Abstract
Purpose
To determine the feasibility and safety of bilateral simultaneous vitreoretinal surgery in pediatric patients.
Design
International, multicenter, interventional, retrospective case series.
Participants
Patients 17 years of age or younger from 24 centers worldwide who underwent immediate sequential bilateral vitreoretinal surgery (ISBVS)—defined as vitrectomy, scleral buckle, or lensectomy using the vitreous cutter—performed in both eyes sequentially during the same anesthesia session.
Methods
Clinical history, surgical details and indications, time under anesthesia, and intraoperative and postoperative ophthalmic and systemic adverse events were reviewed.
Main Outcome Measures
Ocular and systemic adverse events.
Results
A total of 344 surgeries from 172 ISBVS procedures in 167 patients were included in the study. The mean age of the cohort was 1.3±2.6 years. Nonexclusive indications for ISBVS were rapidly progressive disease (74.6%), systemic morbidity placing the child at high anesthesia risk (76.0%), and residence remote from surgery location (30.2%). The most common diagnoses were retinopathy of prematurity (ROP; 72.7% [P < 0.01]; stage 3, 4.8%; stage 4A, 44.4%; stage 4B, 22.4%; stage 5, 26.4%), familial exudative vitreoretinopathy (7.0%), abusive head trauma (4.1%), persistent fetal vasculature (3.5%), congenital cataract (1.7%), posterior capsular opacification (1.7%), rhegmatogenous retinal detachment (1.7%), congenital X-linked retinoschisis (1.2%), Norrie disease (2.3%), and viral retinitis (1.2%). Mean surgical time was 143±59 minutes for both eyes. Higher ROP stage correlated with longer surgical time (P=0.02). There were no reported intraoperative ocular complications. During the immediate postoperative period, 2 eyes from different patients demonstrated unilateral vitreous haemorrhage (0.6%). No cases of endophthalmitis, choroidal hemorrhage, or hypotony occurred. Mean total anesthesia time was 203±87 minutes. There were no cases of anesthesia-related death, malignant hyperthermia, anaphylaxis, or cardiac event. There was 1 case of reintubation (0.6%) and 1 case of prolonged oxygen desaturation (0.6%). Mean follow-up after surgery was 103 weeks, and anatomic success and globe salvage rates were 89.8% and 98.0%, respectively.
Conclusions
This study found ISBVS to be a feasible and safe treatment paradigm for pediatric patients with bilateral vitreoretinal pathologic features when repeated general anesthesia is undesirable or impractical.
Bilateral involvement is common in pediatric vitreoretinopathies. Systemic aberrations often predispose both eyes to pathologic features, such as premature birth in retinopathy of prematurity (ROP), Wnt signaling defects in familial exudative vitreoretinopathy, and trauma in abusive head trauma (formerly shaken baby syndrome). Furthermore, pediatric vitreoretinal surgical patients present unique challenges. Many—especially premature infants—are systemically fragile with multiple life-threatening comorbidities. They are at high anesthesia risk, and repeated sessions of general anesthesia are undesirable, or occasionally simply not possible.1–3 Second, some pediatric vitreoretinopathies, such as ROP, are prone to rapid symmetrical bilateral progression. Delayed surgical intervention may decrease the likelihood of optimal outcome for the second eye, increasing the probability of life-long visual impairment or blindness.4 Third, relatively few centers specialize in the management of pediatric vitreoretinal surgical diseases.5 A single surgeon or surgical group may draw patients from several states in the United States, or several countries globally. Consequently, the financial challenges posed by out-of-state or out-of-country care with regard to treatment and travel may be prohibitive for repeated visits for a second eye. A single session of general anesthesia to repair both eyes addresses all 3 issues.
Although the rationale outlined just above may be compelling, bilateral same-day intraocular surgery is a controversial topic. Under most circumstances, surgeons stage bilateral surgery by intervening first in the eye in which visual potential is greater and is threatened more urgently, deferring the fellow eye for a separate surgical intervention days or weeks later. This is the standard of care for intraocular surgery in most countries, primarily because of concerns regarding potential complications such as endophthalmitis and toxic anterior segment syndrome.6–8 Interestingly, bilateral intravitreal injections are performed routinely in many practices, and injections have similar rates of endophthalmitis compared with intraocular surgeries.9,10
The debate is most mature as it relates to cataract surgery.6–8,11 The most updated nomenclature for this practice is immediate sequential bilateral cataract surgery (ISBCS). Numerous centers have published their experiences with reported benefits of faster visual recovery and time convenience.12–16 Immediate sequential bilateral cataract surgery in children also has been reported.17–19 However, immediate sequential bilateral vitreoretinal surgery (ISBVS) is addressed rarely in the literature,20 despite pediatric vitreoretinal surgeons anecdotally performing ISBVS for years. We propose ISBVS as a management paradigm for certain pediatric patients, not for convenience or faster recovery, but rather because the probability of optimal ophthalmic outcomes is increased, while systemic risk of morbidity and mortality and financial barriers to specialized surgical care are minimized.
Methods
This study was an international, multicenter, interventional, retrospective case series of pediatric patients (17 years of age or younger at the time of surgery) who underwent ISBVS, defined as vitrectomy, scleral buckle, or lensectomy using the vitreous cutter, performed in both eyes sequentially during the same anesthesia session. Intravitreal injections, indirect laser treatments, and cataract extraction without a vitreous cutter were excluded. Participants were identified via billing codes and surgical logs, with no limitation to the date of surgery. Institutional review board or ethics committee approval was obtained at each participating institution. The study complied with the Health Insurance Portability and Accountability Act of 1996 and adhered to the tenets of the Declaration of Helsinki.
Data collection included demographic information, diagnoses, clinical histories, indications for ISBVS, surgical details, precautions taken against cross-contamination between eyes, operative time, time under anesthesia, intraoperative and postoperative (≤30 days) ocular and systemic adverse events, anatomic and visual outcomes, and need for further surgeries. Snellen and decimal visual acuities were converted to logarithm of the minimum angle of resolution units for statistical analyses. The binomial test was used for single population categorical dependent variables, and the Spearman rank correlation coefficient was used for nonparametric correlation testing. Statistical tests were 2-tailed and significance was defined as P < 0.05. Stata software version 9.0 (StataCorp, LP, College Station, TX) was used for statistical analyses.
Results
We identified 167 patients who underwent 172 ISBVS sessions (5 patients underwent ISBVS twice), for a total of 344 vitreoretinal surgeries. The mean chronologic age of the cohort was 1.3±2.6 years (median, 17.7 weeks; range, 4.0 weeks–13.2 years). Other demographic data are presented in Table 1. Most surgeries in our collaborative group were performed in the United States and East Asia. The dates of surgery ranged from 2002 through 2015.
Table 1.
Demographics of Patients Undergoing Immediate Sequential Bilateral Pediatric Vitreoretinal Surgery
| Feature | No. of Eyes (%) |
|---|---|
| Age (yrs) | |
| Mean (range) | 1.3 (0.1–13.2) |
| Gender | |
| Male | 102 (61.1) |
| Female | 65 (38.9) |
| Race | |
| Asian | 79 (47.3) |
| White | 63 (37.7) |
| Black | 14 (8.4) |
| Latino | 10 (6.0) |
| Unknown | 1 (0.6) |
| Location of surgery | |
| United States | 87 (52.1) |
| East Asia | 67 (40.1) |
| South Asia | 7 (4.2) |
| United Kingdom | 6 (3.6) |
Indications
The nonexclusive indications (many patients had more than 1 indication) for ISBVS were rapidly progressive disease (74.6%), systemic morbidity placing the child at high risk for general anesthesia (76.0%), residence remote from location of surgery (30.2%), and limited financial resources (4.1%). The diagnoses of the patients undergoing ISBVS are summarized in Table 2. More than half of the surgeries were for patients with ROP (P < 0.01).
Table 2.
Indications and Surgical and Anesthesia Times for Immediate Sequential Bilateral Pediatric Vitreoretinal Surgery
| Diagnosis | No. of Eyes (%) | Mean Surgical Time (SD; Minutes) | Mean Anesthesia Time (SD; Minutes) |
|---|---|---|---|
| ROP | 250 (72.7) | 138 (60) | 203 (79) |
| Stage 3* | 12 (4.8) | 134 (40) | 177 (43) |
| Stage 4A | 111 (44.4) | 127 (46) | 194 (66) |
| Stage 4B | 56 (22.4) | 126 (69) | 212 (103) |
| Unspecified stage 4 | 5 (2.0) | — | — |
| Stage 5 | 66 (26.4) | 172 (70) | 219 (86) |
| FEVR | 24 (7.0) | 128 (68) | 188 (98) |
| Abusive head | 14 (4.1) | 113 (55) | 150 (52) |
| trauma | |||
| PFVS | 12 (3.5) | 111 (82) | 148 (74) |
| Norrie disease | 8 (2.3) | 182 (—) | 224 (—) |
| Congenital cataract | 6 (1.7) | 174 (131) | 214 (152) |
| PCO | 6 (1.7) | 50 (—) | 86 (—) |
| RRD | 6 (1.7) | 219 (—) | 267 (—) |
| CXLRS | 4 (1.2) | 105 (31) | 156 (75) |
| Viral retinitis† | 4 (1.2) | 138 (—) | 197 (—) |
| Other‡ | 10 (2.9) | 85 (64) | 106 (72) |
CXLRS =congenital X-linked retinoschisis; FEVR = familial exudative vitreoretinopathy; PCO = posterior capsular opacification; PFV = persistent fetal vasculature; ROP = retinopathy of prematurity; RRD= rhegmatogenous retinal detachment; SD = standard deviation; — = unavailable.
For vitreous hemorrhage.
Cytomegalovirus (2 eyes) and acute retinal necrosis (2 eyes), both with retinal detachment.
Microspherophakia with pupillary block (3 eyes), suprachoroidal hemorrhage in an eye with a history of microspherophakia after lens extraction (1 eye), unspecified retinal dysplasia (2 eyes), unspecified uveitis with tractional retinal detachment (2 eyes), and hemolytic uremic syndrome with tractional retinal detachment (2 eyes).
Focus on Retinopathy of Prematurity
Immediate sequential bilateral vitreoretinal surgery for ROP was performed in 250 eyes of 120 infants (Fig 1). The mean gestational age was 26.3±3.6 weeks (range, 22–38 weeks), and mean birth weight was 930.1±553.9 g (range, 308–3500 g). Prior laser photocoagulation, intravitreal anti–vascular endothelial growth factor treatment, or vitreoretinal surgery were performed in 63.2%, 15.6%, and 13.7% of eyes, respectively. The mean postmenstrual age at the time of ISBVS was 41.5±5.8 weeks (range, 37–50 weeks). The breakdown of the ROP staging is summarized in Table 2.
Figure 1.
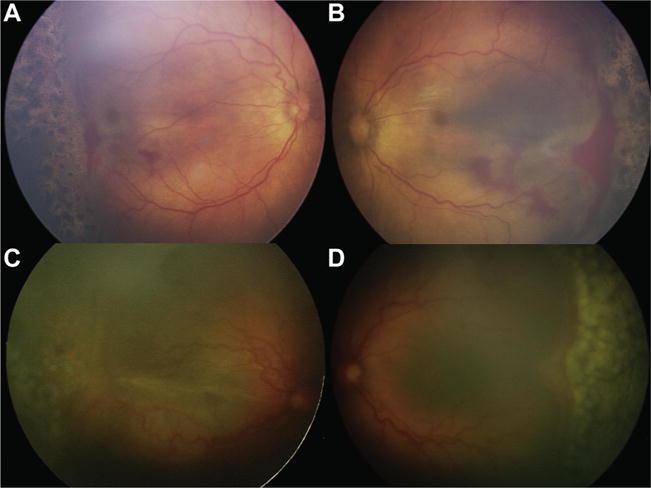
Fundus photographs illustrating immediate sequential bilateral vitreoretinal surgery (ISBVS) for infants with retinopathy of prematurity (ROP). A, B, Premature infant born at 25 weeks gestational age (GA) with a birth weight (BW) of 528 g who demonstrated type 1 ROP and underwent laser photocoagulation at 35 weeks postmenstrual age. The retinopathy continued to progress to stage 4A in both eyes 2 weeks subsequently, and the patient was referred for surgical treatment. Immediate sequential bilateral vitreoretinal surgery was recommended because of bilateral progressive retinopathy in both eyes in an infant with multiple comorbidities, including severe bronchopulmonary dysplasia, intraventricular hemorrhage, and anemia, placing him at high anesthesia risk. Twenty-five–gauge lens-sparing vitrectomy was performed in both eyes. The right eye underwent surgery first, and the left eye was prepared and draped as an independent procedure, using new instruments and intraocular fluids from different lot numbers. Total surgical time was 85 minutes, and time under anesthesia was 113 minutes. There were no ocular or systemic intraoperative or postoperative adverse events. C, D, A premature infant born at 24 weeks GA with a BW of 590 g progressed to stage 4B ROP in the right eye and stage 4A ROP in the left eye, despite 2 rounds of photocoagulation, and was referred for surgical treatment. This infant’s comorbidities included subarachnoid hemorrhage, bronchopulmonary dysplasia, apnea, anemia, patent ductus arteriosus, cirrhosis, and necrotizing enterocolitis. Twenty-five–gauge lens-sparing ISBVS was performed with the same precautions as the first patient without any ocular or systemic adverse events. Total surgical time was 121 minutes, and time under anesthesia was 155 minutes.
Surgeries and Outcomes
Surgeries that involved vitrectomy as the primary procedure comprised 82.3% of all surgeries: lensectomy in 7.0%, scleral buckle in 5.2%, combined scleral buckle and vitrectomy in 3.5%, diagnostic endoscopy in 1.2%, external drainage of subretinal fluid in 0.3%, and other procedures in 0.6%. Twenty-gauge instrumentation was used in 28.6%, 23-gauge instrumentation was used in 66.9%, 25-gauge instrumentation was used in 15.0%, and 27-gauge instrumentation was used in 1.0%. Only disposable instruments were used in 28.8%, only reusable instruments were used in 8.8%, and both disposable and reusable instruments were used in 62.4%. Intraoperative photography was obtained in 95.9% of surgeries. The second eye was reprepared and redraped in 95.9% of surgeries, and completely new sets of instruments were used in 79.8%. (We recommend that 100% of eyes be reprepared and redraped and that 100% of eyes have new instruments used. Please see “Discussion” below.)
Surgical and anesthesia times are summarized in Table 2. Surgical times were available for 129 ISBVS sessions (75%). The mean surgical time for both eyes was 143±59 minutes (median, 132 minutes; range, 36–300 minutes). Surgery lasting less than 120 minutes was possible in 36.4%, and surgery lasting less than 180 minutes was possible in 72.9%. Among infants with ROP, a higher stage of disease correlated with longer surgery (P =0.002). Additional vitreoretinal surgeries were required in 25.7% of eyes, of which 73.8% were unplanned interventions, whereas 26.2% were preplanned staged procedures. Mean follow-up since the date of ISBVS was 723 days (median, 415 days; range, 1–2594 days). Preoperative Snellen or decimal visual acuities were available in only 8 eyes (2.3%). Final Snellen or decimal acuities were available in 62 eyes (43.4%), among which the mean visual acuity was 1.1 logarithm of the minimum angle of resolution units (Snellen equivalent, 20/258). Physician-reported anatomic success was accomplished in 89.8%, and globe salvage at the time of last follow-up was achieved in 98.0%.
Ocular and Systemic Safety
The overall intraoperative and postoperative rates of adverse events were 0.0% and 0.6%, respectively (Table 3). After surgery, 2 different patients demonstrated unilateral vitreous hemorrhage (1 patient with ROP and 1 with retinal dysplasia and tractional membranes). Both patients did not require further intervention. Of note, there were no cases of either unilateral or bilateral endophthalmitis.
Table 3.
Safety of Pediatric Patients Undergoing Immediate Sequential Bilateral Vitreoretinal Surgery
| Complications | Yes | No | % |
|---|---|---|---|
| Ocular | |||
| Intraoperative | |||
| Scleral perforation (if scleral buckle) | 0 | 30 | 0.0 |
| Iatrogenic cataract | 0 | 336 | 0.0 |
| Iatrogenic retinal break | 0 | 336 | 0.0 |
| Choroidal hemorrhage | 0 | 336 | 0.0 |
| Postoperative (≤30 days) | |||
| Hypotony | 0 | 336 | 0.0 |
| Endophthalmitis | 0 | 336 | 0.0 |
| Vitreous hemorrhage* | 2 | 334 | 0.6 |
| Effusive choroidal detachment | 0 | 336 | 0.0 |
| Hemorrhagic choroidal detachment | 0 | 336 | 0.0 |
| Systemic | |||
| Intraoperative and postoperative (≤30 days) | |||
| Death | 0 | 168 | 0.0 |
| Malignant hyperthermia | 0 | 168 | 0.0 |
| Anaphylaxis | 0 | 168 | 0.0 |
| Iatrogenic pneumothorax | 0 | 168 | 0.0 |
| Aspiration pneumonitis | 0 | 168 | 0.0 |
| Prolonged desaturation | 1 | 167 | 0.6 |
| Embolism | 0 | 168 | 0.0 |
| Cardiac events | 0 | 168 | 0.0 |
| Other† | 1 | 167 | 0.6 |
Neither eye required a second surgery to clear the hemorrhage.
Intraoperative reintubation.
Anesthesia was provided via general endotracheal routes in 93.4% of cases and via laryngeal mask airways in 6.6%. No procedures were performed solely with local anesthesia with or without monitored anesthesia care. Duration of anesthesia was available for 101 ISBVS sessions (58.7%). The mean time per session was 203±87 minutes (median, 193 minutes; range, 42–462 minutes). The systemic, presumably anesthesia-related, complication rates are detailed in Table 3. The overall intraoperative and postoperative complication rates were 1.2% and 0.0%, respectively. One patient had prolonged oxygen desaturation during surgery, and 1 patient required reintubation. Both were ROP patients with multiple comorbidities, including bronchopulmonary dysplasia. Of note, there were no permanent morbidities and no deaths related to anesthesia, and the all-cause perioperative mortality rate was 0.0%.
Discussion
Immediate sequential bilateral vitreoretinal surgery is defined as vitreoretinal surgery in both eyes during the same anesthesia session. This is in contrast to delayed bilateral vitreoretinal surgery, where the second eye is staged days to weeks subsequently during a separate anesthesia session. Delayed bilateral vitreoretinal surgery assumes that a second anesthesia session is possible and that the fellow eye is not disadvantaged while waiting. Both assumptions are not guaranteed in often rapidly progressive pediatric vitreoretinopathies, for which surgical interventions are performed on the smallest, youngest, and sickest children at high anesthesia risk. Immediate sequential bilateral vitreoretinal surgery is performed in the interest of optimizing visual and anatomic outcomes while minimizing systemic morbidity and mortality (vide infra).
Numerous studies have reported on ISBCS,12,13,15,16 which is performed more commonly in countries where regulated resources may necessitate delays for elective surgeries.11 On the contrary, ISBCS is limited in the United States, where timely delayed second-eye surgeries are possible routinely, and concern as to the risk of bilateral endophthalmitis figures more prominantly.7,11 The inability to adjust targets based on first-eye results, financial penalization, and legal consequences also are concerns of United States cataract surgeons.8 The American Academy of Ophthalmology recognizes that ISBCS may be appropriate in rare instances.21 Nevertheless, ISBCS may continue to be a controversial topic because cataract surgery is elective, but pediatric retinal surgery often is not, particularly in a time-sensitive disease like ROP.
Indications for Immediate Sequential Bilateral Vitreoretinal Surgery
Our strongest recommendations for ISBVS are for the following patients: (1) preterm infants (2) with rapidly progressive, bilateral, symmetric, stage 4A or 4B ROP (3) who are at substantial risk for anesthesia-related morbidity and mortality and have fluctuating states of illness where a second window for surgery may not be possible, and (4) for whom the surgical intervention would be relatively brief for each eye. Other candidate pathologic features for ISBVS include stage 5 ROP, familial exudative vitreoretinopathy, abusive head trauma, and persistent fetal vasculature. Stage 5 ROP is a highly heterogeneous entity. Early stage 5 detachments have superior outcomes compared with chronically closed funnels22; however, complex stage 4B or stage 5 surgeries that take longer may not be good candidates for ISBVS. Patients with abusive head trauma may have serious comorbidities where multiple intubations may not be advisable, but familial exudative vitreoretinopathy and persistent fetal vasculature patients usually are not critically ill. Such diagnoses typically also do not have a quick tempo of progression. However, other factors may render ISBVS a practical consideration, including patients of families with limited means or limited surgeon availability. In all cases, the consent process should address thoroughly the risks and rationales for ISBVS, including bilateral endophthalmitis, to ensure that they are understood clearly by consenting family members.
Ocular Safety
The risk of postoperative endophthalmitis after vitrectomy is approximately 0.03% to 0.08%.23,24 We observed no unilateral or bilateral cases of endophthalmitis. Although the current series of 344 surgeries is underpowered to assess true endophthalmitis risk, a study of ISBVS powered for that purpose would be impractical because both ISBVS and postvitrectomy endophthalmitis are rare. Although we cannot assume that each eye of a ISBVS is independent of each other6 or that pediatric vitrectomies have similar rates of endophthalmitis as adults, an estimate can be made: the risk for bilateral endophthalmitis lies in the vicinity of 0.03%×0.03% to 0.08%×0.08%, or 0.000009% to 0.000064%. This is equivalent to 1 case in 1 500 000 to 10 000 000 ISBVS procedures. Even if the second eye has a 10-fold increased risk (0.3%–0.8%) of endophthalmitis, the risk for bilateral endophthalmitis would be 1 case in 150 000 to 1 000 000, which is much lower than the risk of death, as discussed below. Interestingly, bilateral intravitreal injections are performed routinely in many practices, and injections have similar to higher rates of endophthalmitis.9,10 Furthermore, although postvitrectomy endophthalmitis can be a devastating complication, it does not result universally in blindness if managed promptly and aggressively. In one large series of postvitrectomy endophthalmitis, 20/40 or better visual acuity was achieved in 75% of patients.23 The underlying pediatric vitreoretinopathy usually yields lower visual outcomes. However, fundamentally in pediatric ISBVS, the risk of bilateral endophthalmitis is weighed against the substantially higher risk of systemic morbidity and mortality.
Systemic Safety
Patients undergoing ROP surgery are perhaps at the highest anesthesia risk of any patient undergoing an ophthalmic surgical procedure. The risk of general anesthesia-related mortality in the general adult population is estimated to be 1 in 100 000.25,26 The rates are less established in children, but studies report rates of as high as 1 in 10 000.27,28 All-cause mortality is 10 to 100 times greater depending on the surgery and is even higher in neonates.27,28 Preterm infants are at further increased risk.1,29,30 Serious anesthesia complications occurred in 1 of 29 patients31 (3.4%) and 4 of 52 patients32 (7.7%) undergoing ROP surgery in 2 small studies.
The benefit of ISBVS is that 1 session of anesthesia is performed. Although the bilateral simultaneous procedure duration is longer than it would be for a unilateral procedure, the cumulative time for 2 separate sessions would be longer. Notwithstanding the issue of procedure length, because induction and extubation are the highest-risk phases of anesthesia, the risk is inherently greater for infants undergoing procedures in 2 separate sittings.33 Although controversial, there is mounting concern that multiple general anesthesia exposures in infants may lead to neurocognitive deficiencies.3,34
Intraoperative Recommendations
We recommend the following guidelines to minimize the risk of adverse events in pediatric patients undergoing ISBVS: that the surgeon and scrubbed assistants (1) should all rescrub, regown, and reglove with (2) new sterile protective equipment; (3) reprepare and redrape the second eye with new sterile protective equipment; (4) use a new set of instruments, (5) disposable instruments when practical or possible, and (6) a separate lot number for any intraocular fluids or medications; (7) work with an experienced pediatric anesthesia team and (8) an experienced scrub or nursing team; and that 2 sets of postoperative eye drops be used to avoid cross-contamination. Almost all ISBVS sessions in the current study underwent redraping of the second eye, but approximately one-fifth of the cases did not use new sets of instruments. These cases were mostly from non-Western nations, where practice patterns and logistics may differ. Nevertheless, we strongly recommend that new sets of instruments should be made available for all pediatric ISBVS and that all investigators agree to this new standard.
Study Limitations
There are several limitations aside from inherent biases of a retrospective study, including selection and recall biases. First, we lack a control arm because of impracticalities in identifying properly matched controls of rare conditions. Second, follow-up was variable because we did not institute a cutoff. The focus of this study was perioperative safety, not long-term outcomes. Third, visual acuities were not available for many patients. Again, this was not a focus of the study, and most patients were infants, and thus too young for accurate testing. Fourth, the definition of anatomic success varies between conditions, because the heterogeneous pathologic features in the study have unique surgical goals. Finally, we do not provide the denominator of total number of pediatric retina surgeries to illustrate how often ISBVS was used. In an effort to capture as many ISBVS surgeries as possible, we did not limit the date of surgery, which makes obtaining a denominator inaccurate. We recognize that ISBVS is still an uncommon approach, and children beyond infancy who meet the treatment criteria are few.
In conclusion, we endorse ISBVS as a treatment option when staged bilateral surgery unduly increases the risk for vision loss, mortality, or both and when 2 rounds of anesthesia are either undesirable or impractical.
Acknowledgments
Supported by unrestricted grants from Research to Prevent Blindness, New York, New York (to the Illinois Eye and Ear Infirmary, University of Illinois [R.V.P.C., F.Y.C., K.B.K.]); the Emory Eye Center, Emory School of Medicine (J.R.); the Department of Ophthalmology & Visual Sciences, University of Utah (M.E.H.); the National Institutes of Health, Bethesda, Maryland (grant nos.: EY014800 [core grant], R01 R01EY015130, and R01EY017011); the March of Dimes (grant no.: 6-FY13-75 [M.E.H.]); the Mukai Fund, Massachusetts Eye and Ear Infirmary (S.M.); the Ministry of Science and Technology, Taipei, Taiwan (grant nos.: NSC101-2314-B-182A-055-MY3 and MOST104-2314-B-182A-100-MY2); Chang Gung Memorial Hospital, Taoyuan, Taiwan (grant nos.: CMRPG3D0251 and CMRPG3D0522 [W.-C.W.]); the Heed Ophthalmic Foundation; and Ronald G. Michel’s Fellowship Foundation (Y.Y.). The funding organizations played no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Abbreviations and Acronyms
- ISBCS
immediate sequential bilateral cataract surgery
- ISBVS
immediate sequential bilateral vitreoretinal surgery
- ROP
retinopathy of prematurity
Footnotes
Presented at: Association of Pediatric Retina Specialists, 2014, Cabo, Mexico; Vail Vitrectomy, 2016, Vail, Colorado; and Club Jules Gonin, 2016, Bordeaux, France.
Financial Disclosure(s):
The author(s) have made the following disclosure(s): A.M.B.: Advisory board – Alcon
R.V.P.C.: Consultant – Alcon
P.J.F.: Consultant – Alcon
T.G.M.: Consultant – Alcon
S.C.W.: Advisory board – DORC International; Endo Optiks Beaver Visitec
P.J.F.: Consultant and Financial support – Alcon
T.G.M.: Consultant – Alcon
K.A.D.: Equity owner – FocusROP; Consultant – Spark Therapeutics; Synergetics
M.T.T.: Equity owner – FocusROP; Consultant – Synergetics
A.C.: Equity owner – FocusROP; Consultant – Spark Therapeutics; Synergetics
Author Contributions:
Conception and design: Yonekawa, Robinson, Thomas, Drenser, Trese, Capone
Analysis and interpretation: Yonekawa, Robinson, Thomas, Drenser, Trese, Capone
Data collection: Yonekawa, Wu, Kusaka, Robinson, Tsujioka, Kang, Shapiro, Padhi, Jain, Sears, Kuriyan, Berrocal, Quiram, Gerber, Chan, Jonas, Wong, Patel, Abbey, Spencer, Blair, Chang, Papakostas, Vavvas, Sisk, Ferrone, Henderson, Olsen, Hartnett, Chau, Mukai, Murray, Thomas, Meza, Drenser, Trese, Capone
Obtained funding: none
Overall responsibility: Yonekawa, Wu, Kusaka, Robinson, Tsujioka, Kang, Shapiro, Padhi, Jain, Sears, Kuriyan, Berrocal, Quiram, Gerber, Chan, Jonas, Wong, Patel, Abbey, Spencer, Blair, Chang, Papakostas, Vavvas, Sisk, Ferrone, Henderson, Olsen, Hartnett, Chau, Mukai, Murray, Thomas, Meza, Drenser, Trese, Capone
References
- 1.Steward DJ. Preterm infants are more prone to complications following minor surgery than are term infants. Anesthesiology. 1982;56:304–6. doi: 10.1097/00000542-198204000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Meza PA. Anesthesia for infants and children. In: Hartnett ME, Trese MT, Capone A Jr, et al., editors. Pediatric Retina. 2nd. Philadelphia: Lippincott Williams & Wilkins; 2014. pp. 577–85. [Google Scholar]
- 3.Sinner B, Becke K, Engelhard K. General anaesthetics and the developing brain: an overview. Anaesthesia. 2014;69:1009–22. doi: 10.1111/anae.12637. [DOI] [PubMed] [Google Scholar]
- 4.Repka MX, Tung B, Good WV, et al. Outcome of eyes developing retinal detachment during the Early Treatment for Retinopathy of Prematurity study. Arch Ophthalmol. 2011;129:1175–9. doi: 10.1001/archophthalmol.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fierson WM, Capone A. American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association of Certified Orthoptists. Tele-medicine for evaluation of retinopathy of prematurity. Pediatrics. 2015;135:e238–54. doi: 10.1542/peds.2014-0978. [DOI] [PubMed] [Google Scholar]
- 6.Schachat AP. Simultaneous bilateral endophthalmitis after immediate sequential bilateral cataract surgery: what’s the risk of functional blindness? Am J Ophthalmol. 2014;158:410–1. doi: 10.1016/j.ajo.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Li O, Kapetanakis V, Claoué C. Simultaneous bilateral endophthalmitis after immediate sequential bilateral cataract surgery: what’s the risk of functional blindness? Am J Ophthalmol. 2014;157:749–751.e1. doi: 10.1016/j.ajo.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Henderson BA, Schneider J. Same-day cataract surgery should not be the standard of care for patients with bilateral visually significant cataract. Surv Ophthalmol. 2012;57:580–3. doi: 10.1016/j.survophthal.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Fileta JB, Scott IU, Flynn HW. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina. 2014;45:143–9. doi: 10.3928/23258160-20140306-08. [DOI] [PubMed] [Google Scholar]
- 10.VanderBeek BL, Bonaffini SG, Ma L. The Association between intravitreal steroids and post-injection endophthalmitis rates. Ophthalmology. 2015;122:2311–2315.e1. doi: 10.1016/j.ophtha.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang DF. Simultaneous bilateral cataract surgery. Br J Ophthalmol. 2003;87:253–4. doi: 10.1136/bjo.87.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph N, David R. Bilateral cataract extraction in one session: report on five years’ experience. Br J Ophthalmol. 1977;61:619–21. doi: 10.1136/bjo.61.10.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arshinoff SA, Strube YNJ, Yagev R. Simultaneous bilateral cataract surgery. J Cataract Refract Surg. 2003;29:1281–91. doi: 10.1016/s0886-3350(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 14.Arshinoff SA, Bastianelli PA. Incidence of postoperative endophthalmitis after immediate sequential bilateral cataract surgery. J Cataract Refract Surg. 2011;37:2105–14. doi: 10.1016/j.jcrs.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Serrano-Aguilar P, Ramallo-Fariña Y, Cabrera-Hernández JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012;38:1734–42. doi: 10.1016/j.jcrs.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Lansingh VC, Eckert KA, Strauss G. Benefits and risks of immediately sequential bilateral cataract surgery: a literature review. Clin Experiment Ophthalmol. 2015;43:666–72. doi: 10.1111/ceo.12527. [DOI] [PubMed] [Google Scholar]
- 17.Totan Y, Bayramlar H, Çekiç Ö, et al. Bilateral cataract surgery in adult and pediatric patients in a single session. J Cataract Refract Surg. 2000;26:1008–11. doi: 10.1016/s0886-3350(00)00380-1. [DOI] [PubMed] [Google Scholar]
- 18.Dave H, Phoenix V, Becker ER, Lambert SR. Simultaneous vs sequential bilateral cataract surgery for infants with congenital cataracts: visual outcomes, adverse events, and economic costs. Arch Ophthalmol. 2010;128:1050–4. doi: 10.1001/archophthalmol.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magli A, Forte R, Rombetto L. Long-term outcome of primary versus secondary intraocular lens implantation after simultaneous removal of bilateral congenital cataract. Graefes Arch Clin Exp Ophthalmol. 2013;251:309–14. doi: 10.1007/s00417-012-1979-7. [DOI] [PubMed] [Google Scholar]
- 20.Shah PK, Narendran V, Kalpana N. Safety and efficacy of simultaneous bilateral 25-gauge lens-sparing vitrectomy for vascularly active stage 4 retinopathy of prematurity. Eye (Lond) 2015;29:1046–50. doi: 10.1038/eye.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Ophthalmology Cataract and Anterior Segment Preferred Practice Patterns Panel. Cataract in the adult eye preferred practice patterns. 2011 Available at: http://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-october-2011. Accessed January 8, 2016.
- 22.Jabbour NM, Eller AE, Hirose T, et al. Stage 5 retinopathy of prematurity. Prognostic value of morphologic findings. Ophthalmology. 1987;94:1640–6. doi: 10.1016/s0161-6420(87)33255-5. [DOI] [PubMed] [Google Scholar]
- 23.Oshima Y, Kadonosono K, Yamaji H, et al. Multicenter survey with a systematic overview of acute-onset endophthalmitis after transconjunctival microincision vitrectomy surgery. Am J Ophthalmol. 2010;150:716–725.e1. doi: 10.1016/j.ajo.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Govetto A, Virgili G, Menchini F, et al. A systematic review of endophthalmitis after microincisional versus 20-gauge vitrectomy. Ophthalmology. 2013;120:2286–91. doi: 10.1016/j.ophtha.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Arbous MS, Grobbee DE, van Kleef JW, et al. Mortality associated with anaesthesia: a qualitative analysis to identify risk factors. Anaesthesia. 2001;56:1141–53. doi: 10.1046/j.1365-2044.2001.02051.x. [DOI] [PubMed] [Google Scholar]
- 26.Lienhart A, Auroy Y, Péquignot F, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105:1087–97. doi: 10.1097/00000542-200612000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Flick RP, Sprung J, Harrison TE, et al. Perioperative cardiac arrests in children between 1988 and 2005 at a tertiary referral center: a study of 92,881 patients. Anesthesiology. 2007;106:226–37. doi: 10.1097/00000542-200702000-00009. [DOI] [PubMed] [Google Scholar]
- 28.van der Griend BF, Lister NA, McKenzie IM, et al. Postoperative mortality in children after 101,885 anesthetics at a tertiary pediatric hospital. Anesth Analg. 2011;112:1440–7. doi: 10.1213/ANE.0b013e318213be52. [DOI] [PubMed] [Google Scholar]
- 29.Coté CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy. A combined analysis. Anesthesiology. 1995;82:809–22. doi: 10.1097/00000542-199504000-00002. [DOI] [PubMed] [Google Scholar]
- 30.McCann ME, Soriano SG. Progress in anesthesia and management of the newborn surgical patient. Semin Pediatr Surg. 2014;23:244–8. doi: 10.1053/j.sempedsurg.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Aoyama K, Kondou Y, Suzuki Y, et al. Anesthesia protocols for early vitrectomy in former preterm infants diagnosed with aggressive posterior retinopathy of prematurity. J Anesth. 2010;24:633–8. doi: 10.1007/s00540-010-0937-y. [DOI] [PubMed] [Google Scholar]
- 32.Sinha R, Talawar P, Ramachandran R, et al. Perioperative management and post-operative course in preterm infants undergoing vitreo-retinal surgery for retinopathy of prematurity: A retrospective study. J Anaesthesiol Clin Pharmacol. 2014;30:258–62. doi: 10.4103/0970-9185.130050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Difficult Airway Society Extubation Guidelines Group. Popat M, Mitchell V, et al. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia. 2012;67:318–40. doi: 10.1111/j.1365-2044.2012.07075.x. [DOI] [PubMed] [Google Scholar]
- 34.Byrne MW, Casale P, Garzon M, et al. Pediatric surgeons and anesthesiologists expand the dialogue on the neurotoxicity question, rationale for early and delayed surgeries, and practice changes while awaiting definitive evidence. J Neurosurg Anesthesiol. 2014;26:391–5. doi: 10.1097/ANA.0000000000000123. [DOI] [PubMed] [Google Scholar]


