Abstract
Asymmetric cell division (ACD) is universally required for the development of multicellular organisms. Unlike animal cells, plant cells have a rigid cellulosic extracellular matrix, the cell wall, which provides physical support and forms communication routes. This fundamental difference leads to some unique mechanisms in plants for generating asymmetries during cell division. However, plants also utilize intrinsically polarized proteins to regulate asymmetric signaling and cell division, a strategy similar to the differentiation mechanism found in animals. Current progress suggests that common regulatory modes, i.e. protein spontaneous clustering and cytoskeleton reorganization, underlie protein polarization in both animal and plant cells. Despite these commonalities, it is important to note that intrinsic mechanisms in plants are heavily influenced by extrinsic cues. To control physical asymmetry in cell division, although our understanding is fragmentary thus far, plants might have evolved novel polarization strategies to orientate cell division plane. Recent studies also suggest that the phytohormone auxin, one of the most pivotal small molecules in plant development, regulates ACD in plants.
Keywords: Protein polarization, Cell polarity, Asymmetric cell division, Auxin signaling, Differential cell identity, Cell division orientation, Plant development
1. Introduction
Asymmetric cell divisions (ACDs) are imperative for multicellular organisms to generate diversified cell types, while maintaining self-renewal stem cell pools (Abrash and Bergmann, 2009; Knoblich, 2008). Both intrinsic and extrinsic factors cause asymmetries during cell division. Intrinsic mechanisms refer to those of which cell fate differentiation occurs prior to cytokinesis of the parental cell (Goldstein and Macara, 2007; Knoblich, 2008). Extrinsic factors drive differentiation outcome by asymmetric placement of the daughter cells into two distinct microenvironments (Fuller and Spradling, 2007). While both processes are important in complex organisms, intrinsic or extrinsic may have more weight over the other under certain conditions.
“Cell polarization”, or “symmetry breaking”, describes spontaneous assembly of cell cortical membrane domains. During this process proteins, mRNAs, organelles, cytoskeletal components among other molecules become distributed unevenly. Polarization occurs in essentially all cellular organisms and is required for fundamental processes in morphogenesis, cell division as well as cell differentiation (Freisinger et al., 2013; Yang and Lavagi, 2012). Cell polarization is a typical intrinsic regulatory measure that is required early in an ACD and is significant for subsequent check-points, e.g. mitotic spindle alignment, division plane positioning and daughter cell fate specification. The molecular mechanisms underpinning stem cell ACD have been described in model systems, such as the worm C. elegans embryos, the fruit fly Drosophila melanogaster nervous systems, and the budding yeast Saccharomyces cerevisiae (Inaba and Yamashita, 2012). In higher plants, the key roles of cell polarization in stem cell ACD are manifested by asymmetrically distributed proteins and signaling pathways. Contrary to the dominant roles of animal polarity proteins being almost entirely intrinsic cues, plant polarity proteins seem to participate in both intrinsic and extrinsic pathways to regulate divisional asymmetries in development. In addition, the phytoshormone auxin is recognized as an important regulator of ACD in multiple developmental contexts (Balcerowicz et al., 2014; Le et al., 2014; Zhang et al., 2014). This review mainly focuses on the current understanding of how ACD participating proteins and related signaling pathways are asymmetrically distributed in plant cells and how auxin activity in addition to these pathways may regulate physical asymmetry and differential identity in cell division. Other mechanisms underlying cell fate asymmetry in plant ACD, but not necessarily associated with cell physical asymmetry, have been discussed previously (Abrash and Bergmann, 2009; Fisher and Sozzani, 2016; Petricka et al., 2009; Pillitteri et al., 2016; Ten Hove and Heidstra, 2008; Wu and Gallagher, 2011).
2. Symmetry breaking at the cortical membrane
Hallmark polarity proteins in animals, e.g. the Cdc42 small GTPase (Chant, 1999; Slaughter et al., 2009) and PAR proteins (Goldstein and Macara, 2007; Nance and Zallen, 2011), have been investigated intensively for the past decades. However, many of these highly conserved proteins found in animals are missing from the plant genome. The discoveries of polarized proteins in higher plants, e.g. the PANGLOSS (PAN) receptor-like proteins and the plant-specific, novel protein BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL) that participate in the regulation of plant ACD, have not been made until a few years ago (Cartwright et al., 2009; Dong et al., 2009). But excitingly, recent progress disclosed new genetic and physical partners in the PAN-mediated pathway (Humphries et al., 2011; Zhang et al., 2012; Facette et al., 2015) and BASL-centered polarity system (Pillitteri et al., 2011; Zhang et al., 2015). Current data suggest that, despite these polarity proteins being plant-specific, common regulatory themes, such as positive feedback loops, cytoskeletal reorganization and spatially organized cell signaling, underpin the cell polarity-driven ACD in both animals and plants.
2.1. Protein polarization: positive feedback loops
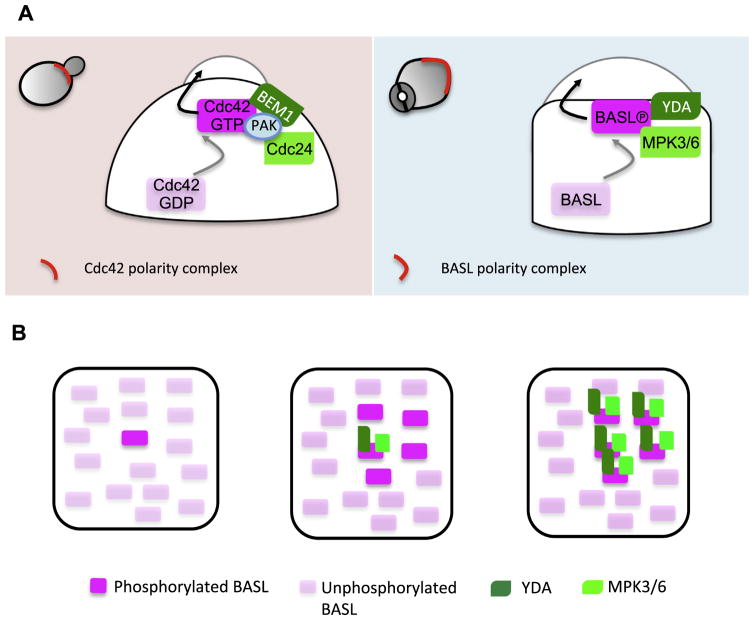
The yeast S. cerevisiae is a single-celled organism that continuously produces small daughter cells via polarizing the mother cell to form a bud, which expands and detaches from the mother. Polarization of the mother cells can be easily recognized as a patch of enriched cytoskeleton and membrane trafficking components at the polarity site, which promote the growth of the daughter cell. The polarity regulator, small Rho GTPase Cdc42, was first identified by Adams and others (Adams et al., 1990) and later established as “the center of cell polarization” ubiquitously from yeast to humans (Etienne-Manneville, 2004; Park and Bi, 2007). Loss of Cdc42 function leads to polarization failure of the mother cell and causes division problems (Adams et al., 1990). The cycling of Cdc42 between active guanosine triphosphate (GTP)-bound and inactive guanosine diphosphate (GDP)-bound forms is controlled by orchestrated activity of activators (guanine nucleotide exchange factors, GEFs), inhibitors (ATPase-activating proteins, GAPs) and other modulators (Rho GTPase-dissociation inhibitors, GDIs) (Vetter and Wittinghofer, 2001). One of the two major pathways that distribute Cdc42 to a highly polarized fashion at the cell cortex is actin-independent and requires a Cdc42-Bem1-Cdc24 centered positive feedback loop (Fig. 1). Bem1 is a scaffold protein and Cdc24 is a GEF activator of Cdc42. In the absence of any spatial cues, stochastically activated Cdc42 molecules may spontaneously cluster to initiate a cortical site where Bem1 is recruited, which locally accumulates Cdc24 that further activates Cdc42 to expand the polarity domain (Butty et al., 2002; Smith et al., 2013). More recently, a Cdc42 effector p21-activated kinase PAK was also found as a part of the complex that binds to Cdc24 and contributes to spontaneous polarization of yeast cells (Kozubowski et al., 2008; Woods et al., 2015). Thus, positive feedback loops provide a base for spontaneous initiation of Cdc42 polarization.
Fig 1.
Symmetry-breaking polarization of Cdc42 and BASL in yeast and Arabidopsis stomatal lineage cells. (A) Left panel: Spontaneous polarization of the Cdc42-Bem1-Cdc24/PAK feedforward loop. In budding yeast, stochastically activated Cdc42-GTP (gray arrow) recruits the scaffold protein Bem1, which locally enrich Cdc24 the Cdc42 GEF activator, to amplify activated Cdc42-mediated protein clustering. This intrinsic positive feedback loop polarizes Cdc42 to the cortical site and promotes daughter cell expansion (black arrow). Right panel: In Arabidopsis stomatal ACD precursor cells, a positive feedback loop of BASL-YDA-MAPK3/6 promotes cell polarization. MPK3/6-mediated phosphorylation triggers BASL cortical polarization (gray arrow). At the cell cortex, phosphorylated BASL functions as a scaffold to recruit both YDA and MPK3/6, which further produces more activated MPK3/6, and thus more activated BASL to polarize. Polarization of the BASL complex was hypothesized to promote local cell expansion (black arrow) (B)Working model for spontaneous clustering of BASL-YDA-MPK3/6 in plant cells. We hypothesize that, similar to Cdc42 polarization, initially activated BASL molecules organize a positive feedback loop by recruiting its activators YDA-MPK3/6, which allows the conversion of neighboring molecules to become active, eventually leading to the formation of a polarized cluster.
In Arabidopsis, the stomatal lineage cells divide asymmetrically to produce highly specialized guard cells that conduct gas exchange between the plant and the atmosphere (Bergmann and Sack, 2007). The initiation of stomatal precursor cells occurs in young developing leaves and their division orientation appears random relative to the leaf axis (Lau and Bergmann, 2012), suggesting an intrinsic polarization property of the cells. This is strongly supported by the discovery of the plant-specific BASL gene (Dong et al., 2009). In the absence of BASL, the stomatal lineage cells lost their full capacity to divide asymmetrically, thus producing an enlarged proliferating population with equal division pattern and disturbed daughter cell fate segregation (Dong et al., 2009). The localization of BASL protein is mainly featured by its strong polar accumulation at the cell cortex that is indispensible for its function (Dong et al., 2009). In plant cells, the molecular mechanisms for protein polarization are better understood for PIN-FORMED (PIN) auxin transports. PINs are membrane integral proteins and often occupy distinct plasma membrane domains in Arabidopsis plants (Friml, 2003). PIN polar targeting and maintenance at the plasma membrane involve rapid and constitutive vesicular recycling between the PM and the endosomes (Feraru and Friml, 2008; Grunewald and Friml, 2010).
How the non-membrane protein BASL polarizes and associates to the PM remains unknown, but interestingly, both positive feedback loop and protein spontaneous clustering appear to be part of the process (Fig. 1). The positive feedback loop involves BASL, the Mitogen Activated Protein Kinase (MAPK) Kinase Kinase YODA (YDA) and MAPK 3 and 6 (MPK3/6), both of which are pivotal signaling molecules in stomatal development (Bergmann et al., 2004; Wang et al., 2007). Activation of BASL polarization can be mediated by MPK3/6 phosphorylation, which promotes nuclear export and cortical polar accumulation (Zhang et al., 2015). At the cell cortex, phosphorylated BASL functions as a scaffold protein and binds to both YDA and MPK3/6, likely promoting signaling specificity and/or efficiency of the YDA MPK3/6 pathway (Zhang et al., 2015). With the interactions established, activating more MPK3/6 can induce further activation of BASL to amplify the polarization effect. In mature tobacco pavement cells, overexpressing BASL protein showed even distribution along the PM. However, when YDA and MPK6 were co-expressed, the three proteins clustered together and accumulated into patches at the cell cortex (Zhang et al., 2015). This suggests a spontaneous self-catalyzing property of the BASL-YDA-MPK3/6 module, which to some extent resembles self-organizing polarization of Cdc42-Bem1-Cdc24 in yeast (Fig. 1).
POLAR is another polarized protein of unknown function in the Arabidopsis stomatal lineage. The cortical polarization pattern of POLAR mirrors that of BASL and is dependent on the presence of BASL (Pillitteri et al., 2011). But how POLAR contributes to the polarity pathway to regulate stomatal ACD remains unknown because mutations in POLAR did not yield discernable defects.
2.2. Cell polarization: cytoskeletal reorganization
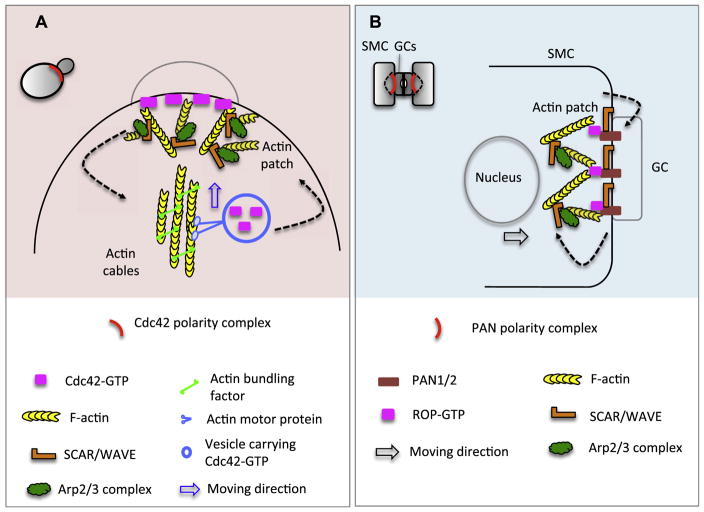
In yeast, the second positive feedback loop in Cdc42 polarization involves active Cdc42-guided, actin-dependent exocytosis (Pruyne et al., 2004; Rohatgi et al., 1999). Two forms of actin structure (patches and cables) are positively regulated by Cdc42. Cdc42-GTP promotes the formation of actin cables, which are nucleated by formin-family proteins (Pruyne et al., 2004) and provide tracks for the delivery of exocytotic vesicles carrying Cdc42-GTP (Evangelista et al., 2002; Pruyne et al., 2004). This in turn enhances Cdc42 enrichment at the polarization site (Marco et al., 2007; Wedlich-Soldner et al., 2003; Wedlich-Soldner et al., 2004) (Fig. 2). Actin patches, a Cdc42-dependent platform for endocytosis and cell wall remodeling, contain a dynamic network of branched actin filaments that are polymerized by the Arp2/3 complex (Moseley and Goode, 2006).
Fig 2.
Actin-dependent positive feedback loops for symmetry-breaking polarization in yeast and maize stomatal cells. (A) Cytoskeletal-dependent polarization of Cdc42 in yeast. Cortically localized active Cdc42-GTP triggers Formin activity (bundling factor) to form actin cables. Actin cables provide tracks for the delivery of exocytotic vesicles carrying Cdc42-GTP to the polarizing site, resulting in further nucleation of actin cables, which in turn enhances the Cdc42 polar enrichment (dashed arrows). Blue arrow shows the moving direction of Cdc42-GTP vesicles driven by actin motor proteins. The formation of actin patches is stimulated by Cdc42-GTP activated SCARE/WAVE and Arp2/3, which promote actin nucleation and branching. Actin patches are necessary for dynamic delivery of PM and cell wall materials. (B)PAN1/2 polarization in maize Subsidiary Mother Cells (SMCs). In SMC, polarized PAN1/2 physically bind to ROP and lead to ROP activation. Polarized ROP activates the SCAR/WAVE and Arp2/3 activity and thus nucleation of actin to form a dense actin patch at the polarity site. Actin patches may help directional nuclear migration (black arrow) during SMC ACD. Interestingly, polarization of SCAR/WAVE is also required for PAN1/2 polarization, suggesting an interdependent activation (dashed arrows).
In plants, the Rho family is diverged into the Rho-like GTPase from Plants (ROP) family that shares the common ancestor of Cdc42 (Gu et al., 2004). Similar to their counterparts in yeast and animals, ROP polarization also involves feedback loops (Hazak et al., 2010; Zhang and McCormick, 2007) that coordinate cytoskeleton organization and vesicular trafficking to establish cell polarity as well as to promote polar growth in plants (Fu et al., 2005; Gu et al., 2005). The roles of ROPs in plant ACD were demonstrated by their functional connection with the PAN leucine-rich repeat receptor-like kinases in maize stomatal development. The polarization of PANs represents an extrinsic-cue induced process.
In maize (Zea mays), each stomatal complex is composed of two guard cells flanked by two subsidiary cells (Fig. 2). The production of subsidiary cells requires an ACD of the precursor subsidiary mother cells (SMC). Prior to an ACD, the SMC polarization is demonstrated by asymmetric accumulation PAN proteins at the plasma membrane abutting the neighboring guard cells, followed by the formation of actin patch at the polarity site (Cartwright et al., 2009). Loss of PAN1 or PAN2 led to failures of SMC premitotic polarization and abnormal SMC division (Cartwright et al., 2009; Zhang et al., 2012). Recent work disclosed that prior to PAN polarization, the SCAR/WAVE complex (activator of the actin nucleating Arp2/3 complex) polarizes in SMCs independent of PANs and is required for PAN polarization (Facette et al., 2015). The early polarization of SCAR/WAVE was unexpected because PAN proteins physically interact and polarize ROP proteins (Humphries et al., 2011), which activate the downstream SCAR/WAVE complex and thus the Arp2/3 complex for promoting formation of actin patches in SMCs (Facette et al., 2015). It remains obscure how the SCAR/WAVE complex becomes polarized and how it functions upstream to determine PAN polarization. As membrane embedded receptors, PANs may require endosomal recycling, during which actin plays important function (Kaksonen et al., 2006; Samaj et al., 2004). It is tantalizing to suspect that the actin network is in-conspicuously reorganized by locally enriched SCAR/WAVE, which then influences membrane trafficking and polarization of PAN proteins in SMCs. What initial cue derived from GCs to induce polarization of SCAR/WAVE and SMCs remains a fascinating question in the field.
3. Divisionally physical asymmetry
Because plant cells are restricted by rigid cell walls, their coordinated division orientation and cell expansion are crucially important for tissue formation and organ patterning in plant development. Division placement has been noted long ago that it is closely related to cell shape in symmetrically dividing cells. In the late 1800’s, Errera hypothesized that the new cell wall takes the shortest path that divides the mother into halves (Smith, 2001). This classic “Errera’s rule” has been re-evaluated by several studies in recent years that integrated microscopic examination and computational simulation (Besson and Dumais, 2011; Sahlin and Jonsson, 2010). The current understanding is that the majority of symmetric cell divisions comply the shortest distance rule, in particular in cells with pronounced length differences in axis. On the other hand, in polygonal cells, the orientation of cell division is stochastic and random, and can be guided by surrounding mechanical forces (Besson and Dumais, 2011; Prusinkiewicz, 2011). However, ACDs that are coordinated with polarity of the mother cells override the influence of cell shape on selection of the division plane and cortical polarity proteins contribute heavily to defining PM domains and thus division plane establishment.
In animals, the cleavage plane that separates two daughter cells is defined by the contractile actomyosin ring, which uses the mitotic spindle as a positional cue (von Dassow, 2009). Correct spindle positioning is the linchpin to deciding placement of the cytokinesis furrow and ensuring cell fate determinants appropriately segregated (Williams and Fuchs, 2013). Conserved Partitioning defective (PAR) polarity proteins play fundamental roles in the regulation of ACD orientation in C. elegans, Drosophila, and vertebrates (Goldstein and Macara, 2007). In Drosophila neuroblast ACD, the PAR complex (PAR3 and PAR6 scaffold proteins and aPKC kinase) is polarized at the apical cortex and accumulates another apical complex (Pins, Gαi, Mud/NuMA) that directs and establishes spindle orientation. Mud/NuMA is a MT-binding protein that localizes to spindle poles and interacts with the dynein motor complex (Siller et al., 2006). The cortical-attached, MT minus-end directed motility of dynein exerts a pulling force to help navigate the mitotic spindle(Kiyomitsu and Cheeseman, 2012). Alternatively, dyneine may serve as a cortical anchor of the plus-end depolymerizing micriotubules that indirectly allow spindle positioning (Grill et al., 2003; Kozlowski et al., 2007). A number of MT plus-end binding proteins, e.g. CLASP, EB1 and the MCAK MT depolymerase have also been identified to contribute to MT dynamics and spindle orientation in animals (reviewed in Lu and Johnston, 2013).
In plant cells, by contrast, the selection of cell division plane in plants is determined prior to the formation of mitotic spindle and relies on a specialized MT apparatus, the preprophase band (PPB), to organize cell division plane. The PPB is a cortical array of cytoskeletal filaments that are composed of microtubules (MTs) and filamentous actins (F-actins) and transiently assembled at G2 phase and dissembled at mitosis (Mineyuki, 1999). The cortical demarcation of the PPB loyally predicts where the division plane is placed, though the formation of PPB is not absolutely required across plant species. But in plant cells where PPBs are normally formed, i.e. in Arabidopsis, the lack of PPB results in severely misorientated cell division planes (Azimzadeh et al., 2008; Camilleri et al., 2002; Kawamura et al., 2006).
3.1. Formation of the PPB
The MT network undergoes tremendous reorganization during plant cell division (Rasmussen et al., 2013). The PPB formation requires disassembly of the interphase cortical MT network, particularly outside of the PPB zone, and concomitant organization of a belt-like array at G2 phase (Rasmussen et al., 2011). In consonance with that, a number of microtubule-associated proteins (MAPs) co-localize with the PPB to initiate and maintain its organization (Rasmussen et al., 2013). Recent research have shed light upon a number of advances in understanding the PPB disassembly, the cortical division zone (CDZ) establishment, phragmoplast guidance and expansion, as well as cell plate synthesis (see recent reviews (Lipka et al., 2015; Muller and Jurgens, 2015; Rasmussen et al., 2011; Rasmussen et al., 2013)). Here we discuss the very early events in PPB formation and how cell polarization might play a role in orientating the PPB.
The initiation of PPB formation is not fully understood yet, but drug treatment and genetic analysis placed protein phosphorylation/dephosphorylation events to a critical position in this process. Application of kinase and phosphatase inhibitors interferes with PPB formation in plants (Ayaydin et al., 2000; Katsuta and Shibaoka, 1992). Two Arabidopsis mutants tonneau1 (ton1) and fass/ton2 do not produce PPBs and cell division planes are randomly placed (Azimzadeh et al., 2008; Kirik et al., 2012; Torres-Ruiz and Jurgens, 1994). TON2/FASS belongs to a heterotrimeric protein phosphatase 2A (PP2A) complex, functioning as a regulatory B subunit (Camilleri et al., 2002). Later, other subunits of the PP2A complex, including the scaffold A subunits and the catalytic C subunits, were co-purified with TON2/FASS from Arabidopsis plants and mutations in these genes recapitulated the cell division defects in ton2 (Spinner et al., 2013). The PPB association of the PP2A holoenzyme is achieved by forming a so called TTP complex (TON1/TRM/PP2A) with two other MT-binding proteins, TON1 and TON1 RECRUITING MOTIF (TRM), both of which share sequence similarity with animal centrosomal proteins (Drevensek et al., 2012). Loss of TTP components results in the absence of PPB and thereby abnormal division plane in Arabidopsis, but spindles and phragmoplasts are not discernably affected (Spinner et al., 2013).
It remains a challenge to identify downstream substrates of the PP2A complex for PPB formation and maintenance. TON1 itself was recognized as a phosphoprotein (Benschop et al., 2007) and can be a direct target of PP2A. Putative candidates also include other PPB-associated MT regulators, e.g. MOR1, SABRE and CLASP, mutations in all of which gave rise to defects in PPB formation and positioning (Ambrose et al., 2011; Kawamura et al., 2006; Pietra et al., 2013; Twell et al., 2002). A conserved eukaryotic protein of unknown function in Arabidopsis, SABRE, weakly associates with the PPB MTs and functions downstream of CLASP (Pietra et al., 2013). The genetic interaction and functional connection of these PPB binding proteins with the TTP family and phosphorylation activity require further in-depth investigation.
The initial position of PPB formation at the cell cortex seems to be uneven and might relate to where the nucleus is located. Three-dimensional analysis of MT array in Arabidopsis leaf epidermal cell divisions disclosed that PPB development initiates from the inner cell cortex (the mesophyll side), but not from the outer side (near the atmosphere). This polarized developmental manner is closely related to the preprophase nuclei that are mainly positioned near the inner cell wall (Lucas and Sack, 2012). Indeed, spatial co-alignment of the nucleus and the PPB in early prophase cells has been noticed for a long time. Early centrifugation experiments using the fern Adiantum protonemata showed that a second PPB is induced around the displaced nucleus (Murata and Wada, 1991). However, the position of the nucleus alone can not determine PPB positioning because when more than one PPB was induced in synchronization of tobacco BY-2 cells, some PPBs do not encircle the nucleus (Granger and Cyr, 2001; Yoneda et al., 2005). In addition, in asymmetrically dividing Arabidopsis stomatal cells, PPB formation precedes nuclear migration and is independent of nucleus position (Geisler et al., 2003). Thus, whether the nucleus leads the PPB or vise versa may depend on cell type and biological context. Particularly in asymmetric divisions, cell polarity is an important factor that influences division plane orientation.
3.2. Orientating the PPB – polarity protein complexes
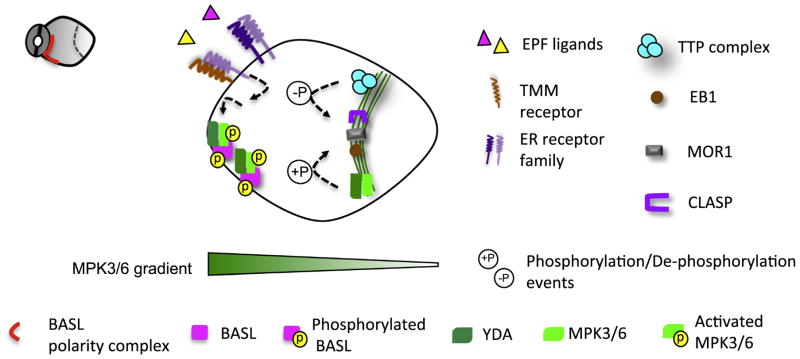
In Arabidopsis stomatal ACD precursor cells, polarization of BASL seems to propel the division plane to form distally (Fig. 3). How BASL functions to orientate the division plane remains a major mystery. However, as the YDA MPK3/6 cascade is tightly associated with polarized BASL (Zhang et al., 2015), it is worth speculating that a MAPK signaling gradient has a potential function in this process (Fig. 3). Besides the BASL positive feedback loop mentioned earlier, a YDA MPK3/6 gradient can also be set up by extrinsic signals triggered by ligand-receptor interaction (Torii, 2012). The extracellular peptide ligands, Epidermal Patterning Factors (EPFs), act upstream of the MAPK cascade in stomatal development (Abrash and Bergmann, 2010; Hara et al., 2007; Hara et al., 2009; Hunt and Gray, 2009; Kondo et al., 2010; Sugano et al., 2010). EPF1 and EPF2 interact with the leucine-rich repeat receptors, Too Many Mouth (TMM) and ERECTA receptor-like kinases (ER, ER-like 1 and ER-like 2), to activate the YDA MPK3/6 pathway (Jewaria et al., 2013; Lee et al., 2015; Nadeau and Sack, 2002; Shpak et al., 2005). In particular, in the loss-of-function mutants epf1 and tmm, the orientated ACD abutting a differentiating stomatal cell cannot fully navigate its polarity and fails to divide properly (Hara et al., 2009; Nadeau and Sack, 2002).
Fig 3.
A hypothetical MAPK gradient and asymmetric placement of cell division plane in the Arabidopsis stomatal lineage. In Arabidopsis stomatal ACD, we hypothesize that the YDA MPK3/6 signaling forms a gradient. Two possible mechanisms may contribute to this gradient. The positive feedback loop with BASL maintains a high concentration of MPK3/6 at the crescent. Extrinsic peptide ligands EPF1/2, derived from the neighboring stomatal cells, are perceived by the membrane receptors TMM and ERECTA family, which may locally activate the downstream YDA-MPK3/6 pathway. The formation of the PPB requires a core TTP complex containing protein phosphatase PP2A enzymes with two MT-binding proteins, TON1 and TRM. The PPB-associated MT regulators, e.g. MOR1, CLASP1 and EB1, might be subject to MAPK- and PP2A-mediate phosphorylation and dephosphorylation, respectively, to initiate and orientate the PPB formation.
More evidence supported that the levels of YDA MPK3/6 activity need to be tightly controlled in plant development, especially in cell division orientation. Both loss-of-function yda and gain-of-function ΔNyda (constitutively active YDA, the N-terminal inhibitory domain deleted) showed similarly dis-orientated cell division profile in Arabidopsis roots (Smekalova et al., 2014). Consistently, mutations in MPK6 recapitulated this phenotype (Muller et al., 2010). Despite genetic data placing YDA as an upstream activator of MPK3/6 in stomatal development and embryogenesis (Bergmann et al., 2004; Lukowitz et al., 2004; Wang et al., 2007), surprisingly, MPK6 protein level was found increased in yda null mutants and that elevated MPK6 may contribute to the phenotype of oblique cell walls in roots. Careful examination of MPK6 localization in yda and ΔNyda showed that YDA activation promotes more physical association of MPK6 with the mitotic MT arrays (Smekalova et al., 2014). It was hypothesized that microtubule-associated protein (MAP) regulators, e.g. MAP65 and MOR1, might be subject to MPK6 phosphorylation and regulation (Muller et al., 2010). The identification of new MPK6 substrates is anticipated to improve our understanding of how YDA MPK3/6 signaling may regulate divisional plane orientation.
An ungrouped plant-specific Kinesin motor-protein, ARK3/At-KINUa, is enriched at the PPB prior to the nuclear envelope breakdown (Malcos and Cyr, 2011; Sakai et al., 2008). Cell type-specific RNAi knocking down the expression of ARK3 in Arabidopsis generated mildly clustered stomatal lineage divisions and paired guard cells, phenocopying that of basl mutants (Lau et al., 2014). The major defects of ark3 mutants seem to center on the orientation of the PPB, but not the formation of the PPB and cell plate per se (Lau et al., 2014). Future genetic analyses between the BASL-MAPK polarity pathway and ARK3 should delineate whether and how they function together to orientate stomatal ACD.
The position of PAN-ROP polarity relative to the cell division plane in maize SMCs opposes how BASL is positioned in the Arabidopsis stomatal lineage cells (Fig. 2B). New cell walls are placed proximately to where PAN is polarized (Cartwright et al., 2009; Zhang et al., 2012). Following PAN polarization and actin patch formation, the nucleus migrates to the division side. Based on inhibitor tests, the directional movement of the nucleus is actin-dependent (Panteris et al., 2006). The microtubule arrays are organized into a specialized prophase “half-spindle” that is connected with the PPB. This perinuclear MT complex may spatially confine the nucleus adjacent to the SMC (Panteris et al., 2006) (Fig. 2B). Thus far, it is not known whether the PAN-ROP pathway contributes to the formation of “half-spindle”-PPB complex, though this complex seems to be stabilized by actin patches (Panteris et al., 2006).
3.3. Orientating the PPB – auxin pathways
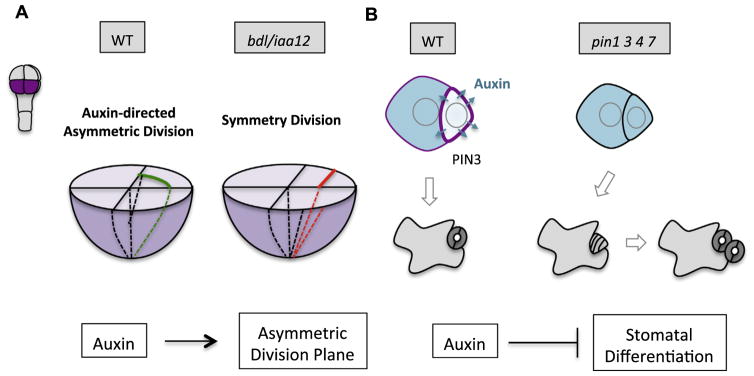
Auxin has profound influences on plant embryogenesis and post-embryonic development (Dharmasiri et al., 2005; Friml et al., 2003; Jürgens et al., 1991; Reinhardt et al., 2000). Recent progress in Arabidopsis stomatal development and early embryo patterning disclosed important roles of auxin signaling in establishing asymmetric division plane placement. By 3-D high-resolution confocal imaging and computational analysis, a complete map of Arabidopsis early embryogenesis (from the zygote to the late hear stage) was constructed (Yoshida et al., 2014). This method greatly enhanced our ability to evaluate cell volume and cell wall surface asymmetries during cell divisions. Interestingly, new ACD patterns in embryos of eight cells or larger were recognized because in the lower tier, the volume of the outer cell (protoderm) is two times larger than that of the inner cell (precursor of vasculature and ground tissues) (Fig. 4). Such a volume difference cannot be revealed by conventional 2-D cross-sections. Furthermore, mutations in BODENLOS (BDL)/IAA12, an inhibitor of auxin response, reverted these divisions to be symmetric, suggesting that asymmetric placement of the division plane is linked to auxin-related pathways (Yoshida et al., 2014). It remains a mystery how auxin response may orientate cell division in embryos, but a concentration gradient may play a role. Indeed, the newly developed auxin markers DR5v2 and R2D2 disclosed a slightly elevated auxin signaling in the inner cells of the early globular embryo (Liao et al., 2015). In addition, both local auxin production and PIN polarization are required for the cell polarization and subsequent apical-basal axis establishment in early embryos (Robert et al., 2013). Alternatively, MP/ARF5 may act through target genes to promote other hormonal pathways, i.e. auxin-elevated cytokinin biosynthesis triggers division plane shift in vasculature tissue (De Rybel et al., 2014; Ohashi-Ito et al., 2014). The major challenge in the field is to provide a mechanistic understanding of how auxin gradient, most likely very small within a cell or between neighboring cells, may alter cell polarity and the PPB positioning.
Fig 4.
Auxin signaling participates in plant ACD. (A) Auxin pathway promotes ACD in early embryos. In 8-cell embryos, ACDs were detected in the lower tier (purple) by 3D high-resolution confocal imaging and computational analyses. The volume of outer cells (protoderm) is about two times that of the inner cells (precursor of vasculature and ground tissues). However, in the auxin inhibitor mutant bdl/iaa12, ACDs were reverted to symmetric. How auxin signaling promotes asymmetric placement of the division plane is unknown. (B)Auxin depletion from the M is linked to stomatal differentiation after an ACD. In the wild-type (WT), auxin level drops when the M differentiates into stomatal guard cells. Auxin depletion is likely mediated by elevated expression level of the PIN3 auxin effluxer (purple). In the loss-of-function mutant pin1 3 4 7, the M maintains high level of auxin and does not differentiate, but divide more times before terminal fate adoption.
Additionally, recent discoveries of the interconnection between mechanical stress and auxin signaling shed new lights into the intricate crosstalk between cell growth/expansion and auxin function. In both Arabidopsis and tomato shoot apex, changes in mechanical tension were found to influence auxin transport and accumulation. In addition, the amount and intracellular localization of PIN1 and the cortical MT orientation are also modulated (Heisler et al., 2010; Nakayama et al., 2012), indicating that cell growth strain feeds back to auxin signaling. Apparently, in our future studies, consideration of auxin signaling should be coupled with growth and division parameters of the cells to interpret the mechanisms of plant ACD.
4. Cell fate asymmetry
Cell-to-cell communication in plants can be achieved through ligand-receptor interactions, intercellular trafficking of hormones, RNAs and proteins that include transcription factors (Wu and Gallagher, 2011). Mobile transcription factors play pivotal roles in plant development (Han et al., 2014) and ACD-involved tissue patterning (Petricka et al., 2009; Ten Hove and Heidstra, 2008). A well-studied case showed that directional movement of the GRAS transcription factor SHORT ROOT (SHR), from the stele into the endodermal cells, helps to establish asymmetric identity of the cortex and endodermis in Arabidopsis roots (Helariutta et al., 2000; Nakajima et al., 2001). Another example showed that the bHLH transcription factor TARGET OF MONOPTEROS 7 (TMO7) travels from the provasculature of the early embryo into the hypophysis to direct asymmetric division (Schlereth et al., 2010). Recently emerging evidence showed that polarizing signals, e.g. proteins and auxin, play important roles in differentiating daughter cells. Here we discuss how polarized cues and auxin signaling may contribute to cell fate specification in plant ACDs.
4.1. Polarized proteins
In animals, the PAR proteins can asymmetrically segregate cell fate-determining factors. In Drosophila neuroblast ACD, the apically polarized PAR complex directs the fate-determining complex, including Numb (an endocytic protein), Brain tumor (BRAT, a translation inhibitor) and Prospero (a transcription factor) to partition into the basal cell (Knoblich, 2008). After mitosis, the cell fate determinants act together to prevent stem cell self-renewal while promoting differentiation (Berdnik et al., 2002; Knoblich et al., 1995; Sonoda and Wharton, 2001; Spana and Doe, 1995).
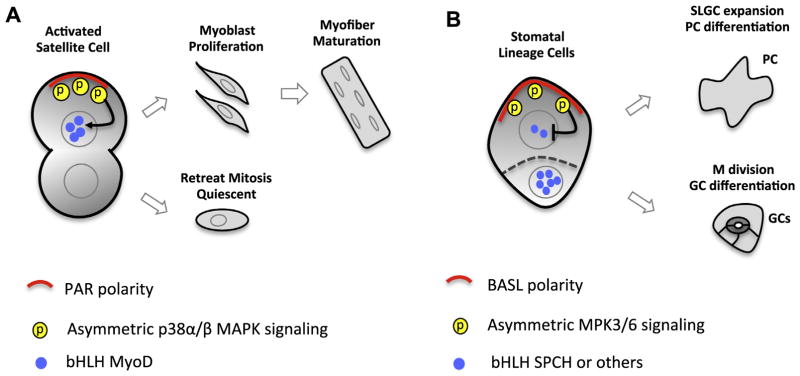
In Arabidopsis, a stomatal lineage precursor cell, Meristemoid Mother Cell (MMC), divides asymmetrically to produce a Meristemoid (M) and a Stomatal Lineage Ground Cell (SLGC). The two daughter cells differ in their stem cell division potential and terminal cell fate. An M divides 3–4 times before terminating into a pair of guard cells and a SLGC may divide one more time before it expands to become a pavement cell. The BASL-YDA-MPK3/6 is polarized premitotically and inherited to the SLGC only. The two daughter cells differ in BASL polarization and the associated MAPK cascade (Zhang et al., 2015). Downstream of MPK3/6, the bHLH transcription factor SPEECHLESS (SPCH) is a direct substrate being phosphorylated for degradation (Lampard et al., 2008). Expression of SPCH initiates stomatal ACD and promotes fate differentiation (Lau et al., 2014; MacAlister et al., 2007). Therefore, the BASL-YDA-MPK3/6 polarity module in the SLGC was hypothesized to exert stronger negative regulation on SPCH expression, thus inducing lowered division potential and cell fate departed from stomatal terminal differentiation (Fig. 5).
Fig 5.
Asymmetrically activated MAPK signaling in muscle satellite cell ACD and Arabidopsis stomatal ACD. (A) Asymmetric p38α/β MAPK activity in mouse muscle satellite cell ACD. Polarized PAR protein complex activates p38α/β MAPK activity in only one daughter cell, which turns on the expression of bHLH transcription factor MyoD to promote amplifying divisions followed by terminal fate differentiation. The other daughter cell exits cell division and returns to the quiescent state. (B)Asymmetric distribution of the YDA-MPK3/6 cascade in Arabidopsis stomatal ACD. Premitotically polarized BASL-YDA-MPK3/6 complex is only inherited to the large daughter cell (SLGC, stomatal lineage ground cell), which results in phosphorylation of the nuclear bHLH SPCH for degradation. Differential SPCH expression levels in two daughters direct their distinct developmental path. The small daughter (M, Meristemoid) undergoes a few divisions and terminates into stomatal guard cells and the large SLGC expands to become a pavement cell (PC).
Spatially organized MAPK activity by polarity proteins is not entirely novel in animal ACD systems (Fig. 5). Mouse muscle satellite cells (quiescent stem cells) are activated by injury and divide asymmetrically to replenish damaged cells (Jones et al., 2005). Polarized PAR proteins were found to concentrate or activate p38α/β MAPK activity in only one daughter cell, which turns on the expression of bHLH transcription factor MyoD to promote amplifying divisions and terminal fate differentiation (Troy et al., 2012). The other daughter cell retreats back and maintains the quiescent status. In plants, MAPK-mediated suppression of SPCH seems to provide more flexibility in stomatal development. When MAPK activities are modulated by environmental changes, stomatal lineage cells are capable of adjusting SPCH level and adapting their division potential to optimize stomatal density and improve growth.
4.2. Asymmetric auxin signaling
Auxin levels and responses fluctuate in Arabidopsis stomatal development. Auxin signaling peaks in the Ms that undergo active stomatal ACD, and drops when an M differentiates into a guard mother cells (GMCs) (Le et al., 2014). Lowered auxin level in the Ms might be mediated by elevated expression of the auxin effluxer PIN3 and was hypothesized to induce stomatal terminal differentiation (Fig. 4). The loss-of-function auxin transporter mutants pin2 3 4 7 and pin1 3 4 7, similar to the receptor mutants tir1 afb1 2 3, showed elevated stomatal index and clustered patterning, suggesting that auxin negatively regulates stomatal production (Le et al., 2014). This connection was further corroborated by Zhang et al. (2014) and Balcerowicz et al. (2014). The crosstalk between auxin signaling and the core stomatal development pathway is partially achieved by the activity of Auxin Response Factor (ARF) 5/MONOPTEROS (MP) that directly binds to the promoter of STOMAGEN and suppresses its expression (Zhang et al., 2014). STOMAGEN is a positive regulator of stomatal development (Kondo et al., 2010; Sugano et al., 2010). As an EPF ligand, STOMAGEN competes with EPF1 and EPF2 to inhibit the TMM/ER receptor-mediated signal transduction (Jewaria et al., 2013; Lee et al., 2015). However, this functional connection cannot well address how auxin depletion from the Ms is related to stomatal differentiation because STOMAGEN is not expressed in the epidermis, but rather functions non-cell-autonomously from the inner mesophyll cells. Future work is anticipated to resolve what determines PIN asymmetric distribution in stomatal ACD pairs and how ARFs crosstalk with the core stomatal fate factors to control cell fate asymmetry.
5. Concluding remarks and perspectives
Orientated cell division and diversified cell fate are key to plant growth and developmental patterning. The discovery of polarized proteins in plant ACD provided a unique opportunity to study the molecular mechanisms underpinning protein polarization at the membrane and how they organize cellular contents to orientate cell division plane and determine differential identity of daughter cells. Although both plants and animals utilize common regulatory modes, e.g. self-organizing molecular amplification and cytoskeleton-dependent forward feedback loops, cell polarization in plants is featured with striking responsiveness to external cues. Finding the GC-derived cues in maize stomatal system will further emphasize position-dependent mechanisms in plant development. In PAR systems, the separation of cortical domains requires phosphorylation-mediated mutual exclusion. Whether polarized BASL and YDA MPK3/6 phosphorylation may propel another set of proteins into the other end is a fascinating question.
Genetic and computational analyses provided in-depth insights into the regulators and general rules in plant symmetric cell divisions. The finding of asymmetrically activated MAPK cascade in stomatal ACD further highlighted regulatory similarities between stomatal lineage cells and muscle satellite cells, both of which are adult stem cell pools. Not only are cell fate transitions orchestrated by bHLH transcription factors in both systems (Matos and Bergmann, 2014), daughter cell differentiation is also achieved by spatially controlled MAPK signaling and differential regulation of bHLH factors. In addition, an exciting wave of research is anticipated to integrate polarized proteins and auxin signaling to the selection of cell division plane in plant ACD. Finding more MAPK substrates and downstream effectors of auxin responses that appear prior to the PPB formation may have the potential to translate cell polarity to division orientation.
Long-term high-resolution 4-dimentional imaging combined with computational quantification has the promise to reveal more plant ACDs that are unrecognized thus far. It remains a challenge to provide a unified view on how auxin regulates plant ACD. Aided by the improved auxin sensors, DR5v2 and R2D2, many plant ACD systems should be revisited so that potential functions of auxin signaling as a general regulator can be better evaluated.
Acknowledgments
We thank Dr. Ying Zhang for critical reading of the manuscript and Helen Xia (Rutgers University) for the help with editing. Our research projects are supported by grants from the U.S. National Institute of General Medical Sciences R01 GM109080 to J.D.. W.S. is supported by Charles and Johanna Busch Predoctoral Fellowship.
Abbreviations
- ACD
asymmetric cell division
- PM
plasma membrane
- MT
microtubule
- F-actin
filamentous actin
- M
meristemoid
- SLGC
stomatal lineage ground cell
- GC
guard cell
- SMC
subsidiary mother cell
Footnotes
Conflict of interest disclosure
The authors declare that there are no conflicts of interest.
References
- Abrash EB, Bergmann DC. Asymmetric cell divisions: a view from plant development. Dev Cell. 2009;16:783–796. doi: 10.1016/j.devcel.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Abrash EB, Bergmann DC. Regional specification of stomatal production by the putative ligand CHALLAH. Development. 2010;137:447–455. doi: 10.1242/dev.040931. [DOI] [PubMed] [Google Scholar]
- Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Allard JF, Cytrynbaum EN, Wasteneys GO. A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis. Nat Commun. 2011;2:430. doi: 10.1038/ncomms1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaydin F, Vissi E, Meszaros T, Miskolczi P, Kovacs I, Feher A, Dombradi V, Erdodi F, Gergely P, Dudits D. Inhibition of serine/threonine-specific protein phosphatases causes premature activation of cdc2MsF kinase at G2/M transition and early mitotic microtubule organisation in alfalfa. Plant J: Cell Mol Biol. 2000;23:85–96. doi: 10.1046/j.1365-313x.2000.00798.x. [DOI] [PubMed] [Google Scholar]
- Azimzadeh J, Nacry P, Christodoulidou A, Drevensek S, Camilleri C, Amiour N, Parcy F, Pastuglia M, Bouchez D. Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell. 2008;20:2146–2159. doi: 10.1105/tpc.107.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcerowicz M, Ranjan A, Rupprecht L, Fiene G, Hoecker U. Auxin represses stomatal development in dark-grown seedlings via Aux/IAA proteins. Development. 2014;141:3165–3176. doi: 10.1242/dev.109181. [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Mohammed S, O’Flaherty M, Heck AJ, Slijper M, Menke FL. Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteom. 2007;6:1198–1214. doi: 10.1074/mcp.M600429-MCP200. [DOI] [PubMed] [Google Scholar]
- Berdnik D, Torok T, Gonzalez-Gaitan M, Knoblich JA. The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell. 2002;3:221–231. doi: 10.1016/s1534-5807(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Sack FD. Stomatal development. Annu Rev Plant Biol. 2007;58:163–181. doi: 10.1146/annurev.arplant.58.032806.104023. [DOI] [PubMed] [Google Scholar]
- Besson S, Dumais J. Universal rule for the symmetric division of plant cells. Proc Natl Acad Sci USA. 2011;108:6294–6299. doi: 10.1073/pnas.1011866108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butty AC, Perrinjaquet N, Petit A, Jaquenoud M, Segall JE, Hofmann K, Zwahlen C, Peter M. A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J. 2002;21:1565–1576. doi: 10.1093/emboj/21.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri C, Azimzadeh J, Pastuglia M, Bellini C, Grandjean O, Bouchez D. The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell. 2002;14:833–845. doi: 10.1105/tpc.010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright HN, Humphries JA, Smith LG. PAN1: a receptor-like protein that promotes polarization of an asymmetric cell division in maize. Science. 2009;323:649–651. doi: 10.1126/science.1161686. [DOI] [PubMed] [Google Scholar]
- Chant J. Cell polarity in yeast. Annu Rev Cell Dev Biol. 1999;15:365–391. doi: 10.1146/annurev.cellbio.15.1.365. [DOI] [PubMed] [Google Scholar]
- De Rybel B, Adibi M, Breda AS, Wendrich JR, Smit ME, Novak O, Yamaguchi N, Yoshida S, Van Isterdael G, Palovaara J, Nijsse B, Boekschoten MV, Hooiveld G, Beeckman T, Wagner D, Ljung K, Fleck C, Weijers D. Plant development. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science. 2014;345:1255215. doi: 10.1126/science.1255215. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jurgens G, Estelle M. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell. 2005;9:109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Dong J, MacAlister CA, Bergmann DC. BASL controls asymmetric cell division in Arabidopsis. Cell. 2009;137:1320–1330. doi: 10.1016/j.cell.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevensek S, Goussot M, Duroc Y, Christodoulidou A, Steyaert S, Schaefer E, Duvernois E, Grandjean O, Vantard M, Bouchez D, Pastuglia M. The Arabidopsis TRM1-TON1 interaction reveals a recruitment network common to plant cortical microtubule arrays and eukaryotic centrosomes (vol 24, pg 178, 2012) Plant Cell. 2012;27:1816–1816. doi: 10.1105/tpc.111.089748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. Cdc42 - the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- Evangelista M, Pruyne D, Amberg DC, Boone C, Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat Cell Biol. 2002;4:260–269. doi: 10.1038/ncb770. [DOI] [PubMed] [Google Scholar]
- Facette MR, Park Y, Sutimantanapi D, Luo A, Cartwright HN, Yang B, Bennett EJ, Sylvester AW, Smith LG. The SCAR/WAVE complex polarizes PAN receptors and promotes division asymmetry in maize. Nat Plants. 2015;1:14024. doi: 10.1038/nplants.2014.24. [DOI] [PubMed] [Google Scholar]
- Feraru E, Friml J. PIN polar targeting. Plant Physiol. 2008;147:1553–1559. doi: 10.1104/pp.108.121756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AP, Sozzani R. Uncovering the networks involved in stem cell maintenance and asymmetric cell division in the Arabidopsis root. Curr Opin Plant Biol. 2016;29:38–43. doi: 10.1016/j.pbi.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Freisinger T, Klunder B, Johnson J, Muller N, Pichler G, Beck G, Costanzo M, Boone C, Cerione RA, Frey E, Wedlich-Soldner R. Establishment of a robust single axis of cell polarity by coupling multiple positive feedback loops. Nat Commun. 2013;4:1807. doi: 10.1038/ncomms2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J. Auxin transport - shaping the plant. Curr Opin Plant Biol. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Geisler MJ, Deppong DO, Nadeau JA, Sack FD. Stomatal neighbor cell polarity and division in Arabidopsis. Planta. 2003;216:571–579. doi: 10.1007/s00425-002-0912-4. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CL, Cyr RJ. Use of abnormal preprophase bands to decipher division plane determination. J Cell Sci. 2001;114:599–607. doi: 10.1242/jcs.114.3.599. [DOI] [PubMed] [Google Scholar]
- Grill SW, Howard J, Schaffer E, Stelzer EH, Hyman AA. The distribution of active force generators controls mitotic spindle position. Science. 2003;301:518–521. doi: 10.1126/science.1086560. [DOI] [PubMed] [Google Scholar]
- Grunewald W, Friml J. The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J. 2010;29:2700–2714. doi: 10.1038/emboj.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol. 2005;169:127–138. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Wang Z, Yang Z. ROP/RAC GTPase: an old new master regulator for plant signaling. Curr Opin Plant Biol. 2004;7:527–536. doi: 10.1016/j.pbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Han X, Kumar D, Chen H, Wu S, Kim JY. Transcription factor-mediated cell-to-cell signalling in plants. J Exp Bot. 2014;65:1737–1749. doi: 10.1093/jxb/ert422. [DOI] [PubMed] [Google Scholar]
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007;21:1720–1725. doi: 10.1101/gad.1550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, Torii KU, Kakimoto T. Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves. Plant Cell Physiol. 2009;50:1019–1031. doi: 10.1093/pcp/pcp068. [DOI] [PubMed] [Google Scholar]
- Hazak O, Bloch D, Poraty L, Sternberg H, Zhang J, Friml J, Yalovsky S. A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol. 2010;8:e1000282. doi: 10.1371/journal.pbio.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler MG, Hamant O, Krupinski P, Uyttewaal M, Ohno C, Jonsson H, Traas J, Meyerowitz EM. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. Plos Biol. 2010:8. doi: 10.1371/journal.pbio.1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101:555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- Humphries JA, Vejlupkova Z, Luo A, Meeley RB, Sylvester AW, Fowler JE, Smith LG. ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. Plant Cell. 2011;23:2273–2284. doi: 10.1105/tpc.111.085597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L, Gray JE. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr Biol. 2009;19:864–869. doi: 10.1016/j.cub.2009.03.069. [DOI] [PubMed] [Google Scholar]
- Inaba M, Yamashita YM. Asymmetric stem cell division: precision for robustness. Cell Stem Cell. 2012;11:461–469. doi: 10.1016/j.stem.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Jewaria PK, Hara T, Tanaka H, Kondo T, Betsuyaku S, Sawa S, Sakagami Y, Aimoto S, Kakimoto T. Differential effects of the peptides Stomagen, EPF1 and EPF2 on activation of MAP kinase MPK6 and the SPCH protein level. Plant Cell Physiol. 2013;54:1253–1262. doi: 10.1093/pcp/pct076. [DOI] [PubMed] [Google Scholar]
- Jones NC, Tyner KJ, Nibarger L, Stanley HM, Cornelison DD, Fedorov YV, Olwin BB. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J Cell Biol. 2005;169:105–116. doi: 10.1083/jcb.200408066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G, Mayer U, Ramon A, Berleth TR, Miséra ST. Genetic analysis of pattern formation in the Arabidopsis embryo. Development. 1991;113:27–38. [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Katsuta J, Shibaoka H. Inhibition by kinase inhibitors of the development and the disappearance of the preprophase band of microtubules in tobacco BY-2 cells. J Cell Sci. 1992;103:397–405. [Google Scholar]
- Kawamura E, Himmelspach R, Rashbrooke MC, Whittington AT, Gale KR, Collings DA, Wasteneys GO. MICROTUBULE ORGANIZATION 1 regulates structure and function of microtubule arrays during mitosis and cytokinesis in the Arabidopsis root. Plant Physiol. 2006;140:102–114. doi: 10.1104/pp.105.069989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik A, Ehrhardt DW, Kirik V. TONNEAU2/FASS Regulates the Geometry of Microtubule Nucleation and Cortical Array Organization in Interphase Arabidopsis Cells. Plant Cell. 2012;24:1158–1170. doi: 10.1105/tpc.111.094367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomitsu T, Cheeseman IM. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat Cell Biol. 2012;14:311–317. doi: 10.1038/ncb2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN. Asymmetric segregation of Numb and Prospero during cell division. Nature. 1995;377:624–627. doi: 10.1038/377624a0. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kajita R, Miyazaki A, Hokoyama M, Nakamura-Miura T, Mizuno S, Masuda Y, Irie K, Tanaka Y, Takada S, Kakimoto T, Sakagami Y. Stomatal density is controlled by a mesophyll-derived signaling molecule. Plant Cell Physiol. 2010;51:1–8. doi: 10.1093/pcp/pcp180. [DOI] [PubMed] [Google Scholar]
- Kozlowski C, Srayko M, Nedelec F. Cortical microtubule contacts position the spindle in C. elegans embryos. Cell. 2007;129:499–510. doi: 10.1016/j.cell.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Kozubowski L, Saito K, Johnson JM, Howell AS, Zyla TR, Lew DJ. Symmetry-breaking polarization driven by a Cdc42p GEF-PAK complex. Curr Biol. 2008;18:1719–1726. doi: 10.1016/j.cub.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampard GR, Macalister CA, Bergmann DC. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science. 2008;322:1113–1116. doi: 10.1126/science.1162263. [DOI] [PubMed] [Google Scholar]
- Lau OS, Bergmann DC. Stomatal development: a plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development. 2012;139:3683–3692. doi: 10.1242/dev.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Davies KA, Chang J, Adrian J, Rowe MH, Ballenger CE, Bergmann DC. Direct roles of SPEECHLESS in the speci3cation of stomatal self-renewing cells. Science. 2014;345:1605–1609. doi: 10.1126/science.1256888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Liu XG, Yang KZ, Chen XL, Zou JJ, Wang HZ, Wang M, Vanneste S, Morita M, Tasaka M, Ding ZJ, Friml J, Beeckman T, Sack F. Auxin transport and activity regulate stomatal patterning and development. Nat Commun. 2014;5:3090. doi: 10.1038/ncomms4090. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hnilova M, Maes M, Lin YC, Putarjunan A, Han SK, Avila J, Torii KU. Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature. 2015;522:439–443. doi: 10.1038/nature14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Smet W, Brunoud G, Yoshida S, Vernoux T, Weijers D. Reporters for sensitive and quantitative measurement of auxin response. Nat Methods. 2015;12:207–210. doi: 10.1038/nmeth.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka E, Herrmann A, Mueller S. Mechanisms of plant cell division. Wiley interdisciplinary reviews. Dev Biol. 2015;4:391–405. doi: 10.1002/wdev.186. [DOI] [PubMed] [Google Scholar]
- Lu MS, Johnston CA. Molecular pathways regulating mitotic spindle orientation in animal cells. Development. 2013;140:1843–1856. doi: 10.1242/dev.087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JR, Sack FD. Polar development of preprophase bands and cell plates in the Arabidopsis leaf epidermis. Plant J: Cell Mol Biol. 2012;69:501–509. doi: 10.1111/j.1365-313X.2011.04809.x. [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Roeder A, Parmenter D, Somerville C. A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell. 2004;116:109–119. doi: 10.1016/s0092-8674(03)01067-5. [DOI] [PubMed] [Google Scholar]
- MacAlister CA, Ohashi-Ito K, Bergmann DC. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature. 2007;445:537–540. doi: 10.1038/nature05491. [DOI] [PubMed] [Google Scholar]
- Malcos JL, Cyr RJ. An ungrouped plant kinesin accumulates at the pre-prophase band in a cell cycle-dependent manner. Cytoskeleton. 2011;68:247–258. doi: 10.1002/cm.20508. [DOI] [PubMed] [Google Scholar]
- Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos JL, Bergmann DC. Convergence of stem cell behaviors and genetic regulation between animals and plants: insights from the Arabidopsis thaliana stomatal lineage. F1000prime Rep. 2014;6:53. doi: 10.12703/P6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineyuki Y. The preprophase band of microtubules: its function as a cytokinetic apparatus in higher plants. In: Kwang WJ, editor. International Review of Cytology. Academic Press Inc; San Diego, CA, USA: 1999. pp. 1–49. [Google Scholar]
- Moseley JB, Goode BL. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol Mol Biol Rev. 2006;70:605–645. doi: 10.1128/MMBR.00013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Beck M, Mettbach U, Komis G, Hause G, Menzel D, Samaj J. Arabidopsis MPK6 is involved in cell division plane control during early root development, and localizes to the pre-prophase band, phragmoplast, trans-Golgi network and plasma membrane. Plant J: Cell Mol Biol. 2010;61:234–248. doi: 10.1111/j.1365-313X.2009.04046.x. [DOI] [PubMed] [Google Scholar]
- Muller S, Jurgens G. Plant cytokinesis-No ring, no constriction but centrifugal construction of the partitioning membrane. Semin Cell Dev Biol. 2015;53:10–18. doi: 10.1016/j.semcdb.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Murata T, Wada M. Effects of centrifugation on preprophase-band formation in Adiantum protonemata. Planta. 1991;183:391–398. doi: 10.1007/BF00197738. [DOI] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. Control of stomatal distribution on the Arabidopsis leaf surface. Science. 2002;296:1697–1700. doi: 10.1126/science.1069596. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 2001;413:307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Smith RS, Mandel T, Robinson S, Kimura S, Boudaoud A, Kuhlemeier C. Mechanical regulation of auxin-mediated growth. Curr Biol. 2012;22:1468–1476. doi: 10.1016/j.cub.2012.06.050. [DOI] [PubMed] [Google Scholar]
- Nance J, Zallen JA. Elaborating polarity: PAR proteins and the cytoskeleton. Development. 2011;138:799–809. doi: 10.1242/dev.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Saegusa M, Iwamoto K, Oda Y, Katayama H, Kojima M, Sakakibara H, Fukuda H. A bHLH complex activates vascular cell division via cytokinin action in root apical meristem. Curr Biol. 2014;24:2053–2058. doi: 10.1016/j.cub.2014.07.050. [DOI] [PubMed] [Google Scholar]
- Panteris E, Apostolakos P, Galatis B. Cytoskeletal asymmetry in Zea mays subsidiary cell mother cells: a monopolar prophase microtubule half-spindle anchors the nucleus to its polar position. Cell Motil Cytoskelet. 2006;63:696–709. doi: 10.1002/cm.20155. [DOI] [PubMed] [Google Scholar]
- Park HO, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev. 2007;71:48–96. doi: 10.1128/MMBR.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka JJ, Van Norman JM, Benfey PN. Symmetry breaking in plants: molecular mechanisms regulating asymmetric cell divisions in Arabidopsis. Cold Spring Harb Perspect Biol. 2009;1:a000497. doi: 10.1101/cshperspect.a000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietra S, Gustavsson A, Kiefer C, Kalmbach L, Horstedt P, Ikeda Y, Stepanova AN, Alonso JM, Grebe M. Arabidopsis SABRE and CLASP interact to stabilize cell division plane orientation and planar polarity. Nat Commun. 2013;4:2779. doi: 10.1038/ncomms3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Guo X, Dong J. Asymmetric cell division in plants: mechanisms of symmetry breaking and cell fate determination. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2290-2. http://dx.doi.org/10.1007/s00018-016-2290-2. [DOI] [PMC free article] [PubMed]
- Pillitteri LJ, Peterson KM, Horst RJ, Torii KU. Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. Plant Cell. 2011;23:3260–3275. doi: 10.1105/tpc.111.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinkiewicz P. Inherent randomness of cell division patterns. Proc Natl Acad Sci USA. 2011;108:5933–5934. doi: 10.1073/pnas.1103212108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Humphries JA, Smith LG. Determination of symmetric and asymmetric division planes in plant cells. Annu Rev Plant Biol. 2011;62:387–409. doi: 10.1146/annurev-arplant-042110-103802. [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Wright AJ, Muller S. The role of the cytoskeleton and associated proteins in determination of the plant cell division plane. Plant J: Cell Mol Biol. 2013;75:258–269. doi: 10.1111/tpj.12177. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12:507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert HS, Grones P, Stepanova AN, Robles LM, Lokerse AS, Alonso JM, Weijers D, Friml J. Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr Biol. 2013;23:2506–2512. doi: 10.1016/j.cub.2013.09.039. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Sahlin P, Jonsson H. A modeling study on how cell division affects properties of epithelial tissues under isotropic growth. PloS One. 2010;5:e11750. doi: 10.1371/journal.pone.0011750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Honing H, Nishioka M, Uehara Y, Takahashi M, Fujisawa N, Saji K, Seki M, Shinozaki K, Jones MA, Smirnoff N, Okada K, Wasteneys GO. Armadillo repeat-containing kinesins and a NIMA-related kinase are required for epidermal-cell morphogenesis in Arabidopsis. Plant J : Cell Mol Biol. 2008;53:157–171. doi: 10.1111/j.1365-313X.2007.03327.x. [DOI] [PubMed] [Google Scholar]
- Samaj J, Baluska F, Voigt B, Schlicht M, Volkmann D, Menzel D. Endocytosis, actin cytoskeleton, and signaling. Plant Physiol. 2004;135:1150–1161. doi: 10.1104/pp.104.040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth A, Moller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jurgens G, Weijers D. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature. 2010;464:913–916. doi: 10.1038/nature08836. [DOI] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science. 2005;309:290–293. doi: 10.1126/science.1109710. [DOI] [PubMed] [Google Scholar]
- Siller KH, Cabernard C, Doe CQ. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat Cell Biol. 2006;8:594–600. doi: 10.1038/ncb1412. [DOI] [PubMed] [Google Scholar]
- Slaughter BD, Smith SE, Li R. Symmetry breaking in the life cycle of the budding. Yeast Csh Perspect Biol. 2009:1. doi: 10.1101/cshperspect.a003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smekalova V, Luptovciak I, Komis G, Samajova O, Ovecka M, Doskocilova A, Takac T, Vadovic P, Novak O, Pechan T, Ziemann A, Kosutova P, Samaj J. Involvement of YODA and mitogen activated protein kinase 6 in Arabidopsis post-embryogenic root development through auxin up-regulation and cell division plane orientation. New Phytol. 2014;203:1175–1193. doi: 10.1111/nph.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG. Plant cell division: building walls in the right places. Nat Rev Mol Cell Biol. 2001;2:33–39. doi: 10.1038/35048050. [DOI] [PubMed] [Google Scholar]
- Smith SE, Rubinstein B, Mendes Pinto I, Slaughter BD, Unruh JR, Li R. Independence of symmetry breaking on Bem1-mediated autocatalytic activation of Cdc42. J Cell Biol. 2013;202:1091–1106. doi: 10.1083/jcb.201304180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Wharton RP. Drosophila brain tumor is a translational repressor. Genes Dev. 2001;15:762–773. doi: 10.1101/gad.870801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spana EP, Doe CQ. The prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in Drosophila. Development. 1995;121:3187–3195. doi: 10.1242/dev.121.10.3187. [DOI] [PubMed] [Google Scholar]
- Spinner L, Gadeyne A, Belcram K, Goussot M, Moison M, Duroc Y, Eeckhout D, De Winne N, Schaefer E, Van De Slijke E, Persiau G, Witters E, Gevaert K, De Jaeger G, Bouchez D, Van Damme D, Pastuglia M. A protein phosphatase 2A complex spatially controls plant cell division. Nat Commun. 2013;4:1863. doi: 10.1038/ncomms2831. [DOI] [PubMed] [Google Scholar]
- Sugano SS, Shimada T, Imai Y, Okawa K, Tamai A, Mori M, Hara-Nishimura I. Stomagen positively regulates stomatal density in Arabidopsis. Nature. 2010;463:241–244. doi: 10.1038/nature08682. [DOI] [PubMed] [Google Scholar]
- Ten Hove CA, Heidstra R. Who begets whom? Plant cell fate determination by asymmetric cell division. Curr Opin Plant Biol. 2008;11:34–41. doi: 10.1016/j.pbi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Torii KU. Mix-and-match: ligand-receptor pairs in stomatal development and beyond. Trends Plant Sci. 2012;17:711–719. doi: 10.1016/j.tplants.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz RA, Jurgens G. Mutations in the FASS gene uncouple pattern formation and morphogenesis in Arabidopsis development. Development. 1994;120:2967–2978. doi: 10.1242/dev.120.10.2967. [DOI] [PubMed] [Google Scholar]
- Troy A, Cadwallader AB, Fedorov Y, Tyner K, Tanaka KK, Olwin BB. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38alpha/beta MAPK. Cell Stem Cell. 2012;11:541–553. doi: 10.1016/j.stem.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twell D, Park SK, Hawkins TJ, Schubert D, Schmidt R, Smertenko A, Hussey PJ. MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat Cell Biol. 2002;4:711–714. doi: 10.1038/ncb844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- von Dassow G. Concurrent cues for cytokinetic furrow induction in animal cells. Trends Cell Biol. 2009;19:165–173. doi: 10.1016/j.tcb.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Wai SC, Schmidt T, Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J Cell Biol. 2004;166:889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Fuchs E. Oriented divisions, fate decisions. Curr Opin Cell Biol. 2013;25:749–758. doi: 10.1016/j.ceb.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods B, Kuo CC, Wu CF, Zyla TR, Lew DJ. Polarity establishment requires localized activation of Cdc42. J Cell Biol. 2015;211:19–26. doi: 10.1083/jcb.201506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Gallagher KL. Mobile protein signals in plant development. Curr Opin Plant Biol. 2011;14:563–570. doi: 10.1016/j.pbi.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Yang Z, Lavagi I. Spatial control of plasma membrane domains: ROP GTPase-based symmetry breaking. Curr Opin Plant Biol. 2012;15:601–607. doi: 10.1016/j.pbi.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Akatsuka M, Hoshino H, Kumagai F, Hasezawa S. Decision of spindle poles and division plane by double preprophase bands in a BY-2 cell line expressing GFP-tubulin. Plant Cell Physiol. 2005;46:531–538. doi: 10.1093/pcp/pci055. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Barbier de Reuille P, Lane B, Bassel, George W, Prusinkiewicz P, Smith, Richard S, Weijers D. Genetic control of plant development by overriding a geometric division rule. Dev Cell. 2014;29:75–87. doi: 10.1016/j.devcel.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang JY, He SB, Li L, Yang HQ. Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proc Natl Acad Sci USA. 2014;111:E3015–E3023. doi: 10.1073/pnas.1400542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Facette M, Humphries JA, Shen Z, Park Y, Sutimantanapi D, Sylvester AW, Briggs SP, Smith LG. Identification of PAN2 by quantitative proteomics as a leucine-rich repeat-receptor-like kinase acting upstream of PAN1 to polarize cell division in maize. Plant Cell. 2012;24:4577–4589. doi: 10.1105/tpc.112.104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:18830–18835. doi: 10.1073/pnas.0705874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang P, Shao W, Zhu JK, Dong J. The BASL polarity protein controls a MAPK signaling feedback loop in asymmetric cell division. Dev Cell. 2015;33:136–149. doi: 10.1016/j.devcel.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]