SUMMARY
During the angiosperm (flowering-plant) life cycle, double fertilization represents the hallmark between diploid and haploid generations [1]. The success of double fertilization largely depends on compatible communication between the male gametophyte (pollen tube) and the maternal tissues of the flower, culminating in precise pollen tube guidance to the female gametophyte (embryo sac) and its rupture to release sperm cells. Several important factors involved in the pollen tube reception have been identified recently [2–6], but the underlying signaling pathways are far from being understood. Here, we report that a group of female-specific small proteins, early nodulin-like proteins (ENODLs, or ENs), are required for pollen tube reception. ENs are featured with a plastocyanin-like (PCNL) domain, an arabinogalactan (AG) glycomodule, and a predicted glycosylphosphatidylinositol (GPI) anchor motif. We show that ENs are asymmetrically distributed at the plasma membrane of the synergid cells and accumulate at the filiform apparatus, where arriving pollen tubes communicate with the embryo sac. EN14 strongly and specifically interacts with the extracellular domain of the receptor-like kinase FERONIA, localized at the synergid cell surface and known to critically control pollen tube reception [6]. Wild-type pollen tubes failed to arrest growth and to rupture after entering the ovules of quintuple loss-of-function EN mutants, indicating a central role of ENs in male-female communication and pollen tube reception. Moreover, overexpression of EN15 by the endogenous promoter caused disturbed pollen tube guidance and reduced fertility. These data suggest that female-derived GPI-anchored ENODLs play an essential role in male-female communication and fertilization.
RESULTS AND DISCUSSION
Isolation of Ovule-Enriched AtENODLs, or ENs
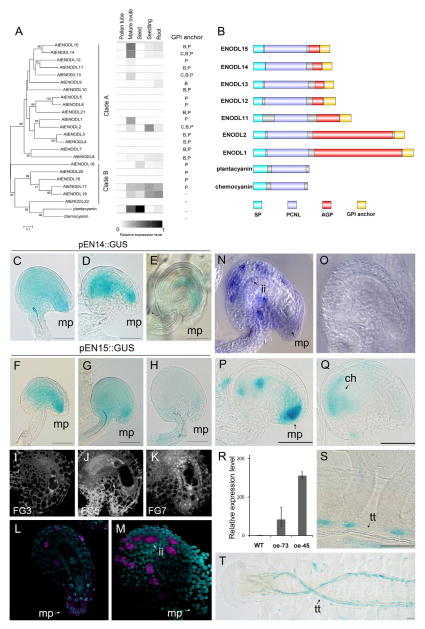
Phytocyanins, a plant-specific subfamily of copper-binding cu-predoxins, are small secretory proteins involved in electron transfer during redox reactions [7]. Previous studies have demonstrated that two members of phytocyanins—plantacyanin and chemocyanin—are female sporophytic signals for directional pollen tube growth in the pistils of Arabidopsis and lily, respectively [8, 9]. Early nodulin (ENOD) is a subclass of phytocyanins that are expressed very early in developing root nodules of legumes and play crucial roles in cell division and dedifferentiation [10]. A number of proteins have sequence similarity to ENOD, including the ENOD-like protein (ENODL, or EN) family, consisting of 22 members in Arabidopsis, 24 members in rice, and 52 members in Chinese cabbage [11]. Many ENODLs were expressed in reproductive organs, suggesting their possible functions in the reproduction process [11]. However, thus far, no specific function has been addressed to any of the ENODLs, likely due to genetic redundancy. According to our RNA-sequencing (RNA-seq)-based transcriptional profiling analysis [12], Clade A genes of the Arabidopsis thaliana ENODLs (AtENODLs, or ENs) showed strong expression in mature ovules but not in pollen tubes (Figure 1A). EN15 and EN14 were most abundantly expressed in mature ovules, while EN11, EN12, and EN13 displayed lowered expression levels [12] (Figure 1A).
Figure 1. Phylogeny, Transcription Profile, and Structure of A. thaliana ENODL Family Proteins and the Detailed Expression Analysis of EN14 and EN15.
(A) Phylogenetic analysis and the transcription profile of all 22 A. thaliana ENODL (EN) family members. Related plantacyanin and lily chemocyanin were used as outgroups in the phylogram. Pollen tube: semi-in-vivo germinated pollen tubes. Lowest expression level is indicated in white, and highest expression level is indicated in black. GPI anchor signals were confirmed by Borner et al. [13] (C) or were predicted by big-PI predictor (B) and PredGPI (P) programs.
(B) Protein structure of selected ENODL proteins of Clade A expressed in mature ovules. Abbreviations: SP, signal peptide; PCNL, plastocyanin-like; AGP, arabinogalactan protein domain; GPI, glycophosphatidylinositol.
(C–K) Dynamic changes of transcription patterns of EN14/EN15 during fertilization. EN14 (C–E) and EN15 (F–H) promoter activity of the GUS reporter before and after fertilization is shown. Scale bars, 50 μm. (Corresponding ovule development stages are shown in (I)–(K). (I) shows an ovule at stage FG3, as in (C) and (F). (J) shows an ovule at stage FG5, as in (D). An ovule at stage FG7 is shown in (G). (K) shows a fertilized ovule (16 HAP) comparable to the stages in (E) and (H). mp, micropyle. Scale bars, 10 μm.
(L and M) F-WISH of EN15 using an antisense probe against its 3′ UTR. Ovules are at stage FG5 (L) and FG7 (M). Nuclei are stained with DAPI. mp, micropyle; ii, inner integument.
(N and O) WISH of EN14 using an antisense probe against its coding region (N). A sense probe is used as negative control (O).
(P and Q) Activity of the GUS reporter in EN15tcsoe trans-activation lines. An early stage of ovule development (P) and a mature ovule (Q) are shown. mp, micropyle; ch, chalaza. Scale bars, 50 μm.
(R) Relative overexpression levels of EN15 in ovules analyzed by qRT-PCR. The error bars represent the SD of three independent biological replicates of qRT-PCR experiments.
(S and T) Activity of the GUS reporter in EN15tcsoe trans-activation lines. Signals at the junction of transmitting tissue and funiculus (S) and an overview showing GUS signals in a mature pistil (T) are shown. The strongest signals are detected at the transmitting tract (tt), pollen tube exit region of the placenta and funiculus. Scale bars, 50 μm. See also Figures S1 and S2.
A glycosylphosphatidylinositol (GPI) anchor motif was predicted at the C-termini of EN11–EN15 (Figures 1A, 1B, and S1B) [13]. With the composition of a GPI anchor, a signal peptide, and an arabinogalactan (AG) glycomodule, EN11–EN15 are similar to “classical” AG proteins (AGPs) [14], which play multiple roles in plant reproductive processes [15, 16]. EN11–EN15 contain an additional plastocyanin-like (PCNL) domain present in sporophytic signaling molecules (discussed earlier). The GPI anchor likely localizes the modified protein at the plasma membrane [15]. After cleavage by phospholipase C, the anchor-free AGPs can be detached from the plasma membrane and released into the extracellular matrix [17, 18]. Both membrane-anchored ENDOLs and the soluble forms would allow them to mediate cell-cell communication between ovules and pollen tubes.
Interestingly, EN11–EN15 appear in a separate clade exclusively composed of angiosperm species, distinct from other ENODL genes in gymnosperms, ferns (Selaginella moellendorffii), bryophytes (Physcomitrella patens), and green algae (Figure S1A). This indicates that functions of EN11–EN15 might be related to angiosperm-specific processes, e.g., double fertilization, which evolved from lower plants and is lost in the gymnosperm lineage.
ENs Are Expressed in the Ovules and the Funiculus
To elucidate their detailed expression profile in Arabidopsis flowers, we analyzed the promoter activity of EN14 and EN15 (strongest expression based on RNA-seq data) by performing a β-glucuronidase (GUS) staining assay using pEN::GUS transgenic lines. The two promoters showed a largely overlapping expression pattern in inflorescences, and the highest GUS activity was found in mature pistils (Figures S2A and S2B). In ovules of stages FG3 to FG7 (FG, female gametophyte) (Figures 1I and 1K), the highest GUS activity was detected at the micropylar region and inner integument surrounding the female gametophyte (Figures 1C–1E for EN14; Figures 1F–1H for EN15). Whole-mount in situ hybridization (WISH) and fluorescent WISH (F-WISH) revealed the strongest expression in individual cells of the inner integument and weak expression at the micropylar region at stage FG7 (Figures 1L–1O). At 16 hr after pollination (HAP), about 7–10 hr after fertilization [19], GUS activity of the EN15 promoter decreased dramatically in the ovules (Figure 1H). Egg apparatus expression of EN11, EN12, and EN13 was previously reported [12, 20]. We also found that EN12, as a representative, was transcribed mainly in the micropylar region of the embryo sac (Figures S2F–S2H). These expression properties provide strong correlation of EN expression with the fertilization process.
To further investigate the expression patterns of EN genes, we transferred the EN15 native promoter into an LhG4/pOp trans-activation system [21] (Figure S3A), in which the GUS reporter gene was activated synchronously with EN15, providing the convenience of visualizing the transcriptional pattern and activity of EN15 in planta. At later developmental stages, we observed an expression level shift of EN15 from the micropyle toward the chalazal end (Figures 1P and 1Q), which nicely correlates with our WISH and F-WISH data. The qRT-PCR results confirmed that the expression levels of EN15 were dramatically elevated in two transgenic lines: EN15tcsoe-73 and EN15tcsoe-45 (Figure 1R; Figures S3B–S3E). Additionally, we were able to observe GUS activity in the funiculus and at the junctions between the transmitting tissue and the funiculus (Figures 1S and 1T). The turning point of the funiculus along the transmitting tissue is a landmark where pollen tubes are guided to grow toward the ovules. The presence of EN15 at the funiculus junction suggests a potential role in pollen tube guidance.
EN Proteins Appear at the Surface of the Placenta and at the Micropylar Surface of the Synergid Cells
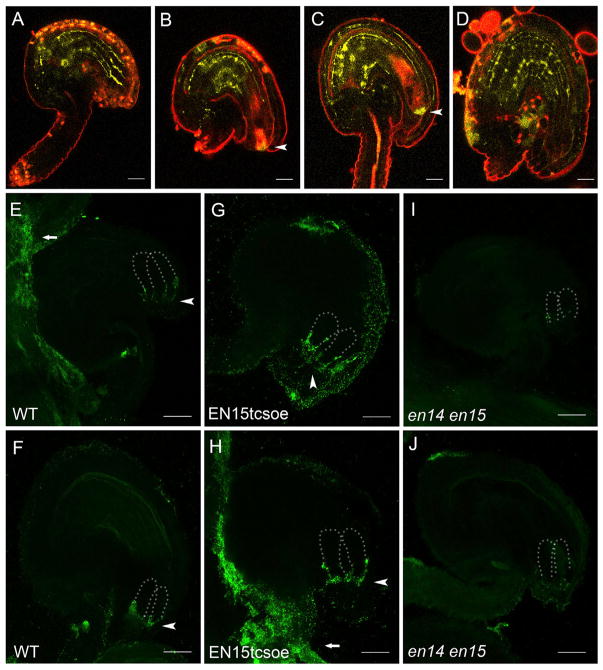
The localization of EN14 and EN15 proteins (EN14/EN15) was recently reported in the stomatal lineage cells [22]. We examined their YFP (yellow fluorescent protein)-fusion-protein expression in reproductive organs and found that EN15 localization was preferentially associated with cell walls (Figures 2A–2D). Notably, a strong signal was observed in the micropylar surface of the synergid cells (Figures 2B and 2C), i.e., the filiform apparatus, a highly thickened structure in the synergid cell wall closely associated with pollentubereception [23, 24]. The micropylar-enriched localization of EN15 suggested its potential function in fertilization, particularly in mediating male-female communication in the ovules.
Figure 2. Protein Localization of EN14/EN15 in WT and EN15tcsoe Ovules.
(A–D) Localization of EN15-YFP fusion protein at different developmental stages of WT ovules. FG3 (A), FG5 (B), FG7 (C), and a fertilized ovule (D) are shown. Arrowheads indicate signals detected at the filiform apparatus. More than 30 out of 50 ovules at each stage with similar expression patterns were observed. Scale bars, 20 μm.
(E and F) Immunofluorescence assay of EN14/EN15 (using an EN14/EN15-specific antibody) in WT ovules. In FG4 ovules, signals were detected at the surface of the placenta (arrow) and accumulate at flank region of synergid cells (arrowhead) (E). In mature ovules, signals were detected at the filiform apparatus (arrowhead) (F). Dotted lines define synergid cells. Out of 14 well-stained ovules, at least ten ovules with the same or similar patterns were observed. Scale bars, 20 μm.
(G and H) Immunofluorescence assay of EN14/EN15 in FG5 EN15tcsoe ovules. In FG4 ovules, signals were detected at the flank region of synergid cells (arrowhead) (G). In FG5 ovules, signals were detected at the filiform apparatus (arrowhead) and surface of the placenta (arrow) (H). Out of 20 well-stained ovules, at least 14 ovules with the same or similar patterns were observed. Scale bars, 20 μm.
(I and J) Immunofluorescence of EN15 in en14 en15 ovules. Signals were almost undetectable in mutant ovules of FG3 (I) and FG5 (J). Out of 15 well-stained ovules, at least 11 ovules with the same or similar patterns were observed. Scale bars, 20 μm. See also Figures S2 and S3 and Table S2.
To validate the protein localization of mature EN14/EN15 in the filiform apparatus at the cellular level, we generated an antibody against specific regions in mature EN14/EN15 (Figure S2I). By using this antibody for immunofluorescence assays, we found that, in more than 70% of the immature ovules, EN14/EN15 exhibited a strikingly polarized localization pattern and accumulated at the micropylar surface of the synergid cells (Figures 2E and 2F). In mature ovules, EN14/EN15 signals were found enriched in the filiform apparatus (Figures 2E and 2F). In the EN15 overexpression lines, the immunofluorescence assay detected enhanced signals and, thus, confirmed protein enrichment both at the flank region of the synergid cells and at the filiform apparatus (Figures 2G and 2H). Some signals appeared at the integuments in the micropylar region, recapitulating what we have observed from the trans-activation and in situ hybridization analyses (Figure 1). EN14/EN15 immunofluorescence signals were almost absent in en14 en15 mutant ovules (Figures 2I and 2J). The expression pattern change from the integuments and flanking region of the synergid cells to the filiform apparatus suggests that the EN15 protein might have had its GPI anchor removed and then released from its original location and diffused toward the filiform apparatus to implement its function. Considering that EN11 and EN12 were also reported to be expressed in the egg apparatus [20], it is very likely that multiple ENs might be involved in the filiform apparatus function and pollen tube-ovule communication.
Loss-of-Function Mutations in ENs Cause Failures of Pollen Tube Burst
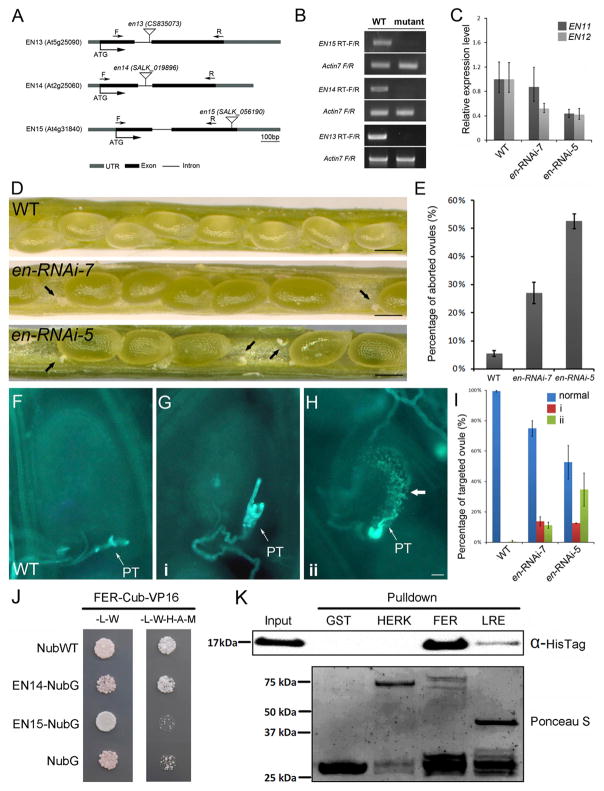
To study the function of polarly localized ENs, we made considerable effort to obtain loss-of-function mutants. However, although en13, en14, and en15 were transfer DNA (T-DNA) insertional null mutations (Figure 3A and 3B), we did not observe any obvious phenotype in single, double, and triple mutants. As EN11 and EN12 clustered together with EN13, EN14, and EN15 (Figure S1A), and they also showed detectable expression in ovules, we further constructed higher order mutants by introducing an RNAi silencing construct for EN11/EN12 into the triple mutant en13 en14 en15. Finally, we created an en-RNAi mutant that contains the loss of function of EN13 EN14 EN15 and lowered expression of EN11 and EN12, respectively (Figure 3C). In these mutant plants, we observed a remarkably reduced seed set due to aborted ovules (52% and 27% in en-RNAi-5 and en-RNAi-7, respectively) (Figures 3D and 3E). Aniline blue staining showed that pollen tubes in these ovules failed to rupture and release their sperm cell cargo (Figures 3F–3H). Two phenotypes might cause these failures: (1) pollen tube overgrowth in the embryo sac, a behavior similar to feronia and lre mutant ovules [4, 6, 25], where pollen tubes kept growing and failed to discharge in the ovule (Figure 3G); and (2) abnormal callose accumulation in the embryo sac (Figure 3H), which likely resulted as a defense reaction to pollen tube overgrowth or structural collapse after fertilization failure.
Figure 3. Pollen Tube Rupture Phenotype in en-RNAi Mutants and Interaction between ENODLs and FERONIA.
(A) Diagram showing T-DNA insertion sites in en13, en14, and en15 mutants.
(B) Semi-qRT-PCR analysis to show the expression levels of EN13, EN14, and EN15 in the corresponding SALK T-DNA mutant lines.
(C) Expression levels of EN11 and EN12 in en-RNAi mutants detected by qRT-PCR assays. The error bars represent the SD of three independent biological replicates of qRT-PCR experiments.
(D) Ovule abortion in en-RNAi siliques. Arrowheads indicate aborted ovules. Scale bars, 200 μm.
(E) Percentage of aborted ovules in two independent quintuple mutant lines compared with the wild-type (WT). Error bars are mean ± SD of three biological replicates; more than 50 siliques from each line were examined.
(F) Fertilized WT ovules. Aniline blue stains a single pollen tube (PT).
(G) Pollen tube failed to rupture and showed overgrowth in an en-RNAi mutant ovule (phenotype 1; i).
(H) Pollen tube failed to rupture and showed callose deposition in ovules of an en-RNAi mutant line (phenotype 2; ii). Arrow, callose deposition. Scale bar, 20 μm.
(I) Histogram to show quantification of both phenotypes. N > 120. Error bars are mean ± SD of three biological replicates; more than 50 siliques from each line were examined.
(J) ENODL14 strongly interacts with FERONIA (FER) in a split-ubiquitin yeast-two-hybrid assay. Cub-VP16, the C-terminal half of an ubiquitin fused with the transcription factor (LexA-VP16) to activate the reporter genes; NubWT, the N-terminal half of a wild-type ubiquitin; NubG, the N-terminal half of a mutated ubiquitin as a negative control; -L, medium without Leu; W, medium without Trp; H, medium without His; A, medium without Ade; M, medium without Met.
(K) ENODL14 strongly and specifically interacts with FER in a pull-down assay. ENODL14-HisTag (top) is pulled with GST-tagged extracellular domains (ECDs) of CrRLK-like receptor kinase FER, but not with its family member HERKULES (HERK). GST-LRE is weakly pulled down with ENODL14-His. The bottom panel shows GST pull-downs with Ponceau S staining.
See also Figure S4.
The receptor-like kinase FERONIA (FER) and the GPI-anchored protein (GPI-AP, GAP) LORELEI (LRE) were reported to be involved in pollen tube reception [4, 6]. Recently, both LRE and its homolog LLG1 were found to interact with the exJM domain of FER and to regulate the function of FER [26]. Because loss of EN function resulted in similar phenotypes of fer and lre, we speculated that ENs might work in the same signaling pathway. To test this hypothesis, we performed a split-ubiquitin yeast two-hybrid assay using the extracellular domain (ECD) of FER as bait and EN14/EN15 as prey [27]. We found that EN14 strongly interacted with the ECD of FER in yeast cells (Figure 3J). We further conducted in vitro pull-down assays to confirm the interaction and found that His-tagged EN14 (EN14-HisTag) was fully pulled down by glutathione S-transferase (GST)-tagged FER but only weakly pulled down by GST-tagged LRE (Figure 3K). GST-tagged HERKULES (HERK), a closely related sister protein of FER, did not interact with EN14-HisTag (Figure 3K). These results support the hypothesis that the interaction between EN14 and the ECD of FER is strong and highly specific. We failed to detect the interaction between EN15 and the ECD of FER in in vitro pull-down assays, possibly due to differences between EN14 and EN15 in post-translational modifications in different organisms. It is worth noting that ENs harbor ECDs that are unrelated to those from LLG1 and LRE [4, 28]. Therefore, ovule-specific ENs may act as adaptors or tethering factors, capturing their specific interaction partner(s), like FER, via their glyco- and PCNL modules at the interface between the filiform apparatus and synergid cell surface for polar activation of the first steps in the FER signaling pathway [15].
ENODLs Are Important for Pollen Tube Entrance into the Ovule, Growth Arrest, and Burst
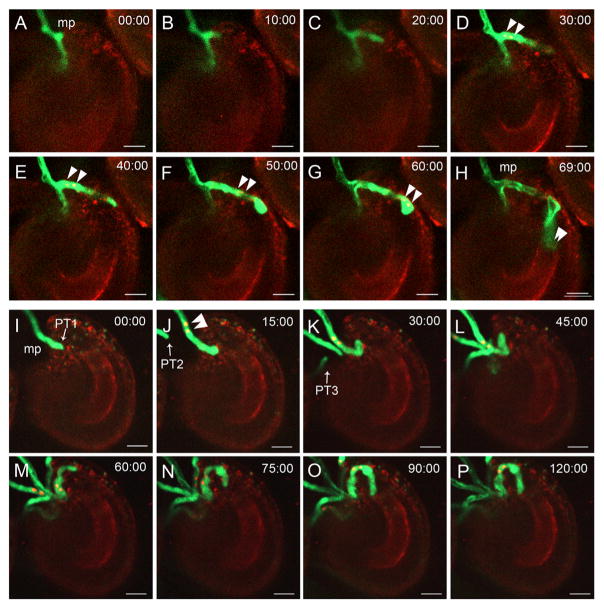
To test whether the ovules of en-RNAi quintuple mutants are recognizable by normal pollen tubes, we performed a semi-in-vivo-fertilization assay [29] using the double marker line pLat52::GFP pHTR10::RFP as a pollen donor (labeling pollen tubes in green and both sperm cells in red). In wild-type (WT) ovules, 92.5% pollen tubes ruptured and released two sperm cells within about 70 min after entering the micropyle (Figures 4A–4H; Figure S4). However, a number of pollen tubes in en-RNAi mutant ovules were attracted but failed to release sperm cells within 120 min (Figures 4I–4P and S4). Moreover, we found that multiple pollen tubes gathered simultaneously at the micropylar opening of the ovule (Figures 4I–4P), which was also observed in vivo (Table S1). However, it is more likely that the observation of polytubey in en-RNAi mutant ovules was a side effect due to fertilization failure. In several ovules with overgrown pollen tubes (phenotype 1 described earlier), a second pollen tube was attracted by the same ovule. This phenomenon was also found in feronia [30] and other fertilization deficiency mutants such as duo3, hap2, ec1, and gex2 [19, 30–33] and represents a fertilization recovery mechanism to enforce successful fertilization when the first pollen tube failed to fertilize [31, 32]. Altogether, based on the phenotype of en-RNAi plants, we hypothesize that the ovule-generated ENs represent mobile extracellular components essential in mediating female-male communication to induce pollen tube reception and burst in Arabidopsis thaliana.
Figure 4. Confocal Live-Cell Imaging of the Fertilization Process in WT and en-RNAi Ovules.
(A–H) Imaging of the fertilization process of wild-type ovules (recording for 69 min).
(I–P) Imaging of the fertilization process of a representative en-RNAi ovule (recording for 120 min).
Arrowheads point toward sperm cells, and arrows point at pollen tubes (PTs). mp, micropyle. Scale bars, 10 μm. See also Table S1.
Notably, EN15tcsoe plants also produced a severely reduced seed set (Figures S3F and S3G) with significantly aborted ovules (Table S2), which emphasized the critical function of ENs in female reproductive organs. Compromised pollen tube guidance was observed in EN15tcsoe ovules (Figures S3S–S3W), and pollen tubes frequently failed to grow onto the funiculus to approach EN15tcsoe ovules (Figures S3H–S3L). Meanwhile, the higher ratio of unfertilized ovules, although normally targeted by pollen tubes, suggested that EN15tcsoe ovules are not fully mature. Among the untargeted EN15tcsoe ovules, we often observed obvious callose accumulation (Figure S3J). In some EN15tcsoe ovules, only one nucleus could be observed in the embryo sac (Figures S3M–S3R). These observations suggest that over-accumulation of EN15 in some ovules interferes with mitosis in female gametophyte development and, as a consequence, with pollen tube guidance.
GAPs are secreted to the space between the plasma membrane and cell walls, which makes them ideal candidates in mediating cell-cell communication [17, 18]. ENODLs are a subset of GPI-anchored secretory proteins that are enriched in the ovule [12], localized to the filiform apparatus [23, 24], and crucial for pollen tube reception. A similar localization was detected with an anti-AGP antibody and was hypothesized to correlate with ovule receptivity [16, 34]. Moreover, a methylated disaccharide 4Me-GlcA-Gal exposed on the side chains of ovular AGPs was recently reported to be essential for pollen tube competency to respond to female signals [35]. Whether ENs, a group of AGP-harboring AG glycomodules, also serve as a source for this competence signal remains to be investigated.
Disturbed expression of ENs, by loss of function or overexpression, led to partial failures in pollen tube reception and double fertilization. In addition, ENs directly interact with FER, the core signal receptor in ovules for pollen tube reception [6]. Thus, we propose that ENs, accumulating in the filiform apparatus from early stage to maturation of the female gametophyte, are used as female cues to facilitate polar anchoring of FER on the filiform apparatus and to mediate the crosstalk between male and female reproductive cells. Since EN14 interacts with the ECD of FER, and LLG1 binds to the juxtamembrane region of FER [26], it is very likely that these proteins form a large complex and cooperate with each other during FER-mediated signaling.
EN proteins appear as multifunctional proteins and may participate in male-female interaction on multiple levels, due to their multiple domains and expression patterns. EN15 preferentially associates with newly formed cell walls [22], raising the possibility that ENs are also involved in cell division of female gametophytes before fertilization. This is consistent with the observation that mitosis of megaspores was affected in some EN15 overexpressor lines. EN proteins also share a PCNL domain. Overexpression of PCN peptides was previously shown to disturb the penetration of pollen tubes into the stigma [9]. Therefore, the PCNL domains of ENs might also be involved in the interactions between pollen tubes and transmitting tissue/funiculus, e.g., during septum penetration and/or polytubey prevention.
GAPs, like ENs, also play key roles in gamete recognition in mammalians [36–38]. Although plants and animals belong to two kingdoms, it is interesting to note that both utilize GAPs for signaling during fertilization, such as COBL10 in pollen tubes, ENs in ovules, and LORELEI in the synergid cells of Arabidopsis [4, 25, 26, 39], while mammals express Juno at the surface of egg cells as a major sperm receptor [38]. Future work is now required to elucidate the detailed molecular interaction between mature ENs, FER, and LRE at the synergid cell surface in order to determine the structure and composition of the FER receptor complex, as well as its polar accumulation and activation.
Highlights.
ENODL11–15 are GPI-anchored proteins, each with a plastocyanin-like domain
ENODL11–15 are predominantly expressed in the ovules and the funiculus
Loss of function or overexpression of ENODLs compromises pollen tube reception
ENODL14 has physical interaction with the extracellular domain of FERONIA
Acknowledgments
We thank Jingjing Liu (Peking University) for technical support and for help with experimental design. We are grateful to Mark Johnson (Brown University) for providing the marker line pLat52::GFP pHTR10::RFP, Ian Moore (Oxford University) for providing the LhG4/pOp trans-activation system, and Dominique Bergmann (Stanford University) for the EN14/15 marker lines. This work was supported by the Natural Science Foundation of China (31230006 and 31370344 to L.-J.Q.), the National Basic Research Program of China (2012CB944801) and the German Research Council (DFG Collaborative Research Center SFB924 to T.D.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, four figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2016.06.053.
AUTHOR CONTRIBUTIONS
L.-J.Q., T.D., J.D., and H.G. conceived and coordinated the study, with input from Y.H. and X.G. Y.H., X.G., L.C., and Y.L. conducted the genetic analyses, phenotypic observations, and yeast two-hybrid assays. Q.H. conducted bioinformatics analysis of the genes. P.C. and A.B. conducted in situ hybridization and pull-down assays. Y.Z. observed the protein localization. L.-J.Q., T.D., and J.D. wrote the paper, with input from other authors.
References
- 1.Yadegari R, Drews GN. Female gametophyte development. Plant Cell. 2004;16(Suppl):S133–S141. doi: 10.1105/tpc.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dresselhaus T, Franklin-Tong N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant. 2013;6:1018–1036. doi: 10.1093/mp/sst061. [DOI] [PubMed] [Google Scholar]
- 3.Qu LJ, Li L, Lan Z, Dresselhaus T. Peptide signalling during the pollen tube journey and double fertilization. J Exp Bot. 2015;66:5139–5150. doi: 10.1093/jxb/erv275. [DOI] [PubMed] [Google Scholar]
- 4.Capron A, Gourgues M, Neiva LS, Faure JE, Berger F, Pagnussat G, Krishnan A, Alvarez-Mejia C, Vielle-Calzada JP, Lee YR, et al. Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell. 2008;20:3038–3049. doi: 10.1105/tpc.108.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleckmann A, Alter S, Dresselhaus T. The beginning of a seed: regulatory mechanisms of double fertilization. Front Plant Sci. 2014;5:452. doi: 10.3389/fpls.2014.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 7.Rydén LG, Hunt LT. Evolution of protein complexity: the blue copper-containing oxidases and related proteins. J Mol Evol. 1993;36:41–66. doi: 10.1007/BF02407305. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA. 2003;100:16125–16130. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong J, Kim ST, Lord EM. Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol. 2005;138:778–789. doi: 10.1104/pp.105.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varkonyi-Gasic E, White DW. The white clover enod40 gene family. Expression patterns of two types of genes indicate a role in vascular function. Plant Physiol. 2002;129:1107–1118. doi: 10.1104/pp.010916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mashiguchi K, Asami T, Suzuki Y. Genome-wide identification, structure and expression studies, and mutant collection of 22 early nodulin-like protein genes in Arabidopsis. Biosci Biotechnol Biochem. 2009;73:2452–2459. doi: 10.1271/bbb.90407. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q, Dresselhaus T, Gu H, Qu LJ. Active role of small peptides in Arabidopsis reproduction: Expression evidence. J Integr Plant Biol. 2015;57:518–521. doi: 10.1111/jipb.12356. [DOI] [PubMed] [Google Scholar]
- 13.Borner GH, Lilley KS, Stevens TJ, Dupree P. Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiol. 2003;132:568–577. doi: 10.1104/pp.103.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Showalter AM, Keppler B, Lichtenberg J, Gu D, Welch LR. A bioinformatics approach to the identification, classification, and analysis of hydroxyproline-rich glycoproteins. Plant Physiol. 2010;153:485–513. doi: 10.1104/pp.110.156554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seifert GJ, Roberts K. The biology of arabinogalactan proteins. Annu Rev Plant Biol. 2007;58:137–161. doi: 10.1146/annurev.arplant.58.032806.103801. [DOI] [PubMed] [Google Scholar]
- 16.Pereira AM, Pereira LG, Coimbra S. Arabinogalactan proteins: rising attention from plant biologists. Plant Reprod. 2015;28:1–15. doi: 10.1007/s00497-015-0254-6. [DOI] [PubMed] [Google Scholar]
- 17.Sharom FJ, Lehto MT. Glycosylphosphatidylinositol-anchored proteins: structure, function, and cleavage by phosphatidylinositol-specific phospholipase C. Biochem Cell Biol. 2002;80:535–549. doi: 10.1139/o02-146. [DOI] [PubMed] [Google Scholar]
- 18.Mayor S. ACEing GPI release. Nat Struct Mol Biol. 2005;12:107–108. doi: 10.1038/nsmb0205-107. [DOI] [PubMed] [Google Scholar]
- 19.Sprunck S, Rademacher S, Vogler F, Gheyselinck J, Grossniklaus U, Dresselhaus T. Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science. 2012;338:1093–1097. doi: 10.1126/science.1223944. [DOI] [PubMed] [Google Scholar]
- 20.Wuest SE, Vijverberg K, Schmidt A, Weiss M, Gheyselinck J, Lohr M, Wellmer F, Rahnenführer J, von Mering C, Grossniklaus U. Arabidopsis female gametophyte gene expression map reveals similarities between plant and animal gametes. Curr Biol. 2010;20:506–512. doi: 10.1016/j.cub.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Craft J, Samalova M, Baroux C, Townley H, Martinez A, Jepson I, Tsiantis M, Moore I. New pOp/LhG4 vectors for stringent glucocorticoid-dependent transgene expression in Arabidopsis. Plant J. 2005;41:899–918. doi: 10.1111/j.1365-313X.2005.02342.x. [DOI] [PubMed] [Google Scholar]
- 22.Adrian J, Chang J, Ballenger CE, Bargmann BOR, Alassimone J, Davies KA, Lau OS, Matos JL, Hachez C, Lanctot A, et al. Transcriptome dynamics of the stomatal lineage: birth, amplification, and termination of a self-renewing population. Dev Cell. 2015;33:107–118. doi: 10.1016/j.devcel.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 24.Márton ML, Fastner A, Uebler S, Dresselhaus T. Overcoming hybridization barriers by the secretion of the maize pollen tube attractant ZmEA1 from Arabidopsis ovules. Curr Biol. 2012;22:1194–1198. doi: 10.1016/j.cub.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto T, Qin Y, Huang Y, Dunatunga D, Palanivelu R. A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J. 2010;62:571–588. doi: 10.1111/j.1365-313X.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Yeh FL, Cheung AY, Duan Q, Kita D, Liu MC, Maman J, Luu EJ, Wu BW, Gates L, et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife. 2015;4:e06587. doi: 10.7554/eLife.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stagljar I, Korostensky C, Johnsson N, te Heesen S. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc Natl Acad Sci USA. 1998;95:5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Premkumar DR, Fukuoka Y, Sevlever D, Brunschwig E, Rosenberry TL, Tykocinski ML, Medof ME. Properties of exogenously added GPI-anchored proteins following their incorporation into cells. J Cell Biochem. 2001;82:234–245. doi: 10.1002/jcb.1154. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Zhong S, Guo X, Hao L, Wei X, Huang Q, Hou Y, Shi J, Wang C, Gu H, Qu LJ. Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol. 2013;23:993–998. doi: 10.1016/j.cub.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- 31.Kasahara RD, Maruyama D, Hamamura Y, Sakakibara T, Twell D, Higashiyama T. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr Biol. 2012;22:1084–1089. doi: 10.1016/j.cub.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 32.Beale KM, Leydon AR, Johnson MA. Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr Biol. 2012;22:1090–1094. doi: 10.1016/j.cub.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori T, Igawa T, Tamiya G, Miyagishima SY, Berger F. Gamete attachment requires GEX2 for successful fertilization in Arabidopsis. Curr Biol. 2014;24:170–175. doi: 10.1016/j.cub.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Coimbra S, Almeida J, Junqueira V, Costa ML, Pereira LG. Arabinogalactan proteins as molecular markers in Arabidopsis thaliana sexual reproduction. J Exp Bot. 2007;58:4027–4035. doi: 10.1093/jxb/erm259. [DOI] [PubMed] [Google Scholar]
- 35.Mizukami AG, Inatsugi R, Jiao J, Kotake T, Kuwata K, Ootani K, Okuda S, Sankaranarayanan S, Sato Y, Maruyama D, et al. The AMOR arabinogalactan sugar chain induces pollen-tube competency to respond to ovular guidance. Curr Biol. 2016;26:1091–1097. doi: 10.1016/j.cub.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 36.Alfieri JA, Martin AD, Takeda J, Kondoh G, Myles DG, Primakoff P. Infertility in female mice with an oocyte-specific knockout of GPI-anchored proteins. J Cell Sci. 2003;116:2149–2155. doi: 10.1242/jcs.00430. [DOI] [PubMed] [Google Scholar]
- 37.Kondoh G, Tojo H, Nakatani Y, Komazawa N, Murata C, Yamagata K, Maeda Y, Kinoshita T, Okabe M, Taguchi R, Takeda J. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat Med. 2005;11:160–166. doi: 10.1038/nm1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, Ge FR, Xu M, Zhao XY, Huang GQ, Zhou LZ, Wang JG, Kombrink A, McCormick S, Zhang XS, Zhang Y. Arabidopsis COBRA-LIKE 10, a GPI-anchored protein, mediates directional growth of pollen tubes. Plant J. 2013;74:486–497. doi: 10.1111/tpj.12139. [DOI] [PubMed] [Google Scholar]