Abstract
The proteasome, a validated cellular target for cancer, is central for maintaining cellular homeostasis, while fatty acid synthase (FAS), a novel target for numerous cancers, is responsible for palmitic acid biosynthesis. Perturbation of either enzymatic machine results in decreased proliferation and ultimately cellular apoptosis. Based on structural similarities, we hypothesized that hybrid molecules of belactosin C, a known proteasome inhibitor, and orlistat, a known inhibitor of the thioesterase domain of FAS, could inhibit both enzymes. Herein, we describe proof-of-principle studies leading to the design, synthesis and enzymatic activity of several novel, β-lactone-based, dual inhibitors of these two enzymes. Validation of dual enzyme targeting through activity-based proteome profiling with an alkyne probe modeled after the most potent inhibitor, and preliminary serum stability studies of selected derivatives are also described. These results provide proof of concept for dual targeting of the proteasome and FAS-TE enabling a new approach for the development of drug-candidates with potential to overcome resistance.
Keywords: beta-lactones, inhibitor, activity-based protein profiling, structure-activity relationship studies, serum stability
Introduction
Intentional and selective polypharmacology has emerged as an exciting concept for rational drug design.[1] In cancer therapy, prototypical chemotherapeutic agents are generally based on the concept of “one-drug, one-target,” whereas selective polypharmacology targets two or more enzyme targets with a single drug. Selective polypharmacological drugs can be efficacious, a notable example being the FDA approved multikinase inhibitor lapatinib, which targets receptor tyrosine kinases (EGFR) and ErbB-2 (HER2), and is currently used for the treatment of metastatic breast cancer. [2] Drugs with single targets may fall victim to drug resistance due to mutations or upregulation of the target protein, whereas polypharmacological anticancer drugs are thought to evade drug resistance due to the need for cells to become resistant across multiple pathways through simultaneous protein/enzyme mutations. [3]
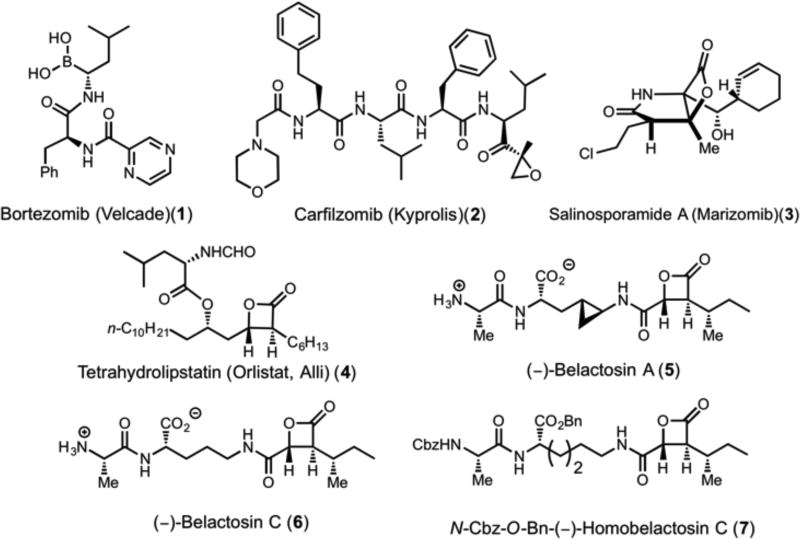
The human proteasome is responsible for the vast majority of intracellular protein degradation, and thus plays a central role in cellular homeostasis. [4] The 20S proteasome is the core particle (CP) of the 26S proteasome, and is composed of 28 subunits arranged as four stacked rings, in which each of the rings contains either seven unique α-chains or seven unique β-chains. The organization of the CP is α1-7b1-7b1-7α1-7, and possesses C2 symmetry [5]. Three of the 7 β-subunits are proteolytically active: β1, β2, and β5, and have been characterized to have peptide cleavage preferences corresponding to caspase-like (C-L, β1), trypsin-like (T-L, β2) and chymotrypsin-like (ChT-L, β5) activity[6]. The proteasome is a member of the N-terminal hydrolase super family, and all three catalytic subunits act to degrade substrates utilizing an N-terminal Thr1 residue. Due to accelerated cell cycles and metabolism in tumor cells, the CP is often upregulated to increase turnover rates of proteins. [7] This dependence on CP activity renders neoplastic cells highly susceptible to proteasome inhibition. In addition, proteasome inhibitors increase the level of tumor suppressors (e.g. cyclin kinase inhibitor p27) which induce tumor apoptosis without significantly affecting quiescent cells.[8] Thus, the proteasome CP has been extensively explored as a drug target against various types of cancer, and many classes of compounds have been found to inhibit proteasome function, both by covalently binding to Thr1 and also non-covalent inhibition [9]. The ChT-L site has been the main focus for the design of antineoplastic therapeutics, however, the T-L and C-L sites have also recently gained attention [10]. Two covalent inhibitors with preference for the ChT-L site have received FDA approval: the boronic acid, bortezomib (1, Velcade®), for the treatment of multiple myeloma and relapsed or refractory mantle cell lymphoma; and the epoxyketone carfilzomib (2, Kyprolis®), for the treatment of refractory multiple myeloma. A third promising class of proteasome inhibitors under development possess a β-lactone which covalently modifies Thr1O to generate an acyl-enzyme adduct.[11] An example is salinosporamide A (Marizomib, 3), which is a β-lactone containing proteasome inhibitor designated as an orphan drug for multiple myeloma[12] and is in Phase I/II clinical studies for the treatment of multiple myeloma, leukemia, and solid tumors (Figure 1).[13] Despite these advances, drug resistance to proteasome inhibitors through active site mutations and upregulation of ChT-L subunits, continues to necessitate the development of novel inhibitors for cancer therapy. [14]
Figure 1.
Representative structures of human or yeast 20S proteasome and fatty acid synthase inhibitors.
Fatty acid synthase (FAS) is a second vital enzyme which is upregulated in rapidly proliferating cancers, including breast, prostate, ovarian, and multiple myeloma. [15] FAS catalyzes the de novo synthesis of fatty acids to provide lipids for membrane formation and energy production via β-oxidation and lipid modification of proteins. FAS inhibition suppresses cell proliferation, adhesion, migration, and invasion. It also leads to suppression of genes involved in production of arachidonic acid and androgen hormones, both of which promote tumor progression.[16] Studies have shown that FAS inhibition induces apoptosis in various cancer cell lines,[17] synergizes with common anti-cancer therapies (HerceptinR and TaxolR) and in some cases reverses auto-resistance to those therapies, inhibits tumor angiogenesis, and FAS inhibitors may even act as chemopreventative agents for cancer making FAS an attractive target for further development.[15a, 18] Tetrahydrolipstatin (4, Orlistat®, Alli®, Xenical®) is a reduced form of the β-lactone-containing natural product lipstatin, and was approved by the FDA in 1999 for the treatment of obesity due to its ability to inhibit pancreatic lipase in the gut. Recently, however, it was determined that orlistat also has antitumor activity, through inhibition of the thioesterase domain of FAS, FAS-TE (IC50 = 1.35 µM).[19] X-ray analysis of the human FAS-TE bound with orlistat by Kridel and Lowther revealed the mechanism of inhibition to be covalent bond formation between Ser2308 of FAS-TE and the β-lactone of orlistat through acylation.[20] Poor solubility and poor oral bioavailability limit the practicality of orlistat as an antitumor drug, therefore it was crucial to overcome these problems by modifying the structure of orlistat to develop the next generation of FAS-TE inhibitors. [18e]
Our group previously reported the synthesis of orlistat and a series of analogs with FAS-TE inhibitory activity.[21] We also described the synthesis of (−)-belactosin C (6) and derivatives, [22] which along with (−)-belactosin A (5) and congeners possess inhibitory activity toward the 20S proteasome. X-ray studies of the belactosin C analog, N-CBz-O-Bn homobelactosin C (6), bound to the yeast 20S proteasome by Groll and coworkers[23] revealed that the Thr1O is acylated by the β-lactone moiety, and that the orientation of the inhibitor differs from other β-lactone-containing proteasome inhibitors such as omuralide, which may contribute to its selectivity for ChT-L subunits. A recent study demonstrated the potential of belactosin derivatives bearing minimal β-lactone fragments to have selectivity for the immunoproteasome.[24] Our concurrent studies of the belactosins and orlistat derivatives led to a hypothesis that hybrid structures of these β-lactones might have the potential to inhibit both the proteasome and FAS-TE. The common reactive acylating moiety in these inhibitors is a (3S,4R)-trans-disubstituted β-lactone providing a starting point for the design of analogs with dual inhibitory activity. Herein, we report our successful design and synthesis of dual inhibitors of the proteasome and FAS-TE, structure-activity studies (SAR) using fluorogenic assays for each enzyme, and verification of the ability of these dual inhibitors to target both FAS and the proteasome in HeLa cells using activity-based protein profiling (ABPP).
Results and Discussion
Initial dual inhibitors
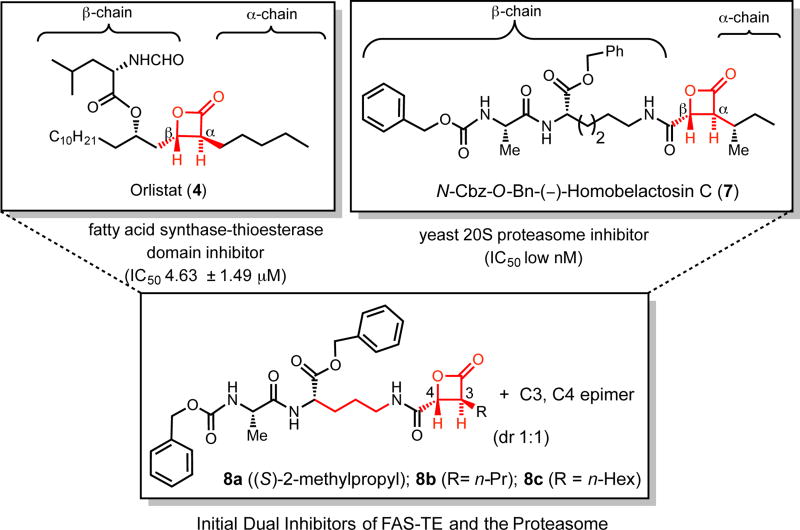
Given the presence of the common (3S, 4R)-trans-disubstituted-β-lactone (red) and similar β-side chains of orlistat and protected belactosins (e.g. N-Cbz-O-Bn-(−)-homobelactosin C (7)), we reasoned that β-lactones 8a–c that were originally synthesized as belactosin derivatives to provide SAR information regarding inhibition of the proteasome,[22a] might also have inhibitory activity toward FAS (Figure 2).
Figure 2.
Representative Initial dual inhibitors 8a–c displaying inhibitory activity toward both FAS-TE and the proteasome and design process based on structures of orlistat (4) and the belactosin C derivative 7.
These initial analogs 8a–c were synthesized as either a 1:1 (8a, 8b) or 1.3:1 (8c) mixture of C3, C4-diastereomers as a result of a non-diastereoselective, tandem Mukaiyama aldol-lactonization with a glyoxamide dipeptide β-side chain, as previously described.[22a] Since these diastereomers were not readily separable, they were assayed as diastereomeric mixtures using fluorogenic substrates for both FAS-TE and the proteasome as previously described. [18e],[25] All three compounds exhibited inhibition of the proteasome with 8c (1.3:1 mixture of N-CBz, O-Bn belactosin C[23b] and its C3,C4 diasteromer) showing the greatest activity as expected. Importantly, all three derivatives were more potent than orlistat toward FAS-TE with IC50 values ranging from ~0.16 to 4.0 µM.[26]
The inhibitory activity of these initial β-lactones verified our hypothesis that simultaneous inhibition of the proteasome and FAS was possible with a single compound by maintaining a (3S,4R)-trans-disubstituted β-lactone pharmacophore with a homobelactosin C dipeptide β-sidechain. Encouraged by these preliminary results, we set out to design and synthesize a series of belactosin C-orlistat hybrids with the great challenge of increasing potency toward both enzymes.
Design and synthesis of dual inhibitors of FAS and the proteasome
A crystal structure of bis-benzylhomobelactosin C bound to the yeast 20S proteasome suggests that the β-dipeptide side-chain plays a role in dictating which proteasomal active site will be targeted via interaction with both the S1 specificity pocket and the primed substrate binding site.[23a] Belactosins are the only class of proteasome inhibitors which bind to the primed region, whereas all other proteasome inhibitors bind to the non-primed sites.[23a] The cyclopropane ring of belactosin A was proposed to adopt a position which blocks the nucleophilic addition of water for enzyme turnover, and in conjunction with cyclopropane stereochemistry, forces the remainder of the compound into the primed site of the CP.
In the case of FAS, the co-crystal structure of FAS-TE with bound orlistat shows the 11-carbon β-chain of orlistat binding predominately in a hydrophobic channel, termed the specificity channel.[20] Given that orlistat possesses a relatively hydrophobic β-side chain also bearing an N-formyl amino ester while N-Cbz, O-Bn-(−)-belactosin C has a protected dipeptide β-chain, we reasoned that this dipeptide might display a similar binding mode and interact with both the specificity channel and interface cavity of FAS-TE. As described previously, the β-chain dipeptide binds to the primed site of the proteasome and optimization of binding interactions with primed sites has led to success in improving potency and selectivity of proteasome inhibitors.[27] Thus, it was reasonable to consider modifications of the β-dipeptide that might alter selectivity to the three proteolytically active sites while at the same time improving interactions with the specificity channel and interface cavity of FAS-TE. We utilized L-(S)-lysine rather than L-(S)-ornithine as the first amino acid of the β-dipeptide side-chain resulting in a four methylene unit spacer rather than three and protection of the terminal nitrogen with a carbobenzyloxy group and the lysine carboxylic acid as the benzyl ester was also maintained in all derivatives given the high potency of N-Cbz, O-Bn homobelactosin C toward the proteasome reported previously.[23a] On the other hand, less is known about the role the small, hydrophobic sec-butyl β-chain might play in binding to the proteasome but the C3-n-hexyl group of orlistat binds to the shortchain pocket in FAS-TE. As described above, replacement of the β-sec-butyl chain with an α-n-hexyl group was tolerated by the proteasome and given the structure of orlistat, we elected to maintain this hydrophobic α-chain in planned derivatives for this study. Both the belactosins and orlistat, bear the same relative (trans) and absolute configuration (3S, 4R) at C2 and C3 of the β-lactone so this was maintained in most derivatives, however the enantiomeric (3R, 4S)-trans-β-lactone was also investigated.
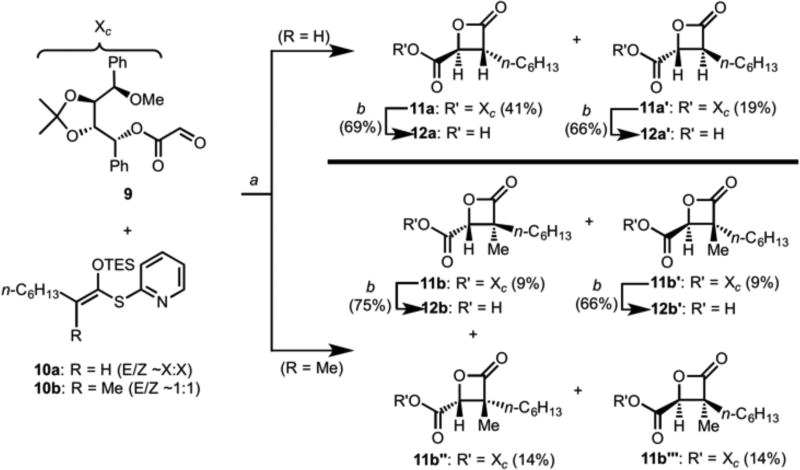
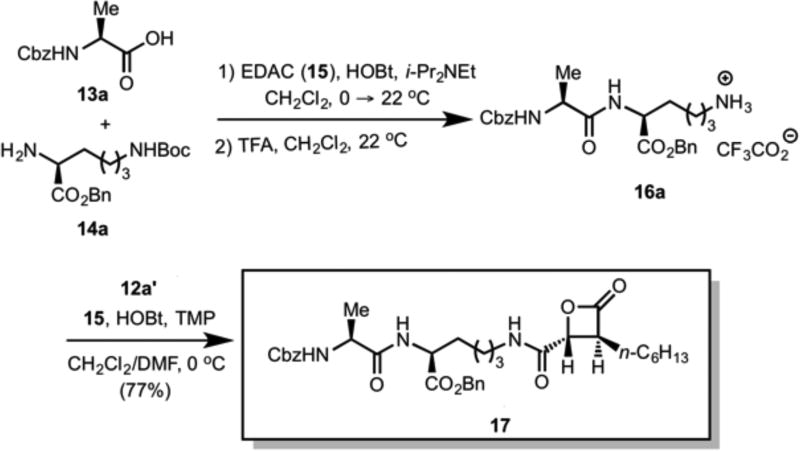
To synthesize the targeted belactosin-orlistat hybrids, we utilized our previously reported strategy toward (−)-belactosin C.[22a] Synthesis of the disubstituted-β-lactones was enabled through a chiral auxiliary-based, tandem Mukaiyama aldol-lactonization (TMAL) process between glyoxylate ester 9 and the silyl ketene acetal 10a to deliver the trans-β-lactone esters 11a and 11b’ (Scheme 1). The tartrate-derived chiral auxiliary, while not significantly impacting the absolute configuration of the newly created C2, C3 stereocenters (dr ~2:1) during the TMAL process, served to facilitate separation of diastereomers 11a/11a’ produced. The two were separated and underwent hydrogenolysis to cleave the chiral and auxiliary and enabled exploration of the enantiomeric trans-β-lactone for SAR purposes. In a similar manner, the α-methyl β-lactone esters 11b, 11b’, 11b”, and 11b’” were synthesized and despite the absence of diastereoselectivity, separation of the diastereomers was again possible and subsequent deprotection of the two trans-diastereomers allowed access to the α-methyl β-lactone acids 11b and 11b’. A series of dipeptides, represented by dipeptide amine 16a, were coupled to the β-lactone carboxylic acids, e.g. 11b, to deliver the dipeptide β-lactone 17 (Scheme 2). In this manner, a series of potential dual inhibitors 17–27 were synthesized through variations of the dipeptide and β-lactone fragments.
Scheme 1.
Synthesis of disubstituted α-lactone acids 12a and 12a’ and α-methyl-β-lactone acids 12b and 12b’ employing a chiral auxiliary-based, tandem Mukaiyama aldol-β-lactonization. (a) ZnCl2, CH2Cl2, 23 °C, 30 min (R = H) or 15 h (R = Me); (b) H2, Pd/C (10% w/w), THF.
Scheme 2.
Representative coupling strategy for synthesis of dual inhibitors as shown for dipeptide β-lactone 17.
Enzymatic Assays: Inhibition of the 20S Proteasome and FAS-TE
With the new analogs in hand, we performed an in vitro enzyme assay to determine their potency as inhibitors of the proteasome and FAS-TE. Both assays measure the rate of hydrolysis of fluorogenic substrates in the presence and absence of inhibitors (see SI for details). In the case of the proteasome, the assay was run three times with a different substrate specific for each of its catalytic sites; chymotrypsin-like (ChT-L(b5)), caspase-like (C-L(b1)), or trypsin-like (T-L(b2)). As summarized in Table 1, the 20S proteasome inhibition assay reveals that all analogs have selectivity for the ChT-L proteasomal subunit, with IC50 values ranging from 0.22 – 3.4 µM. Inhibition is also seen against the C-L subunits with generally higher IC50 values of 2.1–18 µM, however, no inhibition was seen against the TL subunit. Orlistat, used as a control for the FAS-TE assay, showed no inhibition against any of the proteasome catalytic sites at concentrations up to 100 µM (Table 1, entry 1). All of the analogs tested inhibited FAS-TE in the range of ~1.2 – 9.6 µM. The most potent dual inhibitor when considering inhibitory activity toward both enzymes was dipeptide β-lactone 21, which exhibited an IC50 value of 1.50 ± 0.28 µM against FAS-TE, which was 3X more potent compared to orlistat (IC50 4.63 ± 1.49 µM), while also having an IC50 of 0.37 ± 0.01 against the proteasome with high selectivity for ChT-L sites. A more detailed discussion of SAR established from these derivatives is provided below with systematic and sequential changes made in derivatives 18–33 highlighted (aqua) in Table 1.
Table 1.
Structure-activity relationships of novel orlistat-belactosin hybrids against the three proteolytic sites of the human 20S proteasome, human FAS-TE, and HeLa cells.a
| entry | compound | chymotrysin- like (ChT-L) IC50 (µM) |
caspase-like (C-L) IC50 (µM) |
trypsin-like (T-L) IC50 (µM) |
FAS-TE IC50 (µM) |
HeLa Cell Growth IC50 (µM) |
|---|---|---|---|---|---|---|
| 1 |

|
>100 | >100 | >100 | 4.63 ± 1.49 | ND[a] |
| 2 |

|
0.50 ± 0.06 | 3.03 ± 0.05 | >100 | 3.17 ± 0.17 | 62.5 |
| 3 |

|
0.57 ± 0.03 | 3.51 ± 0.04 | >100 | 9.56 ± 0.58 | 51.1 |
| 4 |

|
0.70 ± 0.05 | 2.16 ± 0.05 | >100 | 4.35 ± 1.11 | ND[a] |
| 5 |
|
0.51 ± 0.01 | 2.81 ± 0.24 | >100 | 4.81 ± 1.84 | ND[a] |
| 6 |

|
0.37 ± 0.01 | > 100 | >100 | 1.50 ± 0.28 | 45.8 |
| 7 |

|
0.51 ± 0.02 | 7.93 ± 0.21 | >100 | 5.55 ± 1.33 | ND[a] |
| 8 |

|
2.71 ± 0.17 | >100 | >100 | 12.6 ± 0.6 | ND[a] |
| 9 |

|
1.38 ±0.03 | 8.72 ± 0.05 | >100 | 8.37 ± 0.17 | ND[a] |
| 10 |

|
2.43 ± 0.25 | 3.74 ± 0.04 | > 100 | 2.61 ± 0.94 | 10.0 |
| 11 |

|
2.18 ± 0.08 | 3.98 ± 0.63 | > 100 | 3.11 ±0.75 | 37.5 |
| 12 |

|
3.39 ± 0.08 | 3.62 ± 0.08 | > 100 | 1.19 ± 0.42 | ND[a] |
| 13 |

|
0.26 ± 0.01 | > 100 | > 100 | 3.98 ± 0.74 | ND[a] |
ND = not determined
Variations of the dipeptide fragment
We first targeted variations of the dipeptide side-chain to probe the effects these changes would have on presumed binding to the S1 specificity pocket of the proteasome and the specificity (hydrophobic) channel of FAS-TE. Building on our preliminary data (vide infra), our initial derivative 17 maintained the α-n-hexyl side chain and the N-Cbz-(S)-Ala-O-Bn(S)-Lys dipeptide and served as an embarkation point for further structural modifications. Derivative 17 exhibited IC50 values of 0.50 ± 0.06 and 3.17 ± 0.17 µM toward the proteasome and FAS-TE, respectively (Table 1, entry 2). Removal of a methyl group in the dipeptide by replacement of alanine with glycine in derivative 18 did not impact proteasome inhibition, but led to an ~3-fold drop in potency against FAS-TE (Table 1, entry 3). Removing one methylene unit by replacing L-(S)-lysine with L-(S)-ornithine, as found in the belactosins, provided derivative 19 with similar activity to the ChT-L site of the proteasome and reduced activity toward FAS-TE, thus in all further derivatives (S)-lysine was retained in the dipeptide (Table 1, entry 2 vs. 4). Replacing the terminal benzyl carbamate of derivative 17 with a bromobenzoyl group provided derivative 20, which maintains the hydrophobicity and hydrogen-bonding capabilities, did not significantly alter inhibitory activity (Table 1, entry 5). We also investigated reversed-amide variants in the dipeptide wherein linkage is made through the carboxylic acid rather than the amine of lysine leading to derivative 21 bearing a N-Cbz substituent and terminal benzyl ester. This derivative exhibited the most potent activity toward FAS-TE (1.50 ± 0.28 µM) to this point, while also dramatically increasing specificity toward the ChT-L sites of the proteasome (Table 1, entry 6). A more rigid dipeptide was also explored through synthesis of the cyclic imide urea 22 which also lacked a hydrophobic benzyl substituent relative to other derivatives. The restricted flexibility did not greatly alter ChT-L activity, but reduced active site specificity with activity observed toward the C-L site of the proteasome (IC50 7.93 ± 0.21 µM) and reduced FAS-TE activity (Table 1, entry 7).
Effect of β-lactone absolute configuration
To probe the effect of the β-lactone relative and absolute configuration, analogs 23 and 24 were prepared from diastereomeric β-lactones (cf. 12, Scheme 1). Derivative 23 maintained the reverse amide variation in the dipeptide in analog 21, however the absolute configuration was inverted at both the α- and β-positions of the β-lactone to the 3R, 4S configuration. This alteration reduced the proteasome ChT-L and FAS-TE activity ~8-fold of analog 23 compared to the diastereomeric β-lactone 21 (Table 1, entry 6 vs entry 8). Maintaining the same β-lactone configuration but returning to the previous amide connectivity with a Gly-Lys dipeptide provided analog 24 (Table 1, entry 9) which regained some activity toward both enzymes but did not rival activity previously observed with the 3S, 4R configuration (cf. 18, Table 1, entry 3). The results validated the importance of the β-lactone configuration and is consistent with previous reports indicating the importance of both relative and absolute stereochemistry for β-lactone inhibitors of both FAS-TE and the proteasome. Overall, it is interesting to note that both the reversed amide variation and alternate β-lactone absolute configuration (Table 1, entries 6, 8) led to inhibitors that had complete selectivity for the ChT-L sites of the proteasome over the C-L site.
Effects of the C2-methyl group
Given the potential for hydrolysis of these enzyme inhibitors prior to reaching their targets, we explored introduction of an α-methyl substituent on the β-lactone to slow the rate of hydrolysis. Maintaining the best dipeptide, namely N-Cbz-(S)-Ala-O-Bn-(S)-Lys, we studied three diastereomeric β-lactones 25–27 prepared through a TMAL process with an α-methyl silylketene acetal (cf. 10b, Scheme 1; see SI for further details). In general, and not unexpectedly, the α-quaternary carbon reduced the potency toward both enzymatic targets (IC50 ~1–3 µM, Table 1, entries 10–12) given the expected slower rate of acylation of the active site serine and threonine of the FAS-TE and the proteasome, respectively. However, the stability of these α-quaternary carbon-containing β-lactones was improved in culture media leading to greater cytotoxicity in cellular assays (vida infra). Interestingly, β-lactone 27 with an α-quaternary carbon to the β-lactone but inverted C3 stereochemistry relative to the most potent dual inhibitor 21, rivaled the inhibitory activity against FAS-TE.
Cell cytotoxicity toward HeLa cells
Several of the more potent dual inhibitors, derivatives 17, 18, 21, 25, and 26, identified through enzymatic assays, were selected to assess their cytotoxicity toward HeLa cells using a colorimetric cell viability assay (MTT). All analogs showed IC50 values in the 10–60 µM range with the (3S, 4R)-α-disubstituted β-lactone 25 demonstrating the highest cytotoxicity with an IC50 of 10.0 µM, which was approximately 6X more potent than our initial lead inhibitor 17 (IC50 62.5 µM). While cell permeability or efflux pump effects could be responsible for the disparity observed between cell cytoxicity and enzymatic inhibition, we reasoned that the differential instability of these β-lactones was a more likely explanation. For example, the greatest cytotoxicity, which was observed with derivative 25, did not correspond to the best IC50 values in enzymatic assays, but this could be a reflection of the greater stability to hydrolysis of the α-disubstituted β-lactone. This led us to study the relative rates of hydrolysis of selected β-lactone inhibitors in the buffer and serum used in both enzymatic and cell-based activity studies.
Stability of Selected Inhibitors in Buffer with and without FBS
Given the disparity observed between enzymatic activity and whole cell cytotoxicity and the potential for hydrolysis of these β-lactone containing dual inhibitors, we studied the relative stability of two representative dual inhibitors in media employed in cytotoxicity assays. We chose β-lactone 21 which displayed the greatest potency in the enzymatic assays (IC50 0.37 ± 0.01 and 1.50 ± 0.28 µM vs ChT-L and FAS-TE, respectively) and β-lactone 25 which displayed the greatest cellular cytotoxicity ((IC50 10.0 µM) for these studies. The cell viability assay was run in DMEM buffer (pH 7.5) containing 5% fetal bovine serum (FBS) and for comparison, stability studies were also run in DMEM only. The half-lives of β-lactone inhibitors in buffer with and without added serum was studied through HPLC monitoring over time. In the case of α-monosubstituted β-lactone 21, the impact of added serum is dramatic leading to a change in half-life from an already brief 20 min in buffer to < 3 min with added serum (Table 2). However, the stability of the quaternary α,α-disubstituted-β-lactone 25 did not follow the same trend. The half-life of β-lactone 25 in DMEM buffer alone was nearly 3X that of the α-monosubstituted (t1/2 ~ 70 min). Futhermore, the presence of serum led to a dramatically extended half-life (t1/2 ~ 120 min) for this β-lactone and is consistent with improved stability of labile molecules often observed due to interaction of hydrophobic molecules with serum proteins protecting them from the somewhat basic medium.[28] Furthermore, the hydrolysis products of several α-monosubstituted-β-lactones were observed by LC-MS analysis despite the purity indicated by NMR analysis and this was observed most often with α-monosubstituted β-lactones as expected. Thus, it is reasonable to deduce that α,α-disubstituted β-lactones are generally better drug leads since their increased cytotoxicity may be a reflection of their greater stability to hydrolysis and would be expected to have a longer half-life in the blood stream. These findings are consistent with observations made in recent studies with belactosin A derivatives bearing α,α-disbustituted β-lactones with the exception that increased stability was not observed in the presence of human AB serum.[27a]
Table 2.
Serum stability studies (t1/2) with an α-monosubstituted 21 versus an α-disubstituted 25 β-lactone in DMEM with and without serum (FBS).a.
| β-lactone inhibitor [a] |
DMEM (pH = 7.5) |
DMEM + 5% FBS[b] |
|---|---|---|
| 21 | 20 | <3 |
| 25 | 70 | 120 |
Stability studies were run in triplicate. DMEM = Dulbecco’s modified eagle medium; FBS = fetal bovine serum.
Dual target validation via activity-based protein profiling
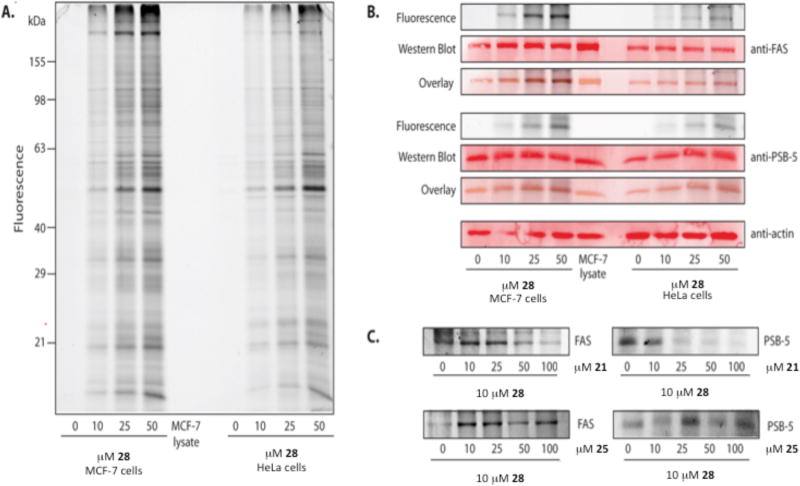
To determine if cytotoxicity observed in cancer cells might be due to dual inhibition of both FAS-TE and the proteasome, we conducted activity-based protein profiling (ABPP) experiments with cell lysates.[29] Previous whole cell ABPP studies led to orlistat as an inhibitor of FAS.[18h] We sought to verify that both FAS-TE and the proteasome were indeed labeled in a sensitive cancer cell line with the designed activity-based probe 28, which is based on the most potent dual inhibitor 21 in terms of enzymatic inhibition. Activity-based probe 28 includes an alkyne group in the para-position of the benzyl ester moiety for subsequent Sharpless-Hüisgen cycloaddition following incubation with a proteome. Given their potency in enzymatic and cell-based assays, dual inhibitors 21 and 25 were selected for competition experiments to validate selectivity if any.[30] Satisfyingly, probe 28 retained good activity compared to the parent inhibitor 21 with slightly improved activity to the ChT-L site of the proteasome and ~2.5X drop in activity toward FAS-TE suggesting no major adverse effects of the alkyne tag (Table 2). Both MCF-7 (breast adenocarcinoma) and HeLa (cervix adenocarcinoma) cells were incubated in situ with varying concentrations (0 → 50 µM) of probe 28 for 1 h at 37 °C. The labeling was followed by cell lysis and attachment of the TAMRA fluorophore under copper (I) catalyzed Sharpless-Hüisgen cycloaddition conditions.[31] SDS-PAGE and in-gel fluorescence scanning were employed to separate and detect labeled proteins, respectively (Figure 3A). Importantly, labeling of several protein targets could be observed with dominant bands at molecular weights of ca. 250, 50, and 20 kDa. To validate the identity of these proteins, we applied western blot analysis with anti-FAS and anti-CT-L (PSB-5) antibodies. The fluorescent bands at 250 kDa and 20 kDa overlapped with the corresponding antibody signals suggesting that these two protein bands represent the expected FAS and CT-L targets (Figure 3B). Finally, we first added the dual inhibitors 21 and 25 in various concentrations (0 → 100 µM) to MCF-7 cells prior to probe addition (Figure 3C). Subsequent labeling with probe 28 revealed a clear concentration dependent reduction in signal intensity only for 21 emphasizing that this inhibitor competes with the ABPP probe 28 for the same active sites. FAS was less sensitive to this competition (10-fold excess resulted in signal reduction) compared to ChT-L (2.5-fold excess resulted in signal reduction) which is in line with the lower IC50 of 21 for FAS. On the other hand, inhibitor 25 was not able to compete effectively with labeling by probe 28 even at 10-fold excess of the compound. This is likely due to slower reaction rates of this inhibitor with actives site nucleophiles (i.e. serine and threonine hydroxyl groups) due to the presence of the more hindered, α-disubstituted β-lactone and the short (30 min) pre-incubation time utilized prior to adding the ABPP probe 28. This observation is consistent with findings made during enzymatic assays with inhibitor 25 which required longer incubation times to achieve comparable IC50 values to other α-monosubstituted β-lactones and also the greater stability to buffer/serum (120 vs < 3 min) leading to greater whole cell cytotoxicity for inhibitor 25 compared to 21 (10.0 vs 45.8 µM, respectively).
Figure 3.
A) In situ ABPP labeling of MCF-7 and HeLa cells with increasing concentrations of alkyne ABPP probe 28. B) Identification of FAS and proteasome beta-5 by Western Blotting using specific anti-FAS and anti-PSB-5 antibodies. C) Competitive labeling with inhibitors 21, 25 and ABPP probe 28. (PSB-5: proteasome subunit beta type, FAS: Fatty acid synthase)
Conclusions
Several dual inhibitors of the proteasome and fatty acid synthase were designed and synthesized based on the original structures of orlistat and belactosin C. Exploiting the trans-disubstituted β-lactone pharmacophore which covalently binds to these two enzyme active sites and modifying the dipeptide, which was optimized based on analysis of the X-ray structures of proteasome-homobelactosin C and FAS-TE-orlistat complexes and structure-activity relationships, we successfully identified a dual inhibitor 21 with greater enzymatic inhibition to both targets (ChT-L: IC50 = 0.37 µM; FAS IC50 = 1.5 µM; HeLa cells IC50 = 45.8 µM) compared to our original lead compound 17. In addition, an α-disubstituted β-lactone dual inhibitor 25 displayed the highest potency in a cell-based cytotoxicity assay (HeLa cells IC50 = 10.0 µM) which along with serum stability studies suggest greater hydrolytic stability of this β-lactone with attendant improved cell-based activity. We confirmed targeting of the proteasome and FAS-TE in HeLa cells through activity based-protein profiling with an alkyne probe modeled after our best enzymatic inhibitor 21. These studies provide proof of concept for the potential of dual inhibition of FAS-TE and the proteasome with orlistat-belactosin hybrid molecules. In addition, it raises the possibility that previously described belactosin derivatives should be analyzed for cross-reactivity with FAS-TE. The ability to target an enzyme that is a validated target for chemotherapy, namely the proteasome, and an enzyme that has attracted great interest for cancer chemotherapy, namely FAS-TE, by a single molecule provides proof-of-principle for targeting both these enzymes with β-lactone-based dual inhibitors.
Experimental Section
General Information
All reactions were carried out under a nitrogen atmosphere in oven-dried glassware. Acetonitrile, dichloromethane, tetrahydrofuran, and methanol were purified by passage through activated molecular sieves or alumina (solvent drying system). DMF was dried over molecular sieves before use. All commercial reagents were used as received. 1H and 13C NMR spectra were recorded on INOVA-500. 1H NMR chemical shifts are reported as δ values in ppm relative to CDCl3 (7.26 ppm,), coupling constants (J) are reported in Hertz (Hz), and multipilicity follows convention. Unless indicated otherwise, deuterochloroform (CDCl3) served as an internal standard (77.2 ppm) for all 13C spectra. Flash column chromatography was performed using 60A silica gel as a stationary phase using a gradient solvent system (EtOAc/n-hexane as eluent unless specified otherwise). Mass spectra were obtained at the Center for Chemical Characterization and Analysis (Texas A&M University). Thin layer chromatography (TLC) was performed using glass-backed silica gel 60F254. LC/MS was carried out using an ion trap HPLC/MS instrument with an Agilent Poroshell EC-C-18 2.7 micron column (50 × 3.0 mm), eluting with a gradient of 5% acetonitrile/95% water → 95% acetonitrile/5% water over 12 min or 20 min. Signals were detected with UV 254 nm and MS ion trap (ionization modes: negative or positive APCI or positive or negative ESI, scan range 100–1000 a.m.u.). Synthetic details and characterization data for thioester (S3) required for the preparation of ketene acetal 10b are provided in the SI. Aldehyde 9 was prepared according to our previously described procedure.[22a] Dipeptide 15c was prepared according to the previously published procedure and data matched that previously reported.[22a] Commercially available protected amino acids, 13a, 13b, 13c, 13d, 13e, 14b were purchased and used as received. Protected amino acids, 14a[23b] and 4-ethynylbenzyl (tert-butoxycarbonyl)-L-alaninate[32] were prepared based on known procedures.
(E/Z)-2-((1-((Triethylsilyl)oxy)oct-1-en-1-yl)thio)pyridine (10a)
The synthesis was performed as previously described[26] and related to a published procedure.[22a] To a solution of S-(pyridin-2-yl) octanethioate[33] (5.30 g, 22.7 mmol, 1 equiv), DMF (2.11 mL, 27.2 mmol, 1.2 equiv) and Et3N (3.78 mL, 27.2, 1.2 equiv) in 100 mL of DCM was added LiHMDS (1M in THF, 52.3 mL, 52.3 mmol, 2.3 equiv) at −78 °C under N2. After stirring for 30 min, TESCl (7.69 mL, 45.4 mmol, 2 equiv) was added. The mixture was continued to stir at −78 °C for another 2 h. The reaction was quenched with pH 7 buffer (40 mL) and the organic layer was separated, dried over MgSO4 and concentrated. The residue was purified by flash chromatography (SiO2, hexanes:MTBE = 30:1) to give ketene acetal 10a as a yellow oil (5.1 g, 64%, ~13:1 ratio of E/Z geometrical isomers). Data provided for major E-olefin isomer: 1H NMR (500 MHz, CDCl3) δ 8.42-8.41 (m, 1H), 7.56-7.52 (m, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.01-6.98 (m, 1H), 5.38 (t, J = 7.3 Hz, 1H), 2.18 (q, J = 7.3 Hz, 2H), 1.44-1.23 (m, 11H), 0.90-0.84 (m, 9H), 0.67-0.62 (m, 6H). 13C NMR (75 MHz, CDCl3) δ 160.6, 149.6, 139.4, 136.7, 124.5, 121.6, 119.8, 31.8, 29.2, 27.0, 22.8, 14.3, 6.7 (2C), 6.0, 5.3 (2C).
Ketene acetal 10b
To a solution of LiHMDS in THF (1 M, 8.76 mL, 2.0 equiv) was added DMF (406 µL, 5.26 mmol, 1.2 equiv) at −78 °C followed by TESCl (742 µL, 4.38 mmol, 1 equiv). After stirring for 10 min, a solution of thioester S3 (1.10 g, 4.38 mmol, 1.0 equiv) in 40 mL of THF was added and the solution was stirred for another 5 h at the same temperature. The reaction was quenched by aq. satd. NH4Cl solution, and was extracted with MTBE (3 × 50 mL). The combined organic layers were washed with brine, dried over anhydrous MgSO4 and concentrated in vacuo. The residue was purified by flash chromatography (SiO2,hexanes:MTBE = 100:1→50:1) to give 10b as an ~1:1 mixture of E- and Z-isomers (1.01 g, 63%). 1H NMR (300 MHz, CDCl3) δ 8.43 (m, 1H), 7.54 (td, J = 7.9, 2.0 Hz, 1H), 7.26 (t, J = 7.9 Hz, 1H), 7.00 (m, 1H), 2.29 (m, 2H), 1.89/1.86 (s, 3H), 1.36-1.24 (m, 8H), 1.03-0.86 (m, 12H), 0.69-0.60 (m, 6H). 13C NMR (75 MHz, CDCl3) δ 200.9 181.7, 151.8, 150.3, 137.4, 130.4, 123.6, 105.2, 34.2, 31.8, 29.4, 27.3, 22.8, 17.7, 14.3, 7.0 (3C), 6.6 (3C).
Tandem Mukaiyama Aldol-Lactonization: Synthesis of β-lactone esters 11 and 11a’
ZnCl2 (1.47 g, 10.82 mmol, 2 equiv) was heated to fuse under vacuum and was cooled to ambient temperature, to which a solution of aldehyde 9 (2.08 g, 5.41 mmol, 1.0 equiv) in 50 mL of CH2Cl2 was added. After stirring at ambient temperature (23 °C) for 10 min, a solution of ketene acetal 10a (2.09 g, 5.95 mmol, 1.1 equiv) in 10 mL of CH2Cl2 was added. After 30 minutes the dark suspension was diluted with MTBE (100 mL), washed with H2O (5 mL) and brine (5 mL), and dried over MgSO4. After concentration the residue was purified by flash chromatography (SiO2, hexanes:MTBE = 5:1) to obtain a diastereomeric mixture of 11a and 11a’. The diastereomeric mixture was further separated by a second flash chromatography ((SiO2, hexanes:DCM:MTBE = 30:10:1) to give 11a (1.13 g, 41%) and 11a’ (530 mg, 19%) as colorless oils. Data for 11a: Rf = 0.33 (hexanes:MTBE = 5:1), [α]D23 −22 (c = 1.0, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.34-7.32 (m, 3H), 7.30-7.27 (m, 3H), 7.14-7.12 (m, 2H), 7.09-7.07 (m, 2H), 5.16 (d, J = 5.3 Hz, 1H), 4.60 (d, J = 4.4 Hz, 1H), 4.17 (dd, J = 6.8, 5.4 Hz, 1H), 4.04 (t, J = 6.7 Hz, 1H), 3.73 (d, J = 6.2 Hz, 1H), 3.63-3.59 (m, 1H), 3.16 (s, 3H), 1.93-1.78 (m, 2H), 1.46 (s, 3H), 1.45 (s, 3H), 1.45-1.41 (m, 2H), 1.35-1.26 (m, 6H), 0.90 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 169.1, 167.2, 137.3, 136.0, 129.1, 128.89 (2C), 128.83 (2C), 128.79, 127.8 (2C), 127.5 (2C), 111.1, 84.4, 80.8, 79.4, 77.1, 71.5, 57.7, 57.0, 31.6, 28.8, 27.84, 27.78, 27.5, 26.8, 22.7, 14.2. LRMS (ESI+): Calcd. For C30H38O7Na ([M+Na]+), 533.3. Found: 533.9. Data for 11a’: Rf = 0.33 (hexanes:MTBE = 5:1), [α]D23 = −0.50 (c = 1.0, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.34-7.32 (m, 3H), 7.31-7.27 (m, 3H), 7.15-7.11 (m, 4H), 5.19 (d, J = 5.4 Hz, 1H), 4.59 (d, J = 4.3 Hz, 1H), 4.18 (dd, J = 7.0, 5.4 Hz, 1H), 4.03 (dd, J = 7.0, 6.2 Hz, 1H), 3.71 (d, J = 6.2 Hz, 1H), 3.68 (ddd, J = 8.7, 6.6, 4.3 Hz, 1H), 3.15 (s, 3H), 1.94-1.79 (m, 2H), 1.47 (s, 3H), 1.45 (s, 3H), 1.34-1.26 (m, 8H), 0.89 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 169.2, 167.2, 137.3, 136.0, 129.1, 128.83 (2C), 128.80 (2C), 128.75, 127.7 (2C), 127.5 (2C), 111.0, 84.2, 80.7, 79.4, 77.1, 71.5, 57.6, 57.0, 31.5, 28.8, 27.8, 27.7, 27.4, 26.7, 22.6, 14.2. LRMS (ESI+): Calcd. For C30H38O7Na ([M+Na]+), 533.3. Found: 533.9.
Tandem Mukaiyama Aldol-Lactonization: Synthesis of β-lactone esters 11b, 11b’, 11b” and 11b’”
ZnCl2 (559 mg, 4.10 mmol, 2.5 equiv) was fused by heating under vacuum and then cooled to ambient temperature and then 0 °C. A solution of aldehyde 9 (630 mg, 1.64 mmol, 1.0 equiv) in 5 mL of CH2Cl2 was added. After stirring for 10 min, a solution of ketene acetal 10b (599 mg, 1.64 mmol, 1 equiv) in 5 mL of CH2Cl2 was added. The mixture was stirred at ambient temperature (23 °C) for 15 h and then the reaction was quenched by adding water (5 mL). The mixture was poured into 125 mL of MTBE and the organic layer was washed with brine. After drying over MgSO4, the organic solution was concentrated. The residue was purified by a flash chromatography (SiO2, hexanes:MTBE = 5:1) to isolate the trans-diastereomers (containing 11b and 11b’) and the cis-diastereomers (containing 11b” and 11b’”). The trans-diastereomers were submitted to a 2nd flash chromatographic separation ((SiO2, hexanes:CH2Cl2:MTBE = 5:4:0.08) to isolate 11b and 11b’. A 2nd flash chromatographic separation enabled separation of the syn-diastereomers 11b” and 11b’” (SiO2, hexanes:CH2Cl2:MTBE = 20:20:1).
(R)-((4S,5S)-5-((R)-methoxy(phenyl)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)(phenyl)methyl (3S, 4R)-3-hexanoyl-3-methyl-4-oxooxetane-2-carboxylate (11b)
Obtained as a colorless oil (72 mg, 9%). [α]D25 + 59 (c = 1.0, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.35-7.27 (m, 6H), 7.15-7.11 (m, 4H), 5.21 (d, J = 5.8 Hz, 1H), 4.69 (s, 1H), 4.22 (dd, J = 6.8, 5.8 Hz, 1H), 4.00 (t, J = 6.3 Hz, 1H), 3.68 (d, J = 6.0 Hz, 1H), 3.16 (s, 3H), 1.80-1.77 (m, 2H), 1.48 (s, 3H), 1.47 (s, 3H), 1.39-1.30 (m, 8H), 1.22 (s, 3H), 0.91 (t, J = 6.6 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.7, 166.64, 137.34, 136.06, 129.11, 128.81 (2C), 128.74 (2C), 128.72, 127.79 (2C), 127.70 (2C), 110.99, 84.13, 80.91, 79.15, 76.94, 75.48, 62.26, 56.95, 35.31, 31.63, 29.30, 27.76, 27.55, 24.43, 22.63, 15.73, 14.17. LRMS (ESI+): Calcd. For C31H40O7Na ([M+Na]+), 547.3. Found: 547.4.
(R)-((4S,5S)-5-((R)-methoxy(phenyl)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)(phenyl)methyl (3R, 4S)-3-hexyl-3-methyl-4-oxooxetane-2-carboxylate (11b’)
Obtained as a colorless oil (72 mg, 9%). [α]D25 + 43 (c = 1.0, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.34-7.25 (m, 6H), 7.12-7.10 (m, 4H), 5.25 (d, J = 5.8 Hz, 1H), 4.70 (s, 1H), 4.22 (dd, J = 6.6, 5.8 Hz, 1H), 4.02 (t, J = 6.2 Hz, 1H), 3.71 (d, J = 6.2 Hz, 1H), 3.15 (s, 3H), 1.76-1.72 (m, 2H), 1.47 (s, 6H), 1.35-1.25 (m, 8H), 1.01 (s, 3H), 0.90 (t, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.61, 166.68, 137.28, 136.03, 129.15, 128.85 (2C), 128.73, 128.68 (2C), 127.93 (2C), 127.76 (2C), 111.14, 84.27, 80.98, 79.20, 77.08, 75.31, 62.26, 56.97, 35.22, 31.66, 29.30, 27.87, 27.59, 24.45, 22.63, 15.46, 14.18. LRMS (ESI+): Calcd. For C31H40O7Na ([M+Na]+), 547.3. Found: 547.4.
(R)-((4S,5S)-5-((R)-methoxy(phenyl)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)(phenyl)methyl (3S, 4S)-3-hexyl-3-methyl-4-oxooxetane-2-carboxylate (11b”)
Obtained as a colorless oil (125 mg, 14%). [α]D25 + 66 (c = 1.0, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.35-7.26 (m, 6H), 7.15-7.11 (m, 4H), 5.19 (d, J = 5.5 Hz, 1H), 4.60 (s, 1H), 4.22 (dd, J = 6.9, 5.5 Hz, 1H), 4.01 (t, J = 6.4 Hz, 1H), 3.70 (d, J = 6.1 Hz, 1H), 3.16 (s, 3H), 1.52 (s, 3H), 1.49 (s, 3H), 1.48 (s, 3H), 1.40-1.30 (m, 2H), 1.27-1.20 (m, 4H), 1.16-1.03 (m, 4H), 0.85 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.51, 166.43, 137.36, 136.04, 129.10, 128.82 (2C), 128.72 (2C), 128.66, 127.90 (2C), 127.71 (2C), 111.12, 84.17, 80.90, 79.19, 77.63, 77.17, 61.83, 56.93, 31.61, 31.44, 29.42, 27.87, 27.51, 23.88, 22.63, 19.59, 14.10. LRMS (ESI+): Calcd. For C31H40O7Na ([M+Na]+), 547.3. Found: 547.4.
(R)-((4S,5S)-5-((R)-methoxy(phenyl)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)(phenyl)methyl (3R, 4R)-3-hexyl-3-methyl-4-oxooxetane-2-carboxylate (11b’”)
Obtained as a colorless oil (125 mg, 14%). [α]D25 + 48 (c = 1.0, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.34-7.24 (m, 6H), 7.15-7.09 (m, 4H), 5.31 (d, J = 5.9 Hz, 1H), 4.62 (s, 1H), 4.26 (t, J = 6.3 Hz, 1H), 4.001 (t, J = 6.3 Hz, 1H), 3.67 (d, J = 6.0 Hz, 1H), 3.14 (s, 3H), 1.494 (s, 3H), 1.486 (s, 3H), 1.47 (s, 3H), 1.30-1.27 (m, 2H), 1.20-1.12 (m, 4H), 1.04-0.97 (m, 4H), 0.82 (t, J = 7.3 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.40, 166.46, 137.35, 135.83, 129.16, 128.80 (2C), 128.71 (2C), 128.64, 128.08 (2C), 127.74 (2C), 111.16, 84.07, 81.06, 79.10, 77.45, 77.32, 61.79, 56.97, 31.60, 31.34, 29.37, 27.90, 27.61, 23.88, 22.62, 19.67, 14.10. LRMS (ESI+): Calcd. For C31H40O7Na ([M+Na]+), 547.3. Found: 547.4.
Representative procedure for hydrogenolytic cleavage of the chiral auxiliary: Synthesis of (3S, 4R)-3-hexyl-4-oxooxetane-2-carboxylic acid (12a)
In a round-bottomed flask was added Pd/C (80 mg, 10% w/w, 0.076 mmol, 0.09 equiv) and the flask was evacuated by vacuum for ~30 minutes, and then refilled with N2. A THF solution of 11a (429 mg, 0.840 mmol, 1 equiv, 0.05M) was added. The flask was charged with a hydrogen balloon and the reaction was kept at ambient temperature (23 °C) for 15 h with stirring. The mixture was filtered through a short celite pad and washed with EtOAc (5 mL). After concentration the crude product was purified by silica gel flash chromatography (hexanes:MTBE = 2:1) to give 12a as a colorless oil (116 mg, 69%). [α]D25 −20 (c = 5.8, CHCl3). 1H NMR (300 MHz, CDCl3) δ 10.19 (brs, 1H), 4.65 (d, J = 4.4 Hz, 1H), 3.82 (ddd, J = 8.7, 6.7, 4.3 Hz, 1H), 2.01-1.80 (m, 2H), 1.54-1.23 (m, 8H), 0.88 (t, J = 6.8 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 173.8, 168.9, 71.0, 58.2, 31.6, 28.8, 27.9, 26.7, 22.6, 14.2. HRMS (ESI−): Calcd. for C10H15O4 ([M-H]−), 199.0976. Found: 199.0982.
(3S, 4R)-3-Hexyl-4-oxooxetane-2-carboxylic acid (12a’)
Based on the representative hydrogenolysis procedure, the enantiomeric β-lactone carboxylic acid 12a’ was obtained as a colorless oil (135 mg, 66%). [α]D25 +18 (c = 6.8, CHCl3). 1H NMR and 13C NMR spectra were identical to that of 12a. HRMS (ESI+): Calcd. For C10H15O4 ([M-H]−), 199.0976. Found: 199.0963.
(3S, 4R)-3-hexyl-3-methyl-4-oxooxetane-2-carboxylic acid (12b)
According to the representative hydrogenolysis procedure, anti-β-lactone acid 12b was prepared from ester 11b as a colorless oil (20.5 mg, 75%). [α]D25 +11.6 (c = 2.1, CHCl3). 1H NMR (500 MHz, CDCl3) δ 8.49 (brs, 1H), 4.75 (s, 1H), 1.83-1.80 (m, 2H), 1.53-1.46 (m, 1H), 1.36 (s, 3H), 1.33-1.24 (m, 7H), 0.89 (t, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.52, 172.36, 75.2, 62.45, 35.24, 31.66, 29.32, 24.47, 22.67, 15.81, 14.19. HRMS (ESI−): Calcd. For C11H17O4 ([M-H]−), 213.1132. Found: 213.1131.
(3R, 4S)-3-hexyl-3-methyl-4-oxooxetane-2-carboxylic acid (12b’)
According to the general hydrogenolysis procedure, the enantiomeric anti-β-lactone 12b’ was prepared from ester 11b’ as a colorless oil (12.8 mg, 68%). [α]D25 −10.5 (c = 1.3, CHCl3). The 1H NMR and 13C NMR were identical to the enantiomeric anti-β-lactone acid 12b. HRMS (ESI−): Calcd.
(3R, 4R)-3-hexyl-3-methyl-4-oxooxetane-2-carboxylic acid (12b”)
According to the general hydrogenolysis procedure, the diastereomeric syn-β-lactone 12b” was prepared as a colorless oil (16.7 mg, 64%). [α]D25 −3.7 (c = 1.7, CHCl3). 1H NMR (500 MHz, CDCl3) δ 8.49 (brs, 1H), 4.75 (s, 1H), 1.83-1.80 (m, 2H), 1.53-1.46 (m, 1H), 1.36 (s, 3H), 1.33-1.24 (m, 7H), 0.89 (t, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.52, 172.36, 75.2, 62.45, 35.24, 31.66, 29.32, 24.47, 22.67, 15.81, 14.19. HRMS (ESI−): Calcd. For C11H17O4 ([M-H]−), 213.1132. Found: 213.1131.
The relative configuration of β-lactone acids 12a, 12a’, 11b, 11b’, 11b”, 11b’”was assigned based on coupling constant analysis or nOe correlations (via ROESY experiments). The absolute configuration was assigned by comparison of optical rotations to known, related β-lactones.[27e, 34] See Supporting Information for details.
4-Ethynylbenzyl-L-ala (14c)
To the solution of 4-ethynylbenzyl (tertbutoxycarbonyl)-L-alaninate[32] (1.00 g, 3.30 mmol) in 12 mL of DCM was slowly added TFA (1.5 mL) at 0 °C. The solution was kept in a 4 °C refrigerator for 40 h. After concentration the crude product was purified on silica gel chromatography (DCM:MeOH = 20:1) to give the product as a yellow oil (880 mg, 89%) in the form of a TFA salt. 1H NMR (500 MHz, CDCl3) δ 8.43 (brs, 3H), 7.44 (d, J = 8.1 Hz, 2H), 7.23 (d, J = 8.1 Hz, 2H), 5.14 (d, J = 12.5 Hz, 1H), 5.08 (d, J = 12.5 Hz, 1H), 4.08 (q, J = 7.2 Hz, 1H), 3.09 (s, 1H), 1.55 (d, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 170.2, 135.1, 132.6, 128.2, 122.8, 83.1, 78.2, 67.9, 49.3, 15.9. HRMS (ESI+): Calcd. for C12H14N2O+ ([M]+), 204.1019. Found: 204.1023.
Cbz-Ala-BnO-Lys(Boc) dipeptide (15a)
Based on the literature procedure,[23b] N-Cbz-Ala (1.1 g, 4.92 mmol) (13a) coupled with amine 14a (1.51 g, 4.47 mmol) to give the known dipeptide 15a (2.12 g, 87% yield) as a white solid. The characterization data are identical to the literature.[23b]
Cbz-Gly-BnO-Lys(Boc) dipeptide (15b)
N-Cbz-Gly (13b) (1.03 g, 4.92 mmol) was dissolved in dry CH2Cl2 (20 mL) in a dry, round-bottomed flask under a positive atmosphere of nitrogen at 22 °C and then the solution was cooled to 0 °C. The EDAC (1.03 g, 5.37 mmol), HOBt (0.726 g, 5.37 mmol) and i-Pr2NEt (2.34 mL, 13.43 mmol) were then added sequentially and the resulting solution was stirred at 0 °C for 30 min. BnO-Lys(Boc) (14a) (1.51 g, 4.47 mmol) was then added via cannula dissolved in dry CH2Cl2 (10 mL). After the addition was complete, the reaction mixture was allowed to warm to 22 °C and stirred at this temperature for 4 h. The reaction was quenched by addition of water (30 mL), the layers were separated, and the organic fraction was dried over Na2SO4, filtered and concentrated under reduced pressure. The crude mixture was purified by MPLC (SiO2, gradient: 100% hexane →100% EtOAc) to afford the desired dipeptide (2.01 g, 85% yield) as a viscous colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.36-7.30 (m, 10H), 6.89 (bs, 1H), 5.75 (bs, 1H), 5.18 (d, J = 12.0 Hz, 1H), 5.12 (d, J = 12.0 Hz, 1H), 5.11 (s, 2H), 4.72 (bs, 1H), 4.64-4.60 (m, 1H), 3.91 (d, J = 5.0 Hz, 2H), 3.05-2.96 (m, 2H), 1.87-1.78 (m, 1H), 1.70-1.62 (m, 1H), 1.46-1.38 (m, 2H), 1.42 (s, 9H), 1.29-1.22 (m, 2H). 13C NMR (125 MHz, CDCl3) δ 172.16, 169.19, 156.79, 156.26, 136.33, 135.37, 128.78, 128.66 (2C), 128.45 (2C), 128.31, 128.19, 79.27, 67.34, 67.26, 52.23, 44.54, 40.11, 31.81, 29.55, 28.57 (3C), 22.39. FT-IR (neat, cm−1): 3326, 2934, 1682, 1520. FT-IR (neat, cm−1): 3326, 2934, 1682, 1520. HRMS (ESI+): Calcd. for C28H38N3O7 ([M+H]+), 528.2710. Found: 528.2681.
4-BrBzAla-BnO-Lys(Boc) dipeptide (15d)
4-BrBz-Ala-OH (13c) (0.536 g, 1.97 mmol) was dissolved in dry CH2Cl2 (10 mL) in a round-bottomed flask under positive atmosphere of nitrogen at 22 °C then the solution was cooled to 0 °C. EDAC (0.412 g, 2.15 mmol), HOBt (0.290 g, 2.15 mmol), and i-Pr2-NEt (0.935 mL, 5.37 mmol) were then added sequentially and the resulting solution was stirred at 0 °C for 30 minutes. BnO-Lys(Boc) (14a) (0.602 g, 1.79 mmol) was then added via cannula dissolved in dry CH2Cl2 (5 mL). After the addition was completed, the reaction mixture was allowed to warm to 22 °C and stirred at this temperature for 12 h. To quench the reaction, the solution was diluted with deionized water (15 mL), the organic fraction was dried over MgSO4, filtered and concentrated under reduced pressure. The crude was purified by MPLC (SiO2, gradient: 100% hexane →100% EtOAc) to afford 15d (0.326 g, 31% yield) as a white solid and as a 2:1 mixture of rotamers. Data for the major rotamer: 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J = 8.5 Hz, 2H), 7.54 (d, J = 8.5 Hz, 2H), 7.35-7.29 (m, 5H), 7.18 (d, J = 8.0 Hz, 1H), 7.10 (d, J = 4.5 Hz, 1H), 5.16 (d, J = 12.0 Hz, 1H), 5.08 (d, J = 12.0 Hz, 1H), 4.81-4.74 (m, 1H), 4.66-4.59 (m, 2H), 3.09-2.97 (m, 2H), 1.90-1.84 (m, 1H), 1.76-1.69 (m, 1H), 1.49 (d, J = 7.0, 3H) 1.47-1.40 (m, 2H), 1.44 (s, 9H), 1.36-1.26 (m, 2H). 13C NMR (125 MHz, CDCl3) δ 172.49, 172.04, 166.40, 156.29, 135.37, 132.77, 131.96 (2C), 128.94 (2C), 128.79 (2C), 128.69 (2C), 128.46, 126.67, 79.38, 67.37, 52.38, 49.37, 40.10, 31.79, 29.60, 28.62 (3C), 22.52, 18.89. FT-IR (neat, cm−1): 3417, 1642, 1535, 1171. HRMS (ESI+): Calcd. For C28H37BrN3O6 ([M+H]+), 590.1866. Found: 590.1895.
BnO-Ala-Cbz-Lys(Boc) dipeptide (15e)
Cbz-Lys(Boc)-OH (13d) (2.0 g, 5.26 mmol) was dissolved in dry CH2Cl2 (20 mL) in a dry round-bottomed flask under a positive atmosphere of nitrogen at 22 °C and then the solution was cooled to 0 °C. The EDAC (1.21 g, 6.31 mmol), HOBt (0.852 g, 6.31 mmol) and i-Pr2NEt (2.75 mL, 15.77 mmol) were then added sequentially and the resulting solution was stirred at 0 °C for 30 min. The H-Ala-OBn p-tosylate (14b) (2.03 g, 5.78 mmol) was then added via cannula as a solution in dry CH2Cl2 (10 mL). After the addition was completed, the reaction mixture was allowed to warm to 22 °C and stirred at this temperature for 4 h. To quench the reaction, the solutions was diluted with water (30 mL), the organic fraction was dried over Na2SO4, filtered and concentrated under reduced pressure. The crude was purified by MPLC (SiO2, gradient: 100% hexane →100% EtOAc) to afford the desired dipeptide 15e (2.52 g, 89% yield) as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.37-7.32 (m, 10H), 6.93 (bs, 1H), 5.68 (bs, 1H), 5.20 (d, J = 12.0 Hz, 1H), 5.12 (d, J = 12.0 Hz, 1H), 5.08 (s, 2H), 4.80 (bs, 1H), 4.60 (p, J = 7.0 Hz, 1H), 4.24 (t, J = 7.0 Hz, 1H), 3.09-3.03 (m, 2H), 1.84-1.78 (m, 1H), 1.67-1.60 (m, 1H), 1.48-1.35 (m, 4H), 1.41 (s, 9H), 1.37 (d, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.67, 171.72, 156.39, 156.29, 136.33, 135.42, 128.72 (2C), 128.60 (2C), 128.54, 128.27 (2C), 128.24, 128.15 (2C), 79.17, 67.26, 67.08, 54.64, 48.25, 39.88, 32.37, 29.50, 28.55 (3C), 22.36, 18.01. FT-IR (neat, cm−1): 3306, 2937, 1694, 1538. FT-IR (neat, cm−1): 3306, 2937, 1694, 1538. HRMS (ESI+) Calcd. for C29H40N3O7 ([M+H]+): 542.2866. Found: 542.2850.
BnO-Ala-Fmoc-Lys(Boc) dipeptide (15f)
Fmoc-Lys(Boc)-OH (13e) (2.0 g, 4.27 mmol) was dissolved in dry CH2Cl2 (20 mL) in a dry round-bottomed flask under a positive atmosphere of nitrogen at 22 °C and then the solution was cooled to 0 °C. The EDAC (0.982 g, 5.12 mmol), HOBt (0.692 g, 5.12 mmol) and i-Pr2NEt (2.23 mL, 12.81 mmol) were then added sequentially and the resulting solution was stirred at 0 °C for 30 min. The H-Ala-OBn p-tosylate (14b) (1.65 g, 4.70 mmol) was then added via cannula as a solution in dry CH2Cl2 (10 mL). After the addition was complete, the reaction was allowed to warm to 22 °C and stirred at this temperature for 1 h. To quench the reaction, the solution was diluted with water (30 mL), the organic fraction was dried over Na2SO4, filtered and concentrated under reduced pressure. The crude was purified by MPLC (SiO2, gradient: 100% hexane →100% EtOAc) to afford the desired dipeptide 15f (2.28 g, 85% yield) as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 7.5 Hz, 2H), 7.60 (d, J = 7.0 Hz, 2H), 7.40 (t, J = 7.5 Hz, 2H), 7.36-7.29 (m, 7H), 6.73 (bs, 1H), 5.60 (bs, 1H), 5.20 (d, J = 12.0 Hz, 1H), 5.13 (d, J = 12.0 Hz, 1H), 4.73 (bs, 1H), 4.62 (p, J = 7.0 Hz, 1H), 4.38 (d, J = 7.0 Hz, 2H), 4.21 (t, J = 7.0 Hz, 2H), 3.15-3.03 (m, 2H), 1.89-1.82 (m, 1H), 1.70-1.63 (m, 1H), 1.48-1.39 (m, 4H), 1.44 (s, 9H), 1.42 (d, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.69, 171.57, 156.36, 144.02, 141.4, 135.43, 128.80 (2C), 128.64 (2C), 128.38 (2C), 128.33 (2C), 127.90 (2C), 127.26 (2C), 125.29 (2C), 120.16 (2C), 79.34, 77.43, 67.42, 54.72, 48.39, 47.30, 39.93, 32.45, 29.62, 28.63 (3C), 22.45, 18.24. FT-IR (neat, cm−1): 3311, 2934, 1684, 1532. HRMS (ESI+) Calcd. for C36H44N3O7 ([M+H]+): 630.3179. Found: 630.3203.
Boc-hydantoin (15ff)
BnO-Ala-Fmoc-Lys(Boc) dipeptide (15f) (0.5 g, 0.793 mmol) was dissolved in dry CH2Cl2 (5 mL) in a dry round-bottomed flask under a positive atmosphere of nitrogen at 22 °C and then the triphosgene was added (94.2 mg, 0.318 mmol). The solution was then cooled to 0 °C and then the DBU (0.475 mL, 3.18 mmol) was added dropwise. The reaction mixture was stirred at 0 °C for 3 h and then at 50 °C for 8 h. The mixture was then concentrated under reduced pressure and the crude residue was purified by MPLC (SiO2, gradient: 100% hexanes →100 % EtOAc) to afford the desired hydantoin 15ff (0.141 g, 41% yield) as a colorless viscous oil. 1H NMR (500 MHz, CDCl3) δ 7.37-7.31 (m, 5H), 6.43 (bs, 1H), 5.20 (d, J = 12.5 Hz, 1H), 5.13 (d, J = 12.5 Hz, 1H), 4.79 (q, J = 7.5 Hz, 1H), 4.62 (bs, 1H), 4.01 (t, J = 6.0 Hz, 1H), 3.12-3.03 (m, 2H), 1.89-1.82 (m, 1H), 1.70-1.61 (m, 1H), 1.66 (d, J = 7.5 Hz, 3H), 1.48-1.32 (m, 4H), 1.45 (s, 9H). 13C NMR (125 MHz, CDCl3) δ 173.55, 169.56, 156.78, 156.42, 135.40, 128.78 (2C), 128.61, 128.48 (2C), 79.58, 67.77, 57.05, 48.38, 40.04, 31.15, 29.72, 28.67 (3C), 21.63, 15.00. FT-IR (neat, cm−1): 3333, 2931, 1717, 1510. HRMS (ESI+) Calcd. for C22H32N3O6 ([M+H]+): 434.2291. Found: 434.2276.
4-Ethynylbenzyl N-Cbz-N-Boc-L-lysyl-L-alaninate (15g)
A solution of N-Cbz-N-Boc-L-lysine (13d) (253 mg, 0.666 mmol, 1.0 equiv), TFA salt of 4-ethynylbenzyl-L-ala (14c) (200 mg, 0.666 mmol, 1 equiv), EDCI (255 mg, 1.33 mmol, 2 equiv), HOBt (90 mg, 0.666 mmol, 1 equiv) in 3 mL of DMF and 12 mL of DCM was cooled to 0 °C and TMP (87 mL, 0.666 mmol, 1 equiv) was added. The solution was kelp in a 4 °C refrigerator for 15 h, diluted with 50 mL of EtOAc and washed with H2O (5 mL) and brine (5mL). The organic layer was dried over MgSO4 and concentrated. The residue was purified on a silica gel chromatography (hexanes:EtOAc = 4:1) to give the product as a yellow oil (250 mg, 66%). 1H NMR (500 MHz, CDCl3) δ 7.41 (d, J = 8.1 Hz, 2H), 7.28-7.23 (m, 5H), 7.22 (d, J = 8.1 Hz, 2H), 5.76 (d, J = 8.0 Hz, 1H), 5.12 (d, J = 12.6 Hz, 1H), 5.04 (d, J = 12.6 Hz, 1H), 5.02 (s, 2H), 4.82 (brs, 1H), 4.58-4.48 (m, 1H), 4.21-4.14 (m, 1H), 3.06 (s, 1H), 30.3-2.95 (m, 2H), 1.80-1.69 (m, 1H), 1.63-1.51 (m, 1H), 1.42-1.30 (m, 4H), 1.36 (s, 9H), 1.33 (d, J = 7.3 Hz, 3H).). 13C NMR (125 MHz, CDCl3) δ 172.6, 171.9, 156.5, 136.1, 132.5 (2C), 128.7 (2C), 128.2 (2C), 128.1 (2C), 127.2, 122.4, 116.5, 111.8, 83.3, 79.5, 78.0, 67.2, 66.7, 54.8, 48.4, 39.9, 32.2, 29.5, 28.5, 22.4, 18.0. HRMS (ESI+): Calcd. for C31H40N3O7+ ([M+H]+), 566.2861. Found: 566.2884.
Cbz-Ala-BnO-Lys dipeptide (16a)
Cbz-Ala-BnO-Lys(Boc) dipeptide (15a) (1.0 g, 1.85 mmol) was dissolved in dry CH2Cl2 (10 mL) in a dry, round-bottomed flask under a positive atmosphere of nitrogen at 22 °C and then TFA was added (1.41 mL, 18.46 mmol). The reaction mixture was stirred for 8 h at 22 °C and then concentrated under reduced pressure and diluted with toluene (2 × 10 mL) and concentrated to provide the desired Cbz-Ala-BnO-Lys dipeptide (1.03 g, 98% yield) in the form a TFA salt as a viscous colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.97 (bs, 3H), 7.44 (d, J = 7.5 Hz, 1H), 7.35-7.27 (m, 10H), 6.01 (d, J = 7.5 Hz, 1H), 5.16 (d, J = 12.0 Hz, 1H), 5.08 (d, J = 12.0 Hz, 1H), 5.06 (d, J = 12.0 Hz, 1H), 4.99 (d, J = 12.0 Hz, 1H), 4.56-4.51 (m, 1H), 4.32-4.29 (m, 1H), 2.82-2.74 (m, 2H), 1.82-1.75 (m, 1H), 1.67-1.56 (m, 2H), 1.55-1.46 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H), 1.37-1.25 (m, 2H). 13C NMR (125 MHz, CDCl3) δ 173.41, 171.84, 162.67 (q, J = 26.75 Hz, CF3CO2−), 156.64, 136.28, 135.38, 128.77 (2C), 128.72, 128.67 (2C), 128.46, 128.35 (2C), 127.95 (2C), 116.75 (q, J = 288.75 Hz, CF3CO2−), 67.42, 67.18, 52.19, 50.50, 39.28, 30.88, 26.63, 21.93, 18.61. FT-IR (neat, cm−1): 3300, 2939, 1673, 1531. HRMS (ESI+): Calcd. for C24H32N3O5+ ([M]+), 442.2336. Found: 442.2365.
Cbz-Gly-BnO-Lys dipeptide (16b)
Cbz-Gly-BnO-Lys(Boc) dipeptide (15b, 1.0 g, 1.90 mmol) was dissolved in dry CH2Cl2 (10 mL) in a dry, round-bottomed flask under a positive atmosphere of nitrogen at 22 °C and then TFA was added (1.41 mL, 18.46 mmol). The reaction mixture was stirred for 8 h at 22 °C and then concentrated under reduced pressure and diluted with toluene (2 × 10 mL) and concentrated to provide the desired Cbz-Gly-BnO-Lys dipeptide (16b, 1.04 g, 99% yield) as a viscous colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.67 (bs, 3H), 7.44 (d, J = 7.5 Hz, 1H), 7.35-7.26 (m, 10H), 6.12 (bs, 1H), 5.14 (d, J = 12.5 Hz, 1H), 5.09 (d, J = 12.5 Hz, 1H), 5.04 (s, 2H), 4.55-4.50 (m, 1H), 3.83 (d, J = 5.0 Hz, 2H), 2.86-2.77 (m, 2H), 1.83-1.75 (m, 1H), 1.65-1.49 (m, 3H), 1.34-1.23 (m, 2H). 13C NMR (125 MHz, CDCl3) δ 172.05, 170.66, 162.67 (q, J = 37.50 Hz, CF3CO2−), 157.46, 136.16, 135.20, 128.84 (2C), 128.79 (2C), 128.75 (2C), 128.43 (2C), 127.96, 116.05 (q, J = 290.62 Hz, CF3CO2−), 67.67, 67.49, 52.24, 44.21, 39.68, 30.96, 26.52, 21.80. FT-IR (neat, cm−1): 3385, 2987, 1781, 1650, 1586. HRMS (ESI+) Calcd. for C23H30N3O5+ ([M]+): 428.2180. Found: 428.2205.
N-CBz-Ala-O-Bn-Orn dipeptide (16c)
N-CBz-Ala-O-Bn-Orn(Boc) (15c)32 (300 mg, 569 µmol) was dissolved in dry CH2Cl2 (5 mL) in a round bottomed flask under positive atmosphere of nitrogen at 22 °C. TFA was added (0.4 mL, 5.54 mmol) to the solution. The reaction mixture was stirred for 8 h at 22 °C and then concentrated under reduced pressure and diluted with toluene (2 × 4 mL) and concentrated to provide 16c (315 mg, 99% yield) as viscous yellow oil in the form of a TFA salt. 1H NMR (500 MHz, CD3OD) δ 7.37-7.30 (m,10 H), 5.18 (d, J = 12.2 Hz, 1H), 5.16 (d, J = 12.2 Hz, 1H), 5.10 (d, J = 12.4 Hz, 1H), 5.04 (d, J = 12.4Hz, 1H), 4.56-4.52 (m, 1H), 4.11 (q, J = 7.3 Hz, 1H), 2.92 (t, J = 7.5 Hz, 2H), 2.04-1.97 (m, 1H), 1.78-1.73 (m, 2H), 1.30 (d, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CD3OD) δ 176.0, 172.6, 163.0 (q, J = 34.5 Hz, CF3CO2−), 158.3, 138.1, 137.0, 129.6 (2C), 129.5 (2C), 129.42, 129.37 (2C), 129.0, 128.7, 118.2 (q, J = 289.4 Hz, CF3CO2−), 68.2, 67.6, 52.8, 51.9, 40.1, 29.2, 24.9, 18.1. HRMS (ESI+) Calcd. for C23H30N3O5+ ([M]+): 428.2180. Found: 428.2289.
4-BrBzAla-BnO-Lys dipeptide (16d)
4-BrBz-Ala-BnO-Lys(Boc) (15d) (326 mg, 552.06 µmol) was dissolved in dry CH2Cl2 (5 mL) in a round-bottomed flask under a positive atmosphere of nitrogen at 22 °C. TFA was added (0.4 mL, 5.54 mmol) to the solution. The reaction mixture was stirred for 8 h at 22 °C and then concentrated under reduced pressure and diluted with toluene (2 × 4 mL). Concentration in vacuo provided the desired 4-BrBz-Ala-BnO-Lys dipeptide (330 mg, 99% yield) as viscous yellow oil and and as a 2:1 mixture of rotamers. Data for the major rotamer: 1H NMR (500 MHz, CDCl3) δ 7.98 (bs, 3H), 7.67 (bs, 1H), 7.59 (d, J = 8.5 Hz, 2H), 7.50 (d, J = 8.5, 2H), 7.34-7.24 (m, J = 7.8, 5H), 5.43 (bs, 1H), 5.17-5.09 (m, 2H), 4.69-4.46 (m, 2H), 3.05-2.85 (m, 2H), 1.85-1.57 (m, 4H), 1.4-1.35 (m, 2H), 1.41 (d, J = 6.0, 3H). 13C NMR (125 MHz, CDCl3) δ 172.65, 171.76, 166.79, 135.22, 131.90, 129.08 (2C), 128.76 (2C), 128.66 (2C), 128.36 (2C), 128.32, 126.79, 67.51, 52.60, 49.83, 39.57, 30.67, 26.83, 22.24, 18.11. FT-IR (neat, cm−1): 3275, 3068, 1740, 1675, 1541, 1204. HRMS (ESI+) Calcd. For C23H29BrN3O4+ ([M]+): 490.1336. Found: 490.1320.
BnO-Ala-Cbz-Lys dipeptide (16e)
BnO-Ala-Cbz-Lys(Boc) dipeptide (15e) (1.0 g, 1.85 mmol) was dissolved in dry CH2Cl2 (10 mL) in a dry round-bottomed flask under a positive atmosphere of nitrogen at 22 °C and then TFA was added (1.41 mL, 18.46 mmol). The reaction mixture was stirred for 8 h at 22 °C and then concentrated under reduced pressure and diluted with toluene (2 × 10 mL) and concentrated to provide the desired BnO-Ala-Cbz-Lys dipeptide 16e (1.015 g, 99% yield) as a viscous colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.04 (bs, 3H), 7.61 (d, J = 6.5 Hz, 1H), 7.32-7.27 (m, 10H), 6.08 (d, J = 8.0 Hz, 1H), 5.13 (d, J = 12.5 Hz, 1H), 5.05 (d, J = 12.0 Hz, 1H), 5.03 (d, J = 12.0 Hz, 1H), 5.01 (d, J = 12.0 Hz, 1H), 4.51 (q, J = 7.0 Hz, 1H), 4.23 (t, J = 6.0 Hz, 1H), 2.82 (bs, 2H), 1.75-1.67 (m, 1H), 1.63-1.54 (m, 3H), 1.42-1.29 (m, 2H), 1.35 (d, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.85, 172.01, 162.04 (q, J = 35.75 Hz, CF3CO2−), 156.65, 136.24, 135.44, 128.75 (2C), 128.67, 128.56 (2C), 128.33 (2C), 128.21(2C), 127.99, 116.56 (q, J = 289.5 Hz, CF3CO2−), 67.30, 67.21, 54.10, 48.39, 39.30, 32.20, 26.49, 21.95, 17.31. FT-IR (neat, cm−1): 3280, 2939, 1681, 1538. HRMS (ESI+) Calcd. for C24H32N3O5+ ([M]+): 442.2336. Found: 442.2341.
Hydantoin (16f)
Boc-hydantoin dipeptide (15ff) (100 mg, 0.230 mmol) was dissolved in dry CH2Cl2 (3 mL) in a dry, round-bottomed flask under a positive atmosphere of nitrogen at 22 °C and then TFA was added (0.176 mL, 2.31 mmol). The reaction mixture was stirred for 8 h at 22 °C and then concentrated under reduced pressure and diluted with toluene (2 × 3 mL) and concentrated to provide the desired hydantoin dipeptide (103 mg, 99% yield) as a viscous colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.70 (bs, 3H), 7.40 (bs, 1H), 7.36-7.29 (m, 5H), 5.18 (d, J = 12.5 Hz, 1H), 5.12 (d, J = 12.5 Hz, 1H), 4.76 (q, J = 7.5 Hz, 1H), 4.04 (dd, J = 7.0, 4.5 Hz, 1H), 2.93 (bs, 2H), 1.87-1.80 (m, 1H), 1.63-1.56 (m, 3H), 1.62 (d, J = 7.5 Hz, 3H), 1.47-1.38 (m, 2H). 13C NMR (125 MHz, CDCl3) δ 173.64, 169.72, 161.61 (q, J = 38.50 Hz, CF3CO2−), 157.57, 135.25, 128.77 (2C), 128.67, 128.30 (2C), 116.05 (q, J = 286.12 Hz, CF3CCO2−), 67.84, 56.84, 48.43, 39.78, 30.57, 26.60, 21.07, 14.76. FT-IR (neat, cm−1): 3179, 2948, 1776, 1713, 1673, 1530. HRMS (ESI+): Calcd. for C17H24N3O4+ ([M]+), 334.1761. Found: 334.1750.
4-Ethynylbenzyl N-Cbz-L-lysyl-L-alaninate (16g)
To the solution of 15g (280 mg, 0.495 mmol) in 10 mL of DCM was slowly added TFA (1.2 mL) at 0 °C. The solution was kept in a 4 °C refrigerator for 15 h. Toluene (10 mL) was added and most of the DCM and TFA were evaporated in vacuo. The residue was purified on silica gel chromatography (DCM:MeOH = 20:1) to give the product 16g as a yellow oil (130 mg, 62%) together with unseparable methyl ketone (~ 40%) derived from hydration of the alkyne. 1H NMR (500 MHz, CDCl3) δ 7.83 (brs, 3H), 7.43 (d, J = 8.1 Hz, 2H), 7.29-7.22 (m, 5H), 7.22 (d, J = 8.1 Hz, 2H), 6.02 (brs, 1H), 5.40 (brs, 1H), 5.11 (d, J = 12.5 Hz, 1H), 5.03-4.99 (m, 3H), 4.57-4.51 (m, 1H), 4.28-4.23 (m, 1H), 3.08 (s, 1H), 2.85 (brs, 2H), 1.71-1.52 (m, 4H), 1.38-1.32 (m, 5H). HRMS (ESI+) Calcd. for C29H32N3O5+ ([M]+): 466.2336. Found: 466.2338.
Representative procedure for coupling of dipeptides and carboxylic acids: synthesis of β-lactone dipeptide 17
To a round-bottomed flask was added dipeptide 16a (13.0 mg, 0.023 mmol, 1 equiv), β-lactone acid 12a’ (4.7 mg, 0.023 mmol, 1 equiv), EDCI (8.9 mg, 0.047 mmol, 2.0 equiv), HOBt (3.1 mg, 0.023 mmol, 1 equiv), DMF (0.1 mL), and DCM (0.4 mL), followed by TMP (3.0 µL, 0.023 mmol, 1.0 equiv). The reaction mixture was stirred at 0 °C under N2 for 4 h, transferred to a separatory funnel, and diluted with EtOAc (10 mL) and MTBE (10 mL). The organic layer was washed with aq. NaHSO4 soln. (1 M), water and brine. The solution was dried over MgSO4 and concentrated in vacuo. The residue was purified by a flash chromatography (hexanes/acetone) to afford 17 as a viscous colorless oil (11.3 mg, 77%). [α]D20 −7.0 (c = 2.4, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.39-7.30 (m, 10 H), 6.70 (d, J = 6.3 Hz, 1H), 6.58 (bs, 1H), 5.49 (d, J = 6.3 Hz, 1H), 5.20 (d, J = 12.4 Hz, 1H), 5.13 (d, J = 12.4 Hz, 1H), 5.09 (s, 2H), 4.59-4.52 (m, 2H), 4.30-4.23 (m, 1H), 3.64-3.61 (m, 1H), 3.36-3.29 (m, 1H), 3.16-3.11 (m, 1H), 1.90-1.79 (m, 3H), 1.69-1.64 (m, 1H), 1.57-1.43 (m, 4H), 1.37 (d, J = 7.0 Hz, 3H), 1.34-1.25 (m, 8H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.69, 172.07, 169.83, 168.42, 156.23, 136.49, 135.45, 128.90 (2C), 128.80, 128.78 (2C), 128.60 (2C), 128.42, 128.25 (2C), 73.10, 67.51, 67.20, 57.99, 52.14, 50.72, 45.20, 38.70, 31.76/31.67 (rotamers), 28.98, 28.67, 28.32, 26.71, 22.74, 22.31, 18.63, 14.25. HRMS (ESI+): Calcd. For C34H45N3O8Li ([M+Li]+), 630.3367. Found: 630.3334.
β-lactone dipeptide 18
Prepared according to the representative procedure from β-lactone acid 12a’ (5.0 mg, 0.025 mmol) and dipeptide amine 16b (13.5 mg, 0.025 mmol) and obtained as a viscous colorless oil (9.0 mg, 59%). 1H NMR (500 MHz, CDCl3) δ 7.39-7.31 (m, 10H), 6.66 (d, J = 7.1 Hz, 1H), 6.53 (bs, 1H), 5.55 (bs, 1H), 5.20 (d, J = 12.1 Hz, 1H), 5.15 (d, J = 12.1 Hz, 1H), 5.14 (s, 2H), 4.66-4.62 (m, 1H), 4.54 (d, J = 4.5 Hz, 1H), 3.91 (d, J = 5.7 Hz, 2H), 3.64 (td, J = 7.6 Hz, J = 4.4 Hz, 1H), 3.29-3.18 (m, 2H), 1.93-1.80 (m, 3H), 1.72-1.64 (m, 1H), 1.58-1.45 (m, 4H), 1.37-1.26 (m, 8H), 0.89 (t, J = 6.5 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.09, 169.89, 169.27, 168.27, 156.87, 136.36, 135.33, 128.89 (2C), 128.82, 128.77 (2C), 128.56 (2C), 128.44, 128.23 (2C), 73.07, 67.56, 67.43, 57.98, 52.02, 44.70, 38.83, 31.97, 31.63, 28.95, 28.61, 28.29, 26.66, 22.70, 22.31, 14.23. HRMS (ESI+) Calcd. For C33H44N3O8 ([M+H]+): 610.3128. Found: 610.3114. (~ 85% purity by HPLC).
β-lactone dipeptide 19
Prepared according to the representative procedure from β-lactone acid 12a’ (5.0 mg, 0.025 mmol) and dipeptide amine 16c (13.5 mg, 0.025 mmol) and obtained as a viscous colorless oil (9.5 mg, 62%). 1H NMR (500 MHz, CDCl3) δ 7.40-7.30 (m, 10 H), 6.78 (d, J = 7.7 Hz, 1H), 6.62 (bs, 1H), 5.429 (d, J = 7.2 Hz, 1H), 5.21 (d, J = 12.3 Hz, 1H), 5.14 (d, J = 12.3 Hz, 1H), 5.11 (s, 2H), 4.62 (td, J = 7.6 Hz, J = 5.1 Hz, 1H), 4.49 (d, J = 4.4 Hz, 1H), 4.27 (pent, J = 7.0 Hz, 1H), 3.62 (ddd, J = 8.3 Hz, J = 6.9 Hz, J = 4.4 Hz, 1H), 3.32-3.25 (m, 1H), 3.24-3.17 (m, 1H), 1.92-1.78 (m, 3H), 1.71-1.61 (m, 2H), 1.54-1.42 (m, 3H), 1.38 (d, J = 7.2 Hz, 3H), 1.35-1.27 (m, 6H), 0.89 (t, J = 6.8 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.51, 171.80, 169.74, 168.21, 156.31, 136.39, 135.28, 128.91 (2C), 128.86, 128.76 (2C), 128.62 (2C), 128.43, 128.25 (2C), 73.03, 67.63, 67.27, 57.98, 51.98, 50.73, 38.58, 31.63, 29.91/29.50 (rotamers), 28.96, 28.93, 26.63, 25.33, 22.70, 18.50, 14.23. HRMS (ESI+) Calcd. For C33H44N3O8 ([M+H]+): 610.3128. Found: 610.3105.
β-lactone dipeptide 20
Prepared according to the representative procedure from β-lactone acid 12a’ (5.0 mg, 0.025 mmol) and dipeptide amine 16b (15.1 mg, 0.025 mmol) and obtained as a viscous colorless oil (5.6 mg, 51%). 1H NMR (500 MHz, CDCl3) δ 7.69-7.65 (m, 2H), 7.58-7.56 (m, 2H), 7.38-7.20 (m, 5H), 6.86 (d, J = 7.5 Hz, 1H), 6.73-6.72 (m, 1H), 6.58-6.55 (m, 1H), 5.22-5.16 (m, 1H), 5.12 (d, J = 12.3 Hz, 1H), 4.76-4.67 (m, 1H), 4.54 (d, J = 4.5 Hz, 1H), 3.70-3.62 (m, 1H), 3.34-3.26 (m, 2H), 1.92-1.83 (m, 4H), 1.76-1.71 (m, 1H), 1.66-1.60 (m, 4H), 1.50 (d, J = 7.0 Hz, 3H), 1.37-1.27 (m, 8H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.4, 171.9, 170.0, 168.2, 166.6, 135.3, 132.7, 132.0 (2C), 129.0, 128.9 (2C), 128.84 (2C), 128.79, 128.5 (2C), 73.2, 67.6, 57.9, 52.1, 49.5, 39.0, 32.2, 31.6, 29.9, 28.6, 28.3, 26.6, 22.6, 22.5, 18.6, 14.2. HRMS (ESI−) Calcd. For C33H41BrN3O7 ([M-H]−): 670.2128. Found: 670.2113.
β-lactone dipeptide 21
Prepared according to the representative procedure from β-lactone acid 12a’ (5.0 mg, 0.025 mmol) and dipeptide amine 16e (13.9 mg, 0.025 mmol) and obtained as a viscous, colorless oil (7.0 mg, 47%). [α]D20 −4.0 (c = 0.8, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.39-7.30 (m, 10H), 6.54-6.50 (m, 2H), 5.46 (d, J = 7.7 Hz, 1H), 5.22 (d, J = 12.2 Hz, 1H), 5.15 (d, J = 12.2 Hz, 1H), 5.11 (s, 2H), 4.62 (pent, J = 7.2Hz, 1H), 4.49 (d, J = 4.1 Hz, 1H), 4.21-4.15 (m, 1H), 3.65 (ddd, J = 8.3Hz, J = 7.1 Hz, J = 4.7 Hz, 1H), 3.36 (dq, J = 13.6 Hz, J = 7.0 Hz, 1H), 3.28-3.21 (m, 1H), 1.90-1.80 (m, 3H), 1.71-1.63 (m, 1H), 1.58-1.45 (m, 4H), 1.42 (d, J = 7.0 Hz, 3H), 1.39-1.26 (m, 8H), 0.89 (t, J = 6.8 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.76, 171.34, 169.74, 168.27, 156.41, 136.37, 135.48, 128.85 (2C), 128.77 (2C), 128.68, 128.45, 128.37 (2C), 128.32 (2C), 73.03, 67.48, 67.31, 57.98, 54.64, 48.40, 38.65, 32.18, 31.63, 29.92, 28.95/28.85 (rotamers), 28.32, 26.66, 22.71, 22.26, 18.25, 14.21. HRMS (ESI+) Calcd. For C34H46N3O8 ([M+H]+): 624.3285. Found: 624.3303.
β-lactone dipeptide 22
Prepared according to the representative procedure from β-lactone acid 12a’ (5.0 mg, 0.025 mmol) and dipeptide amine 16f (11.2 mg, 0.025 mmol) and obtained as a viscous, colorless oil (7.4 mg, 57%). [α]D20 −25 (c = 0.8, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.38-7.31 (m, 5H), 6.49 (t, J = 6.3 Hz, 1H), 5.97 (bs, 1H), 5.21 (d, J = 12.4 Hz, 1H), 5.15 (d, J = 12.4 Hz, 1H), 4.79 (q, J = 7.2 Hz, 1H), 4.55 (d, J = 4.3 Hz, 1H), 4.04 (t, J = 5.4 Hz, 1H), 3.68 (ddd, J = 8.1 Hz, J = 6.8 Hz, J = 4.4 Hz, 1H), 3.33-3.23 (m, 2H), 1.96-1.83 (m, 3H), 1.75-1.69 (m, 1H), 1.66 (d, J = 7.2 Hz, 3H), 1.56-1.46 (m, 4H), 1.40-1.26 (m, 8H), 0.89 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 173.29, 169.69, 169.49, 168.42, 156.53, 135.45, 128.79 (2C), 128.61, 128.45 (2C), 73.01, 67.82, 58.13, 56.78, 48.49, 38.55, 31.65, 30.61, 29.92, 29.00/28.95 (rotamers), 28.40, 26.63, 22.72, 21.02, 14.94, 14.23. HRMS (ESI+) Calcd. For C27H36N3O7 ([M-H]−): 514.2553. Found: 514.2576. (~85% purity by HPLC).
β-lactone dipeptide 23
Prepared according to the representative procedure from β-lactone acid 12a (10.0 mg, 0.050 mmol) and dipeptide amine 16e (27.8 mg, 0.050 mmol) and obtained as a viscous colorless oil (9.3 mg, 60%). [α]D20 −13 (c = 3.1, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.39-7.30 (m, 10H), 6.65 (d, J = 6.7 Hz, 1H), 6.60 (t, J = 5.6 Hz, 1H), 5.50 (d, J = 8.1 Hz, 1H), 5.21 (d, J = 12.3 Hz, 1H), 5.15 (d, J = 12.3 Hz, 1H), 5.10 (s, 2H), 4.60 (pent, J = 7.4Hz, 1H), 4.53 (d, J = 4.4 Hz, 1H), 4.20 (q, J = 7.3 Hz, 1H), 3.65 (ddd, J = 8.1Hz, J = 6.9 Hz, J = 4.3Hz, 1H), 3.33 (dq, J = 13.3 Hz, J = 6.6 Hz, 1H), 3.24 (dq, J = 13.3 Hz, J = 6.6 Hz, 1H), 1.93-1.79 (m, 3H), 1.69-1.62 (m, 1H), 1.58-1.44 (m, 4H), 1.41 (d, J = 7.4 Hz, 3H), 1.37-1.28 (m, 8H), 0.88 (t, J = 6.8 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.74, 171.42, 169.86, 168.12, 156.38, 136.37, 135.50, 128.82 (2C), 128.73 (2C), 128.64, 128.39, 128.31 (2C), 128.23 (2C), 73.04, 67.45, 67.27, 57.90, 54.55, 48.39, 38.91, 32.44, 31.61, 29.89, 28.95/28.83 (rotamers), 28.29, 26.63, 22.60, 22.43, 18.14, 14.20. HRMS (ESI+) Calcd. For C34H46N3O8 ([M+H]+): 624.3285. Found: 624.3304.
β-Lactone dipeptide 24
Prepared according to the representative procedure from β-lactone acid 12a (10.0 mg, 0.050 mmol) and dipeptide amine 16b (27.0 mg, 0.050 mmol) and obtained as a viscous, colorless oil (16.0 mg, 52%). 1H NMR (500 MHz, CDCl3) δ 7.40-7.31 (m, 10H), 6.67 (d, J = 6.8 Hz, 1H), 6.53 (bs, 1H), 5.56 (bs, 1H), 5.20 (d, J = 12.1 Hz, 1H), 5.15 (d, J = 12.1 Hz, 1H), 5.14 (s, 2H), 4.68-4.64 (m, 1H), 4.54 (d, J = 4.2 Hz, 1H), 3.91 (d, J = 5.9 Hz, 2H), 3.64 (td, J = 7.6 Hz, J = 4.2 Hz, 1H), 3.31-3.24 (m, 1H), 3.22-3.16 (m, 1H), 1.95-1.82 (m, 3H), 1.73-1.65 (m, 1H), 1.59-1.44 (m, 4H), 1.38-1.24 (m, 8H), 0.90 (t, J = 6.7 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.05, 169.94, 169.33, 168.18, 156.88, 136.36, 135.34, 128.87 (2C), 128.79, 128.75 (2C), 128.53 (2C), 128.42 (2C), 128.20, 73.06, 67.53, 67.43, 57.86, 52.00, 44.73, 38.93, 31.98, 31.61, 28.93, 28.59, 28.27, 26.65, 22.68, 22.27, 14.20. HRMS (ESI+) Calcd. For C33H44N3O8 ([M+H]+): 610.3128. Found: 610.3114.
α-Methyl-β-lactone dipeptide 25
Prepared according to the representative procedure from β-lactone acid 12b (10.0 mg, 0.045 mmol) and dipeptide amine 16a (24 mg, 0.045 mmol) and obtained as a viscous colorless oil (15 mg, 55%). 1H NMR (500 MHz, CDCl3) δ 7.38-7.29 (m, 10H), 6.76-6.70 (m, 1H), 6.58 (brs, 1H), 5.51/5.38 (d, J = 6.4 Hz, 1H), 5.19 (d, J = 12.2 Hz, 1H), 5.13 (d, J = 12.2 Hz, 1H), 5.10 (s, 2H), 4.62-4.56 (m, 1H), 4.59 (s, 1H), 4.30-4.23 (m, 1H), 3.26-3.16 (m, 2H), 1.90-1.82 (m, 1H), 1.76-1.73 (m, 2H), 1.72-1.63 (m, 1H), 1.54-1.42 (m, 3H), 1.37 (d, J = 7.1 Hz, 3H), 1.36-1.25 (m, 9H), 1.23 (s, 3H), 0.88 (t, J = 6.8 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 173.5, 172.4, 171.9, 167.2, 156.2, 136.4, 135.3, 128.8 (2C), 128.74 (2C), 128.70 (2C), 128.6 (2C), 128.4, 128.2, 76.8, 67.4, 67.2, 61.4, 52.1, 50.7, 38.7, 35.4, 31.8, 31.7, 29.4, 28.9, 24.4, 22.7, 22.3, 18.4, 15.9, 14.2. HRMS (ESI−) Calcd. For C35H47ClN3O8 ([M+Cl]−): 672.3057. Found: 672.3026.
α-Methyl-β-lactone dipeptide 26
Prepared according to the representative procedure from β-lactone acid 12b’ (8.0 mg, 0.035 mmol) and dipeptide amine 16a (19.5 mg, 0.035 mmol) and obtained as a viscous colorless oil (11.5 mg, 51%). 1H NMR (500 MHz, CDCl3) δ 7.38-7.30 (m, 10H), 6.67 (d, J = 7.2 Hz, 1H), 6.53 (brs, 1H), 5.47 (d, J = 7.3 Hz, 1H), 5.19 (d, J = 12.2 Hz, 1H), 5.13 (d, J = 12.2 Hz, 1H), 5.10 (s, 2H), 4.64 (s, 1H), 4.60-4.56 (m, 1H), 4.29-4.23 (m, 1H), 3.33-3.27 (m, 1H), 3.21-3.15 (m, 1H), 1.91-1.84 (m, 1H), 1.76-1.68 (m, 3H), 1.56-1.44 (m, 3H), 1.37 (d, J = 7.1 Hz, 3H), 1.34-1.26 (m, 9H), 1.24 (s, 3H), 0.88 (t, J = 6.8 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 173.3, 172.5, 172.0, 167.4, 156.1, 136.5, 135.4, 128.8 (2C), 128.74, 128.70 (2C), 128.6 (2C), 128.4 (2C), 128.2, 76.9, 67.5, 67.2, 61.4, 52.1, 50.7, 38.4, 35.4, 31.7, 29.9, 29.4, 28.8, 24.4, 22.7, 22.2, 18.5, 15.9, 14.2. HRMS (ESI+) Calcd. For C35H48N3O8 ([M+H]+): 638.3441. Found: 638.3455.
α-Methyl-β-lactone dipeptide 27
Prepared according to the representative procedure from β-lactone acid 12b” (10 mg, 0.045 mmol) and dipeptide amine 16a (24 mg, 0.045 mmol) and obtained as a viscous colorless oil (18 mg, 63%). 1H NMR (500 MHz, CDCl3) δ 7.39-7.30 (m, 10H), 6.67 (d, J = 7.3 Hz, 1H), 6.56 (brs, 1H), 5.51 (d, J = 6.9 Hz, 1H), 5.20 (d, J = 12.2 Hz, 1H), 5.13 (d, J = 12.2 Hz, 1H), 5.09 (s, 2H), 4.60-4.56 (m, 1H), 4.56 (s, 1H), 4.26 (pent, J = 7.1 Hz, 1H), 3.38-3.31(m, 1H), 3.17-3.11 (m, 1H), 1.90-1.83 (m, 1H), 1.73-1.66 (m, 1H), 1.62-1.59 (m, 2H), 1.51-1.45 (m, 1H), 1.49 (s, 3H), 1.40-1.36 (m, 2H), 1.37 (d, J = 7.1 Hz, 3H), 1.28-1.23 (m, 9H), 0.85 (t, J = 6.8 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 173.1, 172.5, 172.0, 167.1, 156.1, 136.4, 135.3, 128.8 (2C), 128.74, 128.71 (2C), 128.5 (2C), 128.4, 128.2 (2C), 79.0, 67.5, 67.2, 61.0, 52.0, 50.7, 38.4, 31.8, 31.6, 29.9, 29.7, 28.7, 24.1, 22.7, 22.2, 20.0, 18.7, 14.2. HRMS (ESI−) Calcd. For C35H47ClN3O8 ([M+Cl]−): 672.3057. Found: 672.3058.
ABPP probe 28
Prepared according to the representative procedure from β-lactone acid 12a’ (6.8 mg, 0.034 mmol) and dipeptide amine 16g (19 mg, 0.034 mmol) and obtained as a viscous colorless oil (11 mg, 50%). 1H NMR (500 MHz, CDCl3) δ 7.48 (d, J = 8.2 Hz, 2H), 7.36-7.32 (m, 5H), 7.30 (d, J = 8.2 Hz, 2H), 6.60 (d, J = 6.8 Hz, 1H), 6.65-6.53 (m, 1H), 5.49 (d, J = 7.8 Hz, 1H), 5.19 (d, J = 12.4 Hz, 1H), 5.12 (d, J = 12.4 Hz, 1H), 5.11 (d, J = 12.5 Hz, 1H), 5.08 (d, J = 12.5 Hz, 1H), 4.59 (pent, J = 7.2 Hz, 1H), 4.48 (d, J = 4.2 Hz, 1H), 4.17 (q, J = 6.1 Hz, 1H), 3.64 (td, J = 7.7 Hz, J = 4.6 Hz, 1H), 3.37-3.31 (m, 1H), 3.26-3.20 (m, 1H), 3.10 (s, 1H), 1.90-1.25 (m, 16H), 1.41 (d, J = 7.2 Hz, 3H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 172.6, 171.3, 169.6, 168.2, 156.1, 136.3, 136.2, 132.5 (2C), 128.7 (2C), 128.4, 128.2 (2C), 128.1 (2C), 122.4, 83.3, 78.0, 73.0, 67.3, 66.8, 58.0, 54.7, 48.4, 38.7, 32.2, 316., 29.0, 28.9, 28.3, 26.6, 22.7, 22.3, 18.1, 14.2. HRMS (ESI+) Calcd. For C36H46N3O8 ([M+H]+): 648.3285. Found: 648.3278. (~ 80% purity by HPLC)
Fluorogenic Assay for Detection of 20S Human Proteasome Inhibition
For determination of IC50 values, 50 ng of 20S proteasome, purified from human red blood cells [5], was incubated in assay buffer (20 mM Tris, pH 7.5) at 37 °C with varying concentrations of each inhibitor. After twenty minutes, the reaction was initiated by the addition of fluorogenic substrate for the chymotrypsin-like (Suc-Leu-Leu-Val-Tyr-AMC), trypsin-like (Boc-Leu-Arg-Arg-AMC), or caspase-like (Z-Leu-Leu-Glu-AMC) activity of the proteasome, with final substrate concentrations of 10 µM, 20 µM, and 20 µM respectively. The rates of hydrolysis of the substrates were monitored using a POLARstar Omega microplate reader, with excitation/emission wavelengths of 360 nm/460 nm. The rates of hydrolysis can be directly correlated to the enzymatic activity of the proteasome. The reaction was monitored for 600 seconds, and the linear portion for each curve was used in calculating the IC50 values (inhibitor concentration to cause 50% inhibition of the enzyme active site).This was accomplished by plotting the residual proteasomal activity against the applied inhibitor concentration using GraphPad Prism version 6 for Windows, and fitting the experimental data to the equation: Y=Bottom + (Top-Bottom)/(1+10^((LogIC50-X)*HillSlope)) where X is the logarithm of inhibitor concentration and Y is the residual activity. Results are average of triplicate measurements. IC50 values were deduced from the fitted data. They depend on enzyme concentration and are comparable only within the same experimental settings.
Fluorogenic Assay for Detection of Human FAS-TE Inhibition
The synthetic fluorogenic substrate, 4-methylumbelliferyl heptanoate (4-MUH), was purchased from Sigma (St. Louis, MO). The reaction mixture consisted of 500 nM human FAS-TE in buffer (100mM Tris-HCl, 50 mM NaCl at pH 7.4) which was pre-incubated with 2.5 µL test compounds dissolved in DMSO at final concentrations of 0.32–100 µM and/or 0.08–10 µM at 37 °C for 30 minutes. The reaction was initiated by addition of 5 µL of 1.25 mM 4-MUH in 1:1 DMSO:buffer A. The resulting fluorescence from liberated 4-methylumbelliferone was measured every five minutes at 350/450nm for 60 minutes. 4-MUH incubated without enzyme served as a background control. Results are the average of triplicate time points. IC50 values were determined in similar fashion as described for the proteasome using GraphPad Prism 6 for Windows. IC50 values were deduced from the fitted data. They depend on enzyme concentration and are comparable only within the same experimental settings.
Cell Cytotoxicity Assays
HeLa cells were grown in DMEM containing 10% fetal bovine serum (FBS). Cells were seeded at the density of 5000 cells per well in a 96-well plate (Thermo) and were incubated overnight at 37°C with 5% CO2. The medium was removed and the cells were treated with 100 µL of fresh culture medium with 17, 18, 21, 25 and 26 (1–100 µM) or vehicle in duplicate. After 24 h, a stock solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 10 µL, 5 mM) was added to each well. As a negative control 10 µL of the MTT stock solution was added to 100 µL of medium alone. The plate was incubated at 37 °C for 4 h. All the medium was removed from the wells and 50 µL of DMSO was added to each well and the solution was mixed thoroughly with a pipette. Absorbance was read at 570 nm with a microplate reader (BioTek, Winooski, VT). The data are shown as mean ± SEM based on three independently repeated experiments. IC50 values were derived from dose-response curves. All statistical analyses were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA).. IC50 values were deduced from the fitted data. They depend on enzyme concentration and are comparable only within the same experimental settings.
Stability studies of β-lactones 21 and 25
In DMEM without FBS
A stock solution of 21 or 25 in DMSO (1 mM, 5 µL) was added to 995 µL of DMEM (pH = 7.5) to reach the final concentration of 5 µM. The vial was loosely capped and placed in an incubator at 37 °C with circulating 5% CO2. At regular time intervals 25 µL of the solution was transferred to another vial and mixed with 50 µL of phosphate buffer (pH = 2.0, 1M). The solution was submitted to HPLC analysis (PhenomenexR Gemini-NX 3µ C18, 110A. Size: 150 × 4.60 mm; Mobile Phase A: water; mobile phase B: acetonitrile) with a constant injection volume (20 µL). The % β-lactone remaining was based on integration in comparison to the initial pure β-lactone. All compounds were assayed in triplicate.
In DMEM with 5% FBS
A stock solution of 21 or 25 in DMSO (1 mM, 5 µL) was added to the pre-mixed 5% fetal bovine serum (FBS) in DMEM (995 µL) to reach the final concentration of 5 µM. The vial was loosely capped and placed in an incubator at 37 °C with circulating 5% CO2. At regular time intervals 100 µL of the solution was transferred to another vial and mixed with 100 µL of phosphate buffer (pH = 2.0, 1M) and 150 µL of CH3CN. The vial was vortexed for 1 minute to precipitate out the serum and 80 µL of the clear solution was transferred to another vial for HPLC analysis with a constant injection volume (20 µL) and analyzed by HPLC in a manner identical to that described above. The ‘time vs % β-lactone remaining’ curves are provided in the SI.
Activity-Based In situ proteome labelling
For labeling experiments, cells were grown to ca. 80–90% confluency in a complete medium in a 6-well plate. Then the medium was aspirated, cells were washed with prewarmed PBS (1.0 mL), followed by removal of PBS by suction. Then, 1.0 mL serum-free fresh medium containing either DMSO (as blank control) or probe at the appropriate concentration (10–50 µM) were added. DMSO content in the medium did not exceed 0.1% (1000× dilution from DMSO stock). Cells were incubated for 1 h with varying concentrations of alkyne probe 28 at 37 °C and 5% CO2. Subsequently, the medium was carefully aspirated, cells were washed with cold PBS (2 × 1.0 mL) to remove the excess of the probe and then harvested in 1.0 mL fresh cold PBS by scraping. Cell pellets were isolated by centrifugation (800 g, 3 min, 4 °C), resuspended in 100 µL lysis buffer (1% NP-40, 1% sodium deoxycholate in PBS, supplemented with protease inhibitor cocktail tablets (Roche)) and lysed for 15 min on ice. Soluble and insoluble fractions were separated by centrifugation at 14800 rpm for 45 min at 4 °C. Protein concentration (soluble fraction) was determined using a BCA protein concentration assay (Carl Roth) and adjusted to the same concentration in PBS (typically 1.0 mg/mL).
Activity-Based In situ competitive labelling
For labeling experiments, cells were grown to ca. 80–90% confluency in a complete medium in a 6-well plate. Then the medium was aspirated, cells were washed with prewarmed PBS (1.0 mL), followed by removal of PBS by suction. Then, 1.0 mL serum-free fresh medium containing either DMSO (as blank control), 10 µM competitive dual inhibitor 21 or 25 (for competitive labeling) at the appropriate concentration (0 → 100 µM) were added. DMSO content in the medium did not exceed 0.1% (1000× dilution from DMSO stock). Cells were incubated for 30 min with varying concentration of the competitive inhibitor (10 → 100 µM corresponding to 1, 2.5, 5, 10-fold excess compared to the alkyne probe 28) at 37 °C and 5% CO2. Then, 1 µL 100 mM alkyne probe 28 was added directly to the medium to achieve a 10 µM final concentration. Cells were incubated for another 30 min at 37 °C and 5% CO2. Subsequently, the medium was carefully aspirated, cells were washed with cold PBS (2 × 1.0 mL) to remove the excess of the probe and then harvested in 1.0 mL fresh cold PBS by scraping. Cell pellets were isolated by centrifugation (800 g, 3 min, 4 °C), resuspended in 100 µL lysis buffer (1% NP-40, 1% sodium deoxycholate in PBS, supplemented with protease inhibitor cocktail tablets (Roche)) and lysed for 15 min on ice. Soluble and insoluble fractions were separated by centrifugation at 14800 rpm for 45 min at 4 °C. Protein concentration (soluble fraction) was determined using BCA protein concentration assay (Carl Roth) and adjusted to the same concentration in PBS (typically 1.0 mg/mL).
Sharpless-Hüisgen (Click) Reactions, Protein Electrophoresis (SDS-PAGE) and In-Gel Fluorescence Scanning (IG-FS)
To 88 µL probe 28-bound cell lysate, 10 µL of a freshly prepared master mix containing 2 µL TAMRA-N3 fluorescent tag (5 mM stock in DMSO), 2 µL TCEP (50 mM stock in water) and 6 µL TBTA ligand (1.7 mM stock in DMSO:tert-BuOH 1:4 (v/v)) were added. The samples were gently vortexed and 2 µL CuSO4 (50 mM stock in water) was added to initiate the Sharpless-Hüisgen cycloaddition reaction, giving a total reaction volume of 100 µL. The final concentrations were as follows: 100 µM TAMRA-N3, 1 mM TCEP, 100 µM TBTA and 1 mM CuSO4. Samples were incubated at RT (25 °C) for 1–1.5 h in the dark. After click chemistry reaction, 400 µL pre-chilled acetone were added, the samples were gently vortexed and left overnight at −20 °C. Then, samples were centrifuged at 14800 rpm for 30 min at 4 °C. Supernatant was discarded and the protein pellets were washed with 200 µL pre-chilled MeOH, resuspended by sonication, centrifuged at 14800 rpm for 30 min at 4 °C and supernatant removed (repeated twice if necessary). After removal of MeOH, the samples were left to warm up to RT and dried in air for ca. 5 min. Then, samples were redissolved in 0.2% SDS in PBS, an equal volume of 2 × SDS-PAGE loading buffer (reducing) was added, samples were vortexed and denaturated at 95 °C for 5 min. Proteins were separated by 1D SDS-PAGE on 10% polyacrylamide gels (ca. 25 µg of protein/gel lane) applying 300 V and then visualized by in-gel fluorescence scanning. Fluorescence was recorded using Fujifilm LAS-4000 Luminescent Image Analyzer with a Fujinon VRF43LMD3 Lens and a 575DF20 filter. Gels were then subjected to Coomassie Brilliant Blue staining to verify equivalent protein loading.
Western Blot
Proteins were separated by 1D SDS-PAGE on 10% polyacrylamide gels and visualized by in-gel fluorescence scanning as described above. Then, the proteins were transferred to a PVDF membrane (VWR) with a semi-dry blotter for 70 min at 14 kV. Prior to the transfer, gel was incubated for ca. 5 min in the transfer buffer (48 mM Tris, 39 mM glycine, 0.38 % w/v SDS, 20% v/v MeOH) while the membrane in 100% MeOH. After the transfer, the membrane was saturated with 5% BSA in 0.1% TBS-T pH 8.0 for 1 h at RT. Then, the blot was cut into three parts for probing with three different antibodies. The upper part was incubated with rabbit anti-FAS primary antibody (1:1000 in 5% BSA in 0.1% TBS-T pH 8.0 (Cell Signaling C20G5)) overnight at 4 °C. Middle part was probed with goat anti-actin primary antibody (1: 1000 in 5% BSA in 0.1% TBS-T pH 8.0 (SantaCruz Biotechnology sc-1616)) overnight at 4 °C. Lower part was incubated with rabbit anti-proteasome beta-5 (anti-PSB-5) primary antibody (1: 1000 in 5% BSA in 0.1% TBS-T pH 8.0 (Enzo BML-PW8895)) overnight at 4 °C. The blots were then washed three times 15 min with TBS-T followed by the probing with the secondary antibody. Goat anti-rabbit IgG HRP-conjugated antibody (1:10,000 dilution in 5% BSA in TBS-T pH 8.0 (Pierce 32260)) and donkey anti-goat IgG HRP-conjugated antibody (1:10,000 dilution in 5% BSA in TBS-T pH 8.0 (SantaCruz Biotechnology sc-2020) were incubated for 1 h at RT (25 °C) with anti-FAS, anti-PSB-5 and anti-actin probed blots, respectively. Signals were detected using Amersham™ ECL™ Prime Western Blotting Detection Reagent (GE HealthCare RPN2232). Chemiluminescence was recorded for 10–60 sec using CCD camera of Fujifilm LAS-4000 Luminescent Image Analyzer.
Supplementary Material
Acknowledgments
We thank the NIH (R37 GM052964 to D.R.; R01 CA188694 to J.W.S; R01 CA161158 to W.S.L.), the NSF (CHE 1546973 to D.R.; for support of the Texas A&M University Undergraduate MiniPharma), and the Robert A. Welch Foundation (AA-1280 to D.R.; A-1715 to W.S.L.; A-0015 to J.C.S.) for generous support. We thank the Office of the Vice President for Research and the Dept. of Chemistry at Texas A&M University for support of the Natural Products LINCHPIN Laboratory at Texas A&M and the Undergraduate Research Scholars at Texas A&M for support of TAMU MiniPharma Team Leaders during Summers of 2012, 2013, and 2014. We thank Dr. Lisa M. Perez of the Texas A&M Laboratory for Molecular Simulation and members of the TAMU MiniPharma Molecular Modeling Team, in particular Evelyn Hoover, Deren Koseoglu, Robert Blando, Prithvi Vangal, Clifton Molak, Emily Brackhahn, and Asuka Orr for assistance in analyzing the orlistat-FAS-TE and human 20S proteasome-belactosin X-ray structure which aided this work.
Footnotes
Supporting Information. Synthetic procedures and characterization data for thioester S3 required to prepare ketene acetal 10b. Details of relative and absolute configuration assignment of β-lactone acids 12a, 12a’, 11b, 11b’, 11b”, 11b’”. Details of buffer/serum stability assays of β-lactones 21 and 25. 1H and 13C NMR spectra for all new compounds, and LC-MS data for β-lactone inhibitors 17–28.
References
- 1.a) Wermuth CG. Drug Discov. Today. 2004;9:826–827. doi: 10.1016/S1359-6446(04)03213-1. [DOI] [PubMed] [Google Scholar]; b) Arnaud CH. Chem. Eng. News. 2011;89:32–33. [Google Scholar]; c) Peters J-U. Polypharmacology in Drug Discovery. John Wiley & Sons; New York: 2012. [Google Scholar]; d) Ciceri P, Muller S, O'Mahony A, Fedorov O, Filippakopoulos P, Hunt JP, Lasater EA, Pallares G, Picaud S, Wells C, Martin S, Wodicka LM, Shah NP, Treiber DK, Knapp S. Nat. Chem. Biol. 2014;10:305–312. doi: 10.1038/nchembio.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Reddy AS, Zhang S. Expert Rev. Clin. Pharm. 2013;6:41–47. doi: 10.1586/ecp.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Arooj M, Sakkiah S, Cao Gp, Lee KW. PLoS ONE. 2013;8:e60470. doi: 10.1371/journal.pone.0060470. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Selvam B, Porter SL, Tikhonova IG. J Chem Inf Model. 2013;53:1761–1774. doi: 10.1021/ci400282q. [DOI] [PubMed] [Google Scholar]
- 2.a) Burris HA., 3rd Oncologist. 2004;9(Suppl 3):10–15. doi: 10.1634/theoncologist.9-suppl_3-10. [DOI] [PubMed] [Google Scholar]; b) Medina PJ, Goodin S. Clinical therapeutics. 2008;30:1426–1447. doi: 10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 3.a) Knight ZA, Lin H, Shokat KM. Nature reviews. Cancer. 2010;10:130–137. doi: 10.1038/nrc2787. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hampton T. Jama. 2004;292:419–422. doi: 10.1001/jama.292.4.419. [DOI] [PubMed] [Google Scholar]
- 4.Janin YL. Amino Acids. 2003;25:1–40. doi: 10.1007/s00726-002-0349-x. [DOI] [PubMed] [Google Scholar]
- 5.Harshbarger W, Miller C, Diedrich C, Sacchettini J. Structure. 2015;23:418–424. doi: 10.1016/j.str.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 6.a) Finley D. Ann. Rev. Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Genin E, Reboud-Ravaux M, Vidal J. Curr. Top. Med. Chem. 2010;10:232–256. doi: 10.2174/156802610790725515. [DOI] [PubMed] [Google Scholar]; c) Neefjes J, Jongsma ML, Paul P, Bakke O. Nat. Rev. Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]; d) Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K. Science. 2007;316:1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 7.Rastogi N, Mishra DP. Cell division. 2012;7:26. doi: 10.1186/1747-1028-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Nickeleit I, Zender S, Sasse F, Geffers R, Brandes G, Sorensen I, Steinmetz H, Kubicka S, Carlomagno T, Menche D, Gutgemann I, Buer J, Gossler A, Manns MP, Kalesse M, Frank R, Malek NP. Cancer Cell. 2008;14:23–35. doi: 10.1016/j.ccr.2008.05.016. [DOI] [PubMed] [Google Scholar]; b) Rentsch A, Landsberg D, Brodmann T, Bulow L, Girbig AK, Kalesse M. Angew. Chem. Int. Ed. 2013;52:5450–5488. doi: 10.1002/anie.201207900. [DOI] [PubMed] [Google Scholar]; c) Huber EM, Groll M. Angew. Chem. Int. Ed. 2012;51:8708–8720. doi: 10.1002/anie.201201616. [DOI] [PubMed] [Google Scholar]
- 9.Wang M. Oncology. 2011;25(Suppl 2):19–24. [PubMed] [Google Scholar]
- 10.a) Britton M, Lucas MM, Downey SL, Screen M, Pletnev AA, Verdoes M, Tokhunts RA, Amir O, Goddard AL, Pelphrey PM, Wright DL, Overkleeft HS, Kisselev AF. Chemistry & biology. 2009;16:1278–1289. doi: 10.1016/j.chembiol.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mirabella AC, Pletnev AA, Downey SL, Florea BI, Shabaneh TB, Britton M, Verdoes M, Filippov DV, Overkleeft HS, Kisselev AF. Chemistry & biology. 2011;18:608–618. doi: 10.1016/j.chembiol.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potts BC, Albitar MX, Anderson KC, Baritaki S, Berkers C, Bonavida B, Chandra J, Chauhan D, Cusack JC, Jr, Fenical W, Ghobrial IM, Groll M, Jensen PR, Lam KS, Lloyd GK, McBride W, McConkey DJ, Miller CP, Neuteboom ST, Oki Y, Ovaa H, Pajonk F, Richardson PG, Roccaro AM, Sloss CM, Spear MA, Valashi E, Younes A, Palladino MA. Curr. Cancer Drug Tar. 2011;11:254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L, Diao A. Anti-Cancer Agents in Medicinal Chemistry. 2015;15:298–306. doi: 10.2174/1871520614666141114202606. [DOI] [PubMed] [Google Scholar]
- 13.a) Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Angew. Chem. Int. Ed. 2003;42:355–357. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]; b) Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, Mitsiades C, Mitsiades N, Yasui H, Letai A, Ovaa H, Berkers C, Nicholson B, Chao TH, Neuteboom ST, Richardson P, Palladino MA, Anderson KC. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]; c) Ruschak AM, Slassi M, Kay LE, Schimmer AD. J. Natl. Cancer Inst. 2011;103:1007–1017. doi: 10.1093/jnci/djr160. [DOI] [PubMed] [Google Scholar]; d) Ma G, Nguyen H, Romo D. Org Lett. 2007;9:2143–2146. doi: 10.1021/ol070616u. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Nguyen H, Ma G, Gladysheva T, Fremgen T, Romo D. J. Org. Chem. 2011;76:2–12. doi: 10.1021/jo101638r. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Nguyen H, Ma G, Romo D. Chem. Commun. 2010;46:4803–4805. doi: 10.1039/c0cc00607f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu S, Wang J. Biomarker research. 2013;1:13. doi: 10.1186/2050-7771-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Menendez JA, Lupu R. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]; b) Kuhajda FP. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]; c) Menendez JA, Lupu R. Current opinion in clinical nutrition and metabolic care. 2006;9:346–357. doi: 10.1097/01.mco.0000232893.21050.15. [DOI] [PubMed] [Google Scholar]
- 16.Yoshii Y, Furukawa T, Oyama N, Hasegawa Y, Kiyono Y, Nishii R, Waki A, Tsuji AB, Sogawa C, Wakizaka H, Fukumura T, Yoshii H, Fujibayashi Y, Lewis JS, Saga T. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0064570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) WH Zhou WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi MS, El Meskini R, Ronnett GV, Townsend CA, Kuhajda FP. Cancer Res. 2007;67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]; b) WS Zhou PJ, McFadden JM, Townsend CA, Medghalchi SM, Vadlamudi A, Pinn ML, Ronnett GV, Kuhajda FP. Cancer Res. 2003;63:7330–7337. [PubMed] [Google Scholar]
- 18.a) Abdel-Magid AF. ACS Med. Chem. Lett. 2012;3:612–613. doi: 10.1021/ml3001566. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gordon EJ, Underwood JG. ACS Chem. Biol. 2008;3:594–599. [Google Scholar]; c) Turrado C, Puig T, Garcia-Carceles J, Artola M, Benhamu B, Ortega-Gutierrez S, Relat J, Oliveras G, Blancafort A, Haro D, Marrero PF, Colomer R, Lopez-Rodriguez ML. J. Med. Chem. 2012;55:5013–5023. doi: 10.1021/jm2016045. [DOI] [PubMed] [Google Scholar]; d) Kuhajda FP. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]; e) Richardson RD, Ma G, Oyola Y, Zancanella M, Knowles LM, Cieplak P, Romo D, Smith JW. J. Med. Chem. 2008;51:5285–5296. doi: 10.1021/jm800321h. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Drahl C. Chem. Eng. News. 2008;86:18–23. [Google Scholar]; g) Baron A, Migita T, Tang D, Loda M. J Cell Biochem. 2004;91:47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]; h) Yang PY, Liu K, Ngai MH, Lear MJ, Wenk MR, Yao SQ. J. Am. Chem. Soc. 2010;132:656–666. doi: 10.1021/ja907716f. [DOI] [PubMed] [Google Scholar]; i) Abramson HN. J. Med. Chem. 2011;54:5615–5638. doi: 10.1021/jm2005805. [DOI] [PubMed] [Google Scholar]
- 19.a) Little JL, Kridel SJ. Subcell Biochem. 2008;49:169–194. doi: 10.1007/978-1-4020-8831-5_7. [DOI] [PubMed] [Google Scholar]; b) Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 20.Pemble CWt, Johnson LC, Kridel SJ, Lowther WT. Nat. Struct. Mol. Biol. 2007;14:704–709. doi: 10.1038/nsmb1265. [DOI] [PubMed] [Google Scholar]
- 21.a) Ma G, Zancanella M, Oyola Y, Richardson RD, Smith JW, Romo D. Org Lett. 2006;8:4497–4500. doi: 10.1021/ol061651o. [DOI] [PubMed] [Google Scholar]; b) Zhang W, Richardson RD, Chamni S, Smith JW, Romo D. Bioorg. Med. Chem. Lett. 2008;18:2491–2494. doi: 10.1016/j.bmcl.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 22.a) Cho SW, Romo D. Org Lett. 2007;9:1537–1540. doi: 10.1021/ol070275k. [DOI] [PubMed] [Google Scholar]; b) Asai A, Hasegawa A, Ochiai K, Yamashita Y, Mizukami T. J. Antibiot. 2000;53:81–83. doi: 10.7164/antibiotics.53.81. [DOI] [PubMed] [Google Scholar]; c) Yamaguchi H, Asai A, Mizukami T, Yamashita Y, Akinaga S, Ikeda S-i, Kanda Y. Preparation of UCK 14A2 derivatives as proteasome inhibitors, Vol. Kyowa Hakko Kogyo Co., Ltd.; Japan: 2000. p. 115. [Google Scholar]; d) Mizukami A TA, Yamashita Y, Katahira R, Hasegawa A, Ochiai K, Akinaga S. In: Vol. L. Kyowa Hakko Kogyo Co., Japan, editor. Japan: 1997. [Google Scholar]
- 23.a) Groll M, Larionov OV, Huber R, de Meijere A. Proc. Natl. Acad. Sci. USA. 2006;103:4576–4579. doi: 10.1073/pnas.0600647103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Larionov OV, de Meijere A. Org Lett. 2004;6:2153–2156. doi: 10.1021/ol049409+. [DOI] [PubMed] [Google Scholar]
- 24.Groll M, Korotkov VS, Huber EM, de Meijere A, Ludwig A. Angewandte Chemie. 2015;54:7810–7814. doi: 10.1002/anie.201502931. [DOI] [PubMed] [Google Scholar]
- 25.Liggett A, Crawford LJ, Walker B, Morris TCM, Irvine AE. Leukemia Research. 2010;34:1403–1409. doi: 10.1016/j.leukres.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Cho SW. Total syntheses of β-lactone containing natural products: Total synthesis of belactosin C and synthetic studies toward spongiolactone Vol. Ph.D. Texas: A&M University; 2008. p. 175. [Google Scholar]
- 27.a) Kawamura S, Unno Y, List A, Mizuno A, Tanaka M, Sasaki T, Arisawa M, Asai A, Groll M, Shuto S. J. Med. Chem. 2013;56:3689–3700. doi: 10.1021/jm4002296. [DOI] [PubMed] [Google Scholar]; b) Kawamura S, Unno Y, Hirokawa T, Asai A, Arisawa M, Shuto S. Chem. Commun. 2014;50:2445–2447. doi: 10.1039/c3cc48818g. [DOI] [PubMed] [Google Scholar]; c) Kawamura S, Unno Y, Asai A, Arisawa M, Shuto S. Bioorg. Med. Chem. 2014 doi: 10.1016/j.bmc.2014.04.032. [DOI] [PubMed] [Google Scholar]; d) Kawamura S, Unno Y, Asai A, Arisawa M, Shuto S. J. Med. Chem. 2014;57:2726–2735. doi: 10.1021/jm500045x. [DOI] [PubMed] [Google Scholar]; e) Kawamura S, Unno Y, Asai A, Arisawa M, Shuto S. Org. Biomol. Chem. 2013;11:6615–6622. doi: 10.1039/c3ob41338a. [DOI] [PubMed] [Google Scholar]
- 28.Okpako DT. Principles of Pharmacology: A Tropical Approach. Cambridge University Press; 1991. Ch. 7. [Google Scholar]
- 29.a) Heal WP, Dang THT, Tate EW. Chem. Soc. Rev. 2011;40:246–257. doi: 10.1039/c0cs00004c. [DOI] [PubMed] [Google Scholar]; b) Cravatt BF, Wright AT, Kozarich JW. Ann. Rev. Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]; c) Evans MJ, Cravatt BF. Chem. Rev. 2006;106:3279–3301. doi: 10.1021/cr050288g. [DOI] [PubMed] [Google Scholar]; d) Krysiak JM, Kreuzer J, Macheroux P, Hermetter A, Sieber SA, Breinbauer R. Angew. Chem. Int. Ed. 2012;51:7035–7040. doi: 10.1002/anie.201201955. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Fonovic M, Bogyo M. Expert Rev. Proteomic. 2008;5:721–730. doi: 10.1586/14789450.5.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Hunerdosse D, Nomura DK. Curr. Opin. Biotech. 2014;28C:116–126. doi: 10.1016/j.copbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.a) Kluge AF, Petter RC. Curr. Opin. Chem. Biol. 2010;14:421–427. doi: 10.1016/j.cbpa.2010.03.035. [DOI] [PubMed] [Google Scholar]; b) Böttcher T, Sieber SA. Med. Chem. Commun. 2012;3:408. [Google Scholar]; c) Gersch M, Kreuzer J, Sieber SA. Nat. Prod. Rep. 2012;29:659–682. doi: 10.1039/c2np20012k. [DOI] [PubMed] [Google Scholar]
- 31.a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; b) Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]; c) Yang Y, Yang X, Verhelst SH. Molecules. 2013;18:12599–12608. doi: 10.3390/molecules181012599. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Adibekian A, Martin BR, Wang C, Hsu KL, Bachovchin DA, Niessen S, Hoover H, Cravatt BF. Nat. Chem. Biol. 2011;7:469–478. doi: 10.1038/nchembio.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Liu K, Kuang G, Masuda T, Zhang A. Macromolecules. 2014;47:3288–3296. [Google Scholar]
- 33.Yang P-Y, Liu K, Ngai MH, Lear MJ, Wenk MR, Yao SQ. Journal of the American Chemical Society. 2010;132:656–666. doi: 10.1021/ja907716f. [DOI] [PubMed] [Google Scholar]
- 34.Kumaraswamy G, Padmaja M, Markondaiah B, Jena N, Sridhar B, Kiran MU. J. Org. Chem. 2006;71:337–340. doi: 10.1021/jo0516887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.