Home-based walking intervention for duration of treatment, 150 min/wk, 50%–70% AP-MHR | Participants in High Walkers group experienced improvements in fatigue, activity level, and aerobic capacity compared with participants in Low Walkers group. | na | 70 | No events reported | |
| Mock19 | 119 | Home-based walking intervention for duration of treatment, 150 min/wk, 50%–70% APM-HR | Participants in High Walkers group experienced in improvements in 12MWT and physical activity compared with Low Walkers group. | 50.8 | 90 | 72 | No events reported |
| Schmidt21 | 95 | 12-wk RE intervention, 3 sets, 8–12 reps, 60%–80% 1RM | RE resulted in negligible changes in fatigue, QoL, depression, and cognitive function. | na | 96 | 71 | No events reported |
| Schwartz24 | 66 | RE: Home-based for duration of treatment, 2 sets 8–10 reps. AE: Home-based 4 d/wk, 15–30 min, moderate intensity. | RE yielded improvements in leg extension and seated-row 1RM. AE yielded improvements in 12MWT, leg extension, and seated-row 1RM. | 95 | 93 | n/a | No events reported |
| Vadiraja27 | 88 | 6-wk supervised yoga, 60 min, 3 d/wk | Improvements in anxiety, stress, depression, and 6 am Cortisol levels | 53.3 | 63.6 | na | No events reported |
| Wang17 | 72 | 6-wk home-based AE intervention, 10–30 min, 3–5 d/wk, 40%–60% AP-MHR | Improvements in QoL, sleep, ESE, GLTEQ score | 45 | 80.6 | 93.6 | No events reported |
| Yang18 | 40 | 6-wk supervised AE intervention, 20–30 min, 2–3 d/wk, 40%–65% AP-MHR | Improvements in MET, and MDSAI and POMS scores | na | 90.9 | 77 | No events reported |
AE, aerobic exercise; AP-MHR, age-predicted maximal heart rate; ESE, exercise stress echocardiography; GLTEQ, Godin Leisure-Time Exercise Questionnaire; MDSAI, MD Anderson Symptom Inventory; MET, metabolic equivalent task; min, minute; mo, month; MWT, minute walk test; na, not applicable; POMS, Profile of Mood States; RE, resistance exercise; RM, repetition maximum; VO2, oxygen consumption; QoL, quality of life; wk, week
Recruitment, retention, and adherence rates
A summary of the recruitment, retention, and adherence rates are reported in Table 1. For 11 of the 17 studies, an average of 53% (range, 15%–95%) of eligible participants screened for inclusion, were enrolled for the trials. Retention rates (participants who completed baselines and post assessments) averaged 86% (range, 55%–100%). Of the 17 studies, 6 did not report adherence to the interventions. Adherence (attendance to each exercise session) for the remaining interventions was 71% (range, 58%–95%).
Methodological quality assessment
A summary of the methodological assessment is presented in Table 2. Overall, the studies met an average of 64% of the quality indicators (range, 43%–86%). The most commonly observed problems were a lack of intent-to-treat analysis, testing not conducted by blinded evaluators, and lack of concealment of treatment allocation. Notable methodological strengths of the RCTs were specificity of eligibility criteria, similarity of key outcomes at baseline, and consistent reporting of relevant descriptive statistics.
TABLE 2.
Methodologic quality indicators from the Delphi consensus criteria list
| Study | Randomized | Allocation concealed |
Key outcomes similar at baseline |
Eligibility criteria specified |
Single blind | Key descriptive statistics reported |
Intent-to- treat analysis |
|---|---|---|---|---|---|---|---|
| Battaglini32 | Yes | No | Yes | Yes | No | Yes | No |
| Battaglini20 | Yes | Yes | Yes | Yes | No | Yes | No |
| Cadmus29 | Yes | No | Yes | Yes | No | Yes | No |
| Chandwani28 | Yes | Yes | Yes | Yes | No | Yes | No |
| Courneya22 | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Dolan23 | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Hornsby16 | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Husebø25 | Yes | No | Yes | Yes | Yes | Yes | No |
| Hwang26 | Yes | No | Yes | Yes | No | Yes | No |
| Kim30 | Yes | No | Yes | Yes | No | Yes | No |
| Mock31 | No | No | Yes | Yes | No | Yes | No |
| Mock19 | Yes | No | Yes | Yes | No | Yes | No |
| Schmidt21 | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Schwartz24 | Yes | No | Yes | Yes | No | Yes | No |
| Vadiraja27 | Yes | No | Yes | Yes | No | Yes | No |
| Wang17 | Yes | No | Yes | Yes | No | Yes | No |
| Yang18 | Yes | No | Yes | Yes | No | Yes | No |
Summary of exercise interventions by treatment type and mode of exercise
A brief summary of each study’s sample, outcome assessments, and feasibility measures, and their accompanying effect sizes are outlined in the following section. The studies are organized by type of treatment and mode of exercise. A summary of the effect size changes in the outcomes of each trial is provided in Table 3.
TABLE 3.
Summary of effect sizes for the physiological and psychosocial QoL outcomes
| Study | Design, treatment | Effect sizes | |
|---|---|---|---|
| Physiological outcomes | Psychosocial QoL outcomes | ||
| Battaglini32 | RCT, COMB-S/R/C | Strength 1.39 | Fatigue Wk 8, .4; Wk 11, .17; Wk 15, .92; Wk 21,.43 |
| Battaglini20 | RCT, COMB-S/C | LBM Wk 8, .3; Wk 11,.47; Wk 15, .55; Wk 21, .9 BF% Wk 8, .31; Wk 11, .5; Wk 15, .53, Wk 21,.98 Strength 1.4 | NA |
| Cadmus29 | RCT, MIX – R/C | NA | Happiness .49 Fatigue −.1 FACT-G .30 SF-36 MH .28; SF-36 Soc .58; SF-36 VIT .01; SF-36 ROLE .33; SF-36 PHY .19; SF-36 Pain .42; SF-36 GEN .16; SF-36 ROLP .45 |
| Chandwani28 | RCT, R | NA | Fatigue post, 1.0; 1-month post, 1.4; 3-month post 2.0; 6-month post, 1.13 Depression post, 1.3; 1-month post, −1.4; 3-month post 0.93; 6-month post, 0.90 QoL post, 1.5; 1-month post, 2.1; 3-month post 3.0; 6-month post, 1.8 Sleep quality post, 2.8; 1-month post, 2.1; 3-month post 3.2; 6-month post, 2.17 |
| Courneya22 | RCT, C | Chest press strength RE, .9; AE, .34 Leg press strength RE, .7; AE, .25 VO2peak RE, .21; AE, .06 BW RE, .09; AE, .07 BF% RE, 0; AE, 0 FM RE, .05; AE, .04 LBM RE, .21; AE, .12 | FACT-A RE: mid, .01, post, .3; AE: mid, −.007; post, .33 SE RE: mid, −.24; post,−.18; AE: mid, .00; post, .24 Anxiety RE: mid, .42; post, .45 AE: mid, 0.46; post, .49 Depression RE: mid, .12; post, .32; AE: mid, .06; post, +.32 |
| Dolan23 | RCT, C | Hb RE, 1.7; AE, 1.1 VO2 RE, .21; AE, .06 | NA |
| Hornsby16 | RCT, C | RHR .55 VO2 .35 Vent .74 CO 1.0 LVEF% .13 | FACT-B, .22 FACT-G,.16 |
| Husebø25 | RCT, MIX – R/C | 6MWT post,−.2; FU, .31 MET post, .18; FU, .43 | Fatigue post, −.41; FU, −.02 |
| Hwang26 | RCT, R | NA | QoL, .63 QoL Overall Health, .36 QoL Physical, 1.67 QoL Psychological, .48 QoL Social, .54 QoL Environmental, .11 |
| Kim30 | RCT, MIX-R/C | RHR .5 MHR .26 RBP .24 MBP .41 VO2 .38 | NA |
| Mock31 | RCT, MIX – R/C | 12MWT Hi, .33; Lo, .02 ALRS Hi, .76; Lo, .25 | Fatigue Hi, 1.41; Lo, .48 |
| Mock19 | RCT, MIX – R/C | PF Hi, .2; Lo, .39 12MWT Hi, .57; Lo, −.20 PA Hi, .40; Lo,−. 14 | Fatigue Hi, −.22; Lo, −70 |
| Schwartz24 | RCT, C | 12MWT AE, .79; RE, .13 OHP strength AE, .24; RE, .21 SR strength AE, .61; RE, .5 Leg ext strength AE, .5; RE, .45 | NA |
| Vadiraja27 | RCT, R | Cortisol 6 am, .68; 9 am, .46; 9 pm, .19 | Anxiety .87 Depression .91 Stress 1.01 |
| Wang17 | RCT, MIX – R/C | NA | FACT-GTP1 .15; TP2,−.12; TP3, .65 FACIT-F TP1 .38; TP2, −.15; TP3, .66 PSQITP1 .19; TP2, .40; TP3, .40 ESES TP1 .48; TP2, .68; TP3, .64 GLTEQ TP1 .96; TP2, .87; TP3, 1.36 |
| Yang18 | RCT, C | MET 6 mo, .64; 12 mo, .6 | Fatigue severity 6 mo, −.5; 12 mo, .56 Fatigue intensity 6 mo, −.85; 12 mo, −.78 POMS-SF 6 mo, .68; 12 mo, .59 |
| Schmidt21 | RCT, C | NA | Total fatigue .015 Physical fatigue .02 Affective fatigue . 11 Cognitive fatigue . 18 QLQ-C30 Global .01 CES-D Depression .008 |
6MWT, 6-minute walk test; 12MWT, 12-minute walk test; ALRS, activity level rating scale; BF%, body fat percentage; BW, body weight; CES-D, Center for Epidemiologic Studies Depression Scale; CO, cardiac output; EDV, end diastolic volume; ESES, Exercise Self-Efficacy Scale; ESV, end systolic volume; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; FACT, Functional Assessment of Cancer Therapy (A, Anemia; B, Breast; G, General) FM, fat mass; FU, follow-up; GLTEQ, Godin and Shepherd’s Leisure Time Exercise Questionnaire; Hb, hemoglobin; LBM, lean body mass; leg ext, leg extension; LEVF%, left ventricle ejection fraction; MBP, max blood pressure; MET, metabolic equivalent task; MHR, max heart rate; na, not applicable; O2 pulse, oxygen pulse (mLO2/beat); OHP, overhead press; PA, physical activity, PBP, peak blood pressure; PF, physical function; PHR, peak heart rate; POMS-SF, Profile of Mood State-Short Form; PSQI, Pittsburgh Sleep Quality Index; QLQ-C30, Quality of Life Questionnaire-Core 36; RBP, resting blood pressure; RHR, resting heart rate; SE, self-efficacy, SF-36, Short Form Health Survey (GEN, general health; MH, mental health; PHY, physical functioning; PAIN, bodily pain; ROLE, role limitations-emotional; ROLE, role limitations-physical; SOC, social functioning; VIT, vitality); SR, seated row; Vent, ventilation (LO2/min); VO2, oxygen consumption;
Chemotherapy
AE interventions
Three trials examined AE during chemotherapy. The duration of the interventions varied from 6–12 weeks, with an average of 40 participants (range, 19–62). Adherence to the interventions ranged from 66%–93%. Three nonserious adverse events were reported among the 3 trials. One was a participant experiencing unexplained leg pain that subsided after exercise cessation. Two participants dropped out of a trial after reports of anemia and dizziness. AE yielded improvements in QoL (d = .56; range, .50−.64), sleep (d = .41), self-efficacy (d = .64), behavior (d = 1.30), social well-being (d = .90), metabolic equivalent tasks (MET; d = .60), MD Anderson Symptom Inventory score (severity, d = .56; interference, d = .78) and Profile of Mood States score (d = .59). AE yielded moderate-in-magnitude improvements in measures of aerobic fitness (d = .47; range, .28−.79).16,17,18, 19
RE interventions
Two trials examined RE in participants undergoing chemotherapy. The duration of the trials were 12 and 21 weeks, and the number of participants in the trials was 20 and 72 patients. Adherence to the 1 intervention was 71%; adherence rates were not reported in the other. RE interventions yielded improvements in body fat (d = 0.98), lean body mass (d = 0.90), and strength (d = 0.78; range, 0.43–1.4) with negligible changes in fatigue (total d = 0.02) QoL (global d = 0.01), depression (d = 0.01) and cognitive function (d = 0.30).20,21
AE and RE interventions
Three studies compared the effects of RE and AE interventions during chemotherapy. The 3 interventions examined exercise for the duration of chemotherapy (mean, 17 weeks; range, 9–24 weeks). The number of participants in the studies ranged from 67–242. Adherence to the interventions ranged from 68%–72%. Two nonserious adverse events were reported during 1 intervention and related to nausea, dizziness, and weakness, with both participants recovering quickly. RE yielded improvements in the upper body (d = .47; range, .21−.70) and lower body (d = .60; range, .43−.90), and strength and anxiety (d = .45). A decline in hemoglobin was observed in 1 RE intervention (d = −1.70), with a concurrent maintenance of aerobic capacity. A decline in hemoglobin (d = −1.10) with maintenance of aerobic capacity was also observed with the AE. AE yielded improvements in upper body (d = .61) and lower body (d = .50) strength, and anxiety (d = .47). One intervention examined chemotherapy completion rates, with 84.1% in the usual-care group, compared with 89% in the RE group and 87.4% in the AE group. Both AE and RE served to maintain bone-mineral density throughout chemotherapy (RE: d = .22; AE: d = .10).22,23,24
Combined exercise interventions
Only 1 trial examined the effects of a combined exercise intervention during chemotherapy. Specifically, in a 2-arm randomized controlled trial, Husebø and colleagues compared patients in a home-based exercise (resistance and aerobic) intervention for the duration of chemotherapy with those in a usual-care group in a total of 67 breast cancer patients.25 Of 92 eligible participants, 67 (76%) were enrolled in the study, and 64 participants completed 6-month follow-up. Adherence to the exercise intervention was 58%. Assessments of fatigue (using the 6-item Schwartz Cancer Fatigue Scale), physical activity (International Physical Activity Questionnaires) and physical fitness (6MWT or 6-minute walk test) were obtained at baseline, end of chemotherapy, and 6-month follow-up. There was a significant increase in fatigue at end of chemotherapy (d = −.41), with a return to baseline at 6-month follow-up (d = .02), while there was a negligible change in 6MWT (d = .20) and MET (d = .18) at end of chemotherapy with a significant improvement at 6-month follow-up (6MWT, d = .31; MET, d = .43). There were 2 nonserious adverse events related to the intervention with 1 account of knee pain, and another due to syncope from a secondary chronic condition.
Alternative modes of exercise interventions
No study that met our inclusion criteria focused specifically on alternative modes of exercise, such as yoga, during chemotherapy alone.
Radiation
AE interventions
No studies that met our inclusion criteria focused specifically on AE during radiation treatment alone.
RE interventions
No studies that met our inclusion criteria focused specifically on RE during radiation treatment alone
AE and RE interventions
No studies that met our inclusion criteria focused specifically on AE and RE during radiation treatment alone
Combined exercise interventions
Only 1 trial examined the effect of a combined exercise intervention during radiation treatment. Hwang and colleagues conducted a 2-arm randomized controlled trial to examine the efficacy of a 5-week supervised exercise intervention in 40 breast cancer patients undergoing radiotherapy.26 Assessments of QoL, fatigue (BFI), range of motion (RoM) of the shoulder, and pain were obtained at baseline and 5-week follow-up. Recruitment to the study was not reported. In all, 37 of the 40 enrolled (92.5%) completed baseline and follow-up assessments. No adverse events related to the intervention were reported. The descriptive statistics necessary to calculate effect sizes for fatigue, RoM, and pain were not provided. However, the exercise intervention resulted in an improvement in overall QoL (d = .63).
Alternative modes of exercise interventions
Two studies were included examining the effects of yoga in breast cancer patients undergoing radiation. Both interventions lasted 2 weeks. The number of participants who completed baseline and follow-up assessments were 56 and 132 respectively. Adherence to either intervention wasn’t reported. No adverse events related to the interventions were reported. Yoga resulted in significant improvements in anxiety (d = .61), depression (d = 1.0; range, 0.91–1.30), stress (d = 1.01), fatigue (d = 1.13), sleep (d = 2.10), and QoL (d = 2.00).27,28
Combination treatment (1 or more variations of chemotherapy, radiation, etc.)
AE interventions
Four studies examined AE during mixed therapy during combination treatment. The duration of the interventions ranged from 8 weeks to 6 months. The number of participants in the AE interventions ranged from 41–108. Adherence to the interventions ranged from 64%–78%. No adverse events related to any of the interventions were reported. The AE interventions yielded small effect size improvements in SBP (d = .25), maximum heart rate (MHR; d = .26), mild-to-moderate effect size improvements in depression (d = .38), happiness (d = .49), aerobic capacity (d = .45; range, .31−.57), activity level (d = .66; range, .62−.76), and resting heart rate (RHR; d = .50). Large effect size improvements were seen in fatigue (d = .82; range, .22–1.41). Negligible changes were observed in Functional Assessment of Cancer Therapy-General scores (d = .30), anxiety (d = .13), physical functioning (d = .2), and self-esteem (d = .10).29,30,31
RE interventions
Only 1 trial examined the effect of an RE intervention during combination treatment. Battaglini and colleagues (2006) examined the effects of a 15-week supervised RE intervention on muscular strength and fatigue in 20 breast cancer patients undergoing radiation, chemotherapy, or a combination of both.32 The number of women prescreened for inclusion was not reported. Although all of the participants completed the study, adherence to RE interventions sessions was not reported. No adverse events were reported. The RE intervention resulted in significant improvements in muscular strength (d = 1.39) and fatigue (week 8, .40; week 11, .17; week 15, .92; week 21, .43).
Combined AE and RE exercise
No studies meeting the inclusion criteria focused specifically on combined exercise during mixed combination therapy.
Alternative modes of exercise interventions
No studies meeting the inclusion criteria focused specifically on alternative exercise (eg, yoga) during mixed combination therapy.
Synthesis of overall interventions during combination therapy
One intervention examined RE during mixed therapy, yielding improvements in muscular strength (d = 1.39) and fatigue (week 8, d = .40; week 11, d = .17; week 15, d = .92; week 21, d = .43). Four studies examining AE during mixed therapy were included in the review. Most notable improvements were seen in depression (d = .38), happiness (d = .49), aerobic capacity (d = 0.45; range, .31−.57), activity level (d = .66; range, .62−.76), RHR (d = .50), and fatigue (d = .82; range, .22–1.41) 29,30,31
Overall, our results revealed that the magnitude of improvement observed with exercise interventions during breast cancer treatment varied across outcomes and appeared to be specific to the mode of exercise. RE yielded large average effect size increases in muscular strength (d = .87; range, .21 to 1.40) and low effect size improvements in cardiovascular function (d = .17; range, .13 to .21) and QoL (d = .28; range, .01 to .30). AE was associated with moderate improvements in strength (d = .55; range, .51 to .60), cardiovascular function (d = .45; range, .28 to .79.), and QoL (d = .50; range, .30 to .64).
Discussion
The purpose of this review was to systematically evaluate of the efficacy of implementing exercise interventions in the adjuvant treatment of breast cancer patients undergoing different modes of primary therapy. The results indicate that AE alone, RE alone, interventions combining aerobic and resistance exercise, as well as alternative modes of exercise (eg, yoga) are safe, well-tolerated lifestyle interventions that can not only attenuate many of the adverse effects accompanying treatment, but can yield significant, clinically meaningful improvements in select fitness, physiological, and patient-reported outcomes (PROs) in breast cancer patients who are undergoing cancer treatment. These findings are consistent with those of other studies of exercise in breast cancer patients7–9 and they provide evidence supporting the safety and utility of integrating exercise as a supportive-care intervention for breast cancer patients undergoing different types of active treatment.
The most consistently assessed outcomes across the trials were disease specific QoL and PROs such as mood, depression, and anxiety. Results revealed that irrespective of the type of exercise, interventions yielded comparable, small-to-moderate effect size improvements in both disease-specific indices of QoL and PROs. We view the observation that various modes of exercise yielded similar improvements in key PROs during breast cancer treatment as a promising finding because it provides oncologists and health/fitness professionals multiple options when considering implementing exercise as a supportive-care approach during breast cancer treatment. We also contend this finding provides the opportunity to better personalize the exercise modality to patients’ individual exercise capacity, tolerance, and preference, which could augment the utility of integrating exercise as an approach for managing adverse effects that often accompany active breast cancer treatment.
Although the dearth of studies precludes a comprehensive evaluation of the comparable efficacy of various modes of exercise during different primary breast cancer treatments, there were several particularly relevant benefits accompanying exercise during specific types of primary breast cancer treatment that warrant attention. For example, RE elicited the most pronounced improvements in body composition observed across trials during chemotherapy. Furthermore, the clinically meaningful improvements in select cardiovascular outcomes accompanying AE during both chemotherapy and the combination of radiation and chemotherapy was also notable. It has been well established that chemotherapy is associated with significant decline in cardiovascular function and unfavorable change in body composition among breast cancer patients. The clinically meaningful benefits highlight the potential utility of integrating these modes of exercise as supportive care approaches for breast cancer patients undergoing chemotherapy or the combination of chemotherapy and radiation.
Another particularly relevant finding was the effects of RE and combined aerobic and RE on fatigue observed during the combination of radiation and chemotherapy. Notably, whereas RE resulted in significant improvement in fatigue, an intervention combining aerobic and RE did not yield favorable change in fatigue during the combination treatment. Although this finding may represent an important difference in the effects of exercise on fatigue symptoms of breast cancer patients during combination therapy, the limited number of studies makes such conclusions premature. Nonetheless, given the profound impact of fatigue symptoms on QoL of patients during breast cancer treatment, this finding underscores the critical need to further evaluate the extent to which RE alone, AE alone, and interventions combining these modes of exercise may yield a unique trajectory of change in fatigue during active breast cancer treatment.
The improvements in multiple relevant PROs around yoga are also noteworthy. Yoga was consistently associated with moderate-to-large effect size improvements in patients’ self-reported mood, stress, and QoL. These findings suggest that yoga may be a particularly beneficial supportive-care intervention for managing the distress that is often reported by breast cancer patients during active treatment. In addition, yoga may also represent an alternative form of exercise for many breast cancer patients with limited tolerance or capacity to perform traditional aerobic and/or RE prescriptions.
A prominent observation of this review is the considerable variability in the quality of the interventions. On average, studies met 4 of the 7 Delphi quality indicators (range, 2–6). Based on the percentage of Delphi quality indicators met across the included RCTs, the methodological quality of exercise intervention studies in breast cancer patients undergoing different primary treatments can be classified as moderate. Accordingly, the methodological rigor of future exercise intervention trials targeting breast cancer patients during active treatment should be improved with additional focus on key design considerations such as inclusion of blinded evaluation of key outcomes, concealment of treatment allocation, and intent-to-treat analyses.
Moreover, there was a large degree in variability in the exercise testing and prescription during these trials. For instance, measures of aerobic capacity included VO2max, VO2peak, 6MWT and 12MWT. Consequently, the lack of specificity makes the pooling and aggregation of data difficult. There was a large variability in the exercise prescription with different times, intensities, and overall duration being prescribed. This variation makes it difficult to conclude a dose-response effect of exercise during cancer therapy. Overall, there is a glaring need for standardization of methods of assessment, and exercise prescription to improve clarity on the role of exercise interventions during primary therapy for breast cancer patients.
It should be acknowledged that our approach to quantitatively evaluating the effects of exercise during different treatments using calculation of effect sizes is not as robust as conducting meta-analytic procedures that systematically adjust for bias in the effect size estimate. However, given the limited number of randomized controlled studies evaluating exercise during different breast cancer treatments, we believe the present approach represents an important first step in synthesizing the effects of exercise during multiple breast cancer treatments. As this area of inquiry expands, future reviews incorporating standardized meta-analytic procedures will allow for a more comprehensive assessment of the potential differential effects of exercise during different primary breast cancer treatments.
It has been suggested that for the field of exercise oncology to progress, there is a need for researchers to increase our understanding of how exercise can affect cancer variables (treatment completion rate, tumor biology, disease outcomes, and so on). To date, only 1 study has examined treatment completion rates in breast cancer patients, with an approximate 5% improvement in chemotherapy completion rate among breast cancer patients engaging in resistance training.22 Although these initial results are promising, future research is needed in this area to determine the effect of exercise on treatment response as an outcome to fully elucidate its efficacy.
Delineating the extent to which the various modes of exercise may be of particular benefit during chemotherapy, radiation therapy, or the sequential combination of these treatments, would be of considerable importance in refining exercise prescription approaches during active breast cancer treatment. Consistent with this position, it is critical to develop consistency in the assessment of key clinical, fitness, and PROs across trials in order to advance understanding of best practices in implementing exercise during primary breast cancer treatment. Finally, the extent to which personalizing the exercise prescription to the individual exercise tolerance, capacity, and preferences of patients during breast cancer treatment is also critical in determining the efficacy of implementing exercise as an effective adjuvant supportive care intervention.
In summary, this is one of the first systematic reviews to evaluate the comparable efficacy of exercise as a supportive care intervention during different primary breast cancer treatments. The findings demonstrate that exercise is a safe, feasible intervention that yields significant, clinically meaningful improvements in relevant fitness, physiological, and patient reported outcomes in breast cancer patients undergoing different treatment. Despite these promising findings, the limited number of studies specifically addressing the different modes during each type of treatment limits the ability to adequately assess the comparable efficacy of the various exercise modes during established primary breast cancer treatments. Accordingly, additional research that systematically evaluates the potential unique benefits that various modes of exercise may offer women while undergoing different primary breast cancer treatment is warranted to help guide the oncologists and health/fitness professionals in implementing exercise as a supportive care intervention for breast cancer patients.
Footnotes
Disclosures: The authors report no disclosures or conflicts of interest.
References
- 1.Hsieh CC, Sprod LK, Hydock DS, Carter SD, Hayward R, Schneider CM. Effects of a supervised exercise intervention on recovery from treatment regimens in breast cancer survivors. Oncol Nurs Forum. 2008;35:909–915. doi: 10.1188/08.ONF.909-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 3.Garg A, Leitzel K, Ali S, Lipton A. Antiresorptive therapy in the management of cancer treatment-induced bone loss. Curr Osteoporos Rep. 2015;13:73–77. doi: 10.1007/s11914-014-0252-x. [DOI] [PubMed] [Google Scholar]
- 4.Valachis A, Nilsson C. Cardiac risk in the treatment of breast cancer: assessment and management. Breast Cancer (Dove Med Press) 2015;7:21–35. doi: 10.2147/BCTT.S47227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo AH, Ancukiewicz M, Kozak KR, Yock TI, Padera TP. Cardiac and inflammatory biomarkers do not correlate with volume of heart or lung receiving radiation. Radiat Oncol. 2015;10:5. doi: 10.1186/s13014-014-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong R, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2001;(2):CD002092. doi: 10.1002/14651858.CD002092. [DOI] [PubMed] [Google Scholar]
- 7.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battaglini CL, Mills RC, Phillips BL, et al. Twenty-five years of research on the effects of exercise training in breast cancer survivors: A systematic review of the literature. World J Clin Oncol. 2014;5:177–190. doi: 10.5306/wjco.v5.i2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 10.Meneses-Echávez JF, González-Jiménez E, Correa-Bautista JE, Valle JS-R, Ramírez-Vélez R. [Effectiveness of physical exercise on fatigue in cancer patients during active treatment: a systematic review and meta-analysis] [Article in Spanish] Cad Saude Publica. 2015;31:667–681. doi: 10.1590/0102-311x00114414. [DOI] [PubMed] [Google Scholar]
- 11.Bluethmann SM, Vernon SW, Gabriel KP, Murphy CC, Bartholomew LK. Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. breast cancer research and treatment. 2015;149:331–342. doi: 10.1007/s10549-014-3255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courneya KS. Exercise interventions during cancer treatment: bio-psychosocial outcomes. Exerc Sport Sci Rev. 2001;29:60–64. doi: 10.1097/00003677-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23:3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 16.Hornsby WE, Douglas PS, West MJ, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53:65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y-J, Boehmke M, Wu Y-WB, Dickerson SS, Fisher N. Effects of a 6-week walking program on Taiwanese women newly diagnosed with early-stage breast cancer. Cancer Nurs. 2011;34:E1–E13. doi: 10.1097/NCC.0b013e3181e4588d. [DOI] [PubMed] [Google Scholar]
- 18.Yang C-Y, Tsai J-C, Huang Y-C, Lin C-C. Effects of a home-based walking program on perceived symptom and mood status in postoperative breast cancer women receiving adjuvant chemotherapy. J Adv Nurs. 2011;67:158–168. doi: 10.1111/j.1365-2648.2010.05492.x. [DOI] [PubMed] [Google Scholar]
- 19.Mock V, Pickett M, Ropka ME, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9:119–127. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- 20.Battaglini C, Bottaro M, Dennehy C, et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J. 2007;125:22–28. doi: 10.1590/S1516-31802007000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int J Cancer. 2015;137:471–480. doi: 10.1002/ijc.29383. [DOI] [PubMed] [Google Scholar]
- 22.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 23.Dolan LB, Gelmon K, Courneya KS, et al. Hemoglobin and aerobic fitness changes with supervised exercise training in breast cancer patients receiving chemotherapy. Cancer Epidemiol Biomarkers Prev. 2010;19:2826–2832. doi: 10.1158/1055-9965.EPI-10-0521. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 2007;34:627–633. doi: 10.1188/07.ONF.627-633. [DOI] [PubMed] [Google Scholar]
- 25.Husebø AML, Dyrstad SM, Mjaaland I, Søreide JA, Bru E. Effects of scheduled exercise on cancer-related fatigue in women with early breast cancer. ScientificWorldJournal. 2014;2014:271828–271829. doi: 10.1155/2014/271828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang JH, Chang HJ, Shim YH, et al. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Med J. 2008;49:443–450. doi: 10.3349/ymj.2008.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on Cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009;8:37–46. doi: 10.1177/1534735409331456. [DOI] [PubMed] [Google Scholar]
- 28.Chandwani KD, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32:1058–1065. doi: 10.1200/JCO.2012.48.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psycho oncology. 2009;18:343–352. doi: 10.1002/pon.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim C-J, Kang D-H, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006;29:156–165. doi: 10.1097/00002820-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Mock V, Frangakis C, Davidson NE, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005;14:464–477. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 32.Battaglini C, Bottaro M, Dennehy C, et al. The effects of resistance training on muscular strength and fatigue levels in breast cancer patients. [Accessed April 20,2016];Rev Bras Med Esporte. http://dx.doi.org/10.1590/S1517-86922006000300009 Published May/June 2006.