Abstract
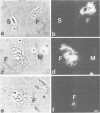
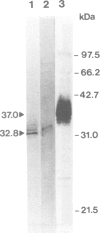
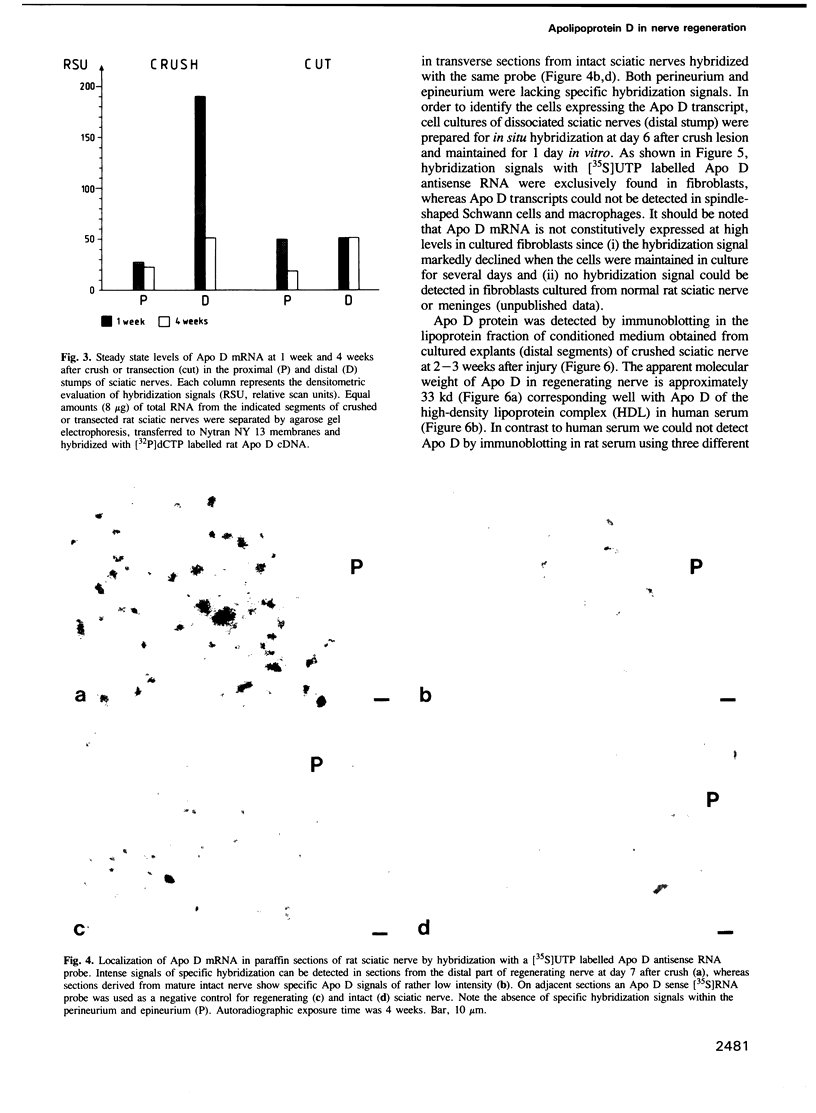
A cDNA clone containing the entire coding region of rat apolipoprotein D (Apo D) was isolated from a cDNA library of regenerating sciatic nerve by differential hybridization. Only small amounts of Apo D mRNA were detected in noninjured mature nerve. Moderately increased levels of Apo D transcripts were found in transected nerves, which were prevented from regeneration by ligation. In contrast, in regenerating crushed nerve, the steady-state level of Apo D mRNA transiently increased at least 40-fold above control levels at the time when axons from the proximal stump grow into the distal nerve segment. Using transverse sections and primary cell cultures from regenerating nerve, Apo D transcripts could be localized by in situ hybridization in endoneurial fibroblasts but not in Schwann cells, macrophages or perineurial and epineurial cells. Apo D protein (Mr 32.8 kd) was secreted and accumulated in the endoneurial extracellular space where it could be detected in lipoprotein fractions by immunoblotting using established antibodies to human Apo D. High level expression of Apo D mRNA seems to be a novel regeneration-associated molecular event of endoneurial fibroblasts indicating a function for Apo D and fibroblasts in nerve repair.
Full text
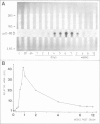
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Johnson M. L. Quantitative histology of Wallerian degeneration: I. Nuclear population in rabbit sciatic nerve. J Anat. 1946 Jan;80(Pt 1):37–50. [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuche W., Friede R. L. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984 Oct;13(5):767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- Boyles J. K., Zoellner C. D., Anderson L. J., Kosik L. M., Pitas R. E., Weisgraber K. H., Hui D. Y., Mahley R. W., Gebicke-Haerter P. J., Ignatius M. J. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989 Mar;83(3):1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley W. G., Asbury A. K. Duration of synthesis phase in neuilemma cells in mouse sciatic nerve during degeneration. Exp Neurol. 1970 Feb;26(2):275–282. doi: 10.1016/0014-4886(70)90125-1. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D., Fielding C., McLean J., Baer B., Castro G., Chen E., Comstock L., Henzel W., Kohr W., Rhee L. Cloning and expression of human apolipoprotein D cDNA. J Biol Chem. 1986 Dec 15;261(35):16535–16539. [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J. A cholesteryl ester transfer complex in human plasma. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3327–3330. doi: 10.1073/pnas.77.6.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R., Lindholm D., Bandtlow C., Meyer M., Radeke M. J., Misko T. P., Shooter E., Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius M. J., Gebicke-Härter P. J., Skene J. H., Schilling J. W., Weisgraber K. H., Mahley R. W., Shooter E. M. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1125–1129. doi: 10.1073/pnas.83.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius M. J., Shooter E. M., Pitas R. E., Mahley R. W. Lipoprotein uptake by neuronal growth cones in vitro. Science. 1987 May 22;236(4804):959–962. doi: 10.1126/science.3576212. [DOI] [PubMed] [Google Scholar]
- Kaplan B. B., Bernstein S. L., Gioio A. E. An improved method for the rapid isolation of brain ribonucleic acid. Biochem J. 1979 Oct 1;183(1):181–184. doi: 10.1042/bj1830181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer L. F., Cornbrooks C. J. Transplants of Schwann cell cultures promote axonal regeneration in the adult mammalian brain. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6330–6334. doi: 10.1073/pnas.82.18.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lemke G., Chao M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development. 1988 Mar;102(3):499–504. doi: 10.1242/dev.102.3.499. [DOI] [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Hengerer B., Thoenen H. Interleukin 1 increases stability and transcription of mRNA encoding nerve growth factor in cultured rat fibroblasts. J Biol Chem. 1988 Nov 5;263(31):16348–16351. [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Meyer M., Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987 Dec 17;330(6149):658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Lubińska L. Early course of Wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 1977 Jul 8;130(1):47–63. doi: 10.1016/0006-8993(77)90841-1. [DOI] [PubMed] [Google Scholar]
- Meier R., Spreyer P., Ortmann R., Harel A., Monard D. Induction of glia-derived nexin after lesion of a peripheral nerve. Nature. 1989 Nov 30;342(6249):548–550. doi: 10.1038/342548a0. [DOI] [PubMed] [Google Scholar]
- Müller H. W., Gebicke-Härter P. J., Hangen D. H., Shooter E. M. A specific 37,000-dalton protein that accumulates in regenerating but not in nonregenerating mammalian nerves. Science. 1985 Apr 26;228(4698):499–501. doi: 10.1126/science.3983637. [DOI] [PubMed] [Google Scholar]
- Müller H. W., Ignatius M. J., Hangen D. H., Shooter E. M. Expression of specific sheath cell proteins during peripheral nerve growth and regeneration in mammals. J Cell Biol. 1986 Feb;102(2):393–402. doi: 10.1083/jcb.102.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Brown M. C., Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987 Apr 1;165(4):1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P. M., McGuinness U. M., Aguayo A. J. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980 Mar 20;284(5753):264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Salonen V., Aho H., Röyttä M., Peltonen J. Quantitation of Schwann cells and endoneurial fibroblast-like cells after experimental nerve trauma. Acta Neuropathol. 1988;75(4):331–336. doi: 10.1007/BF00687785. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T., Friede R. L. The role of endoneurial fibroblasts in myelin degradation. J Neuropathol Exp Neurol. 1981 Mar;40(2):134–154. doi: 10.1097/00005072-198103000-00006. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Shooter E. M. Denervated sheath cells secrete a new protein after nerve injury. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4169–4173. doi: 10.1073/pnas.80.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes G. J., McGuire C. B., Norden J. J., Freeman J. A. Nerve injury stimulates the secretion of apolipoprotein E by nonneuronal cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1130–1134. doi: 10.1073/pnas.83.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G., Griffin J. W., Li C. Y., Trapp B. D. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989 Oct;18(5):671–683. doi: 10.1007/BF01187086. [DOI] [PubMed] [Google Scholar]
- Stoll G., Müller H. W. Macrophages in the peripheral nervous system and astroglia in the central nervous system of rat commonly express apolipoprotein E during development but differ in their response to injury. Neurosci Lett. 1986 Dec 23;72(3):233–238. doi: 10.1016/0304-3940(86)90519-7. [DOI] [PubMed] [Google Scholar]
- Taub F., Thompson E. B. An improved method for preparing large arrays of bacterial colonies containing plasmids for hybridization: in situ purification and stable binding of DNA on paper filters. Anal Biochem. 1982 Oct;126(1):222–230. doi: 10.1016/0003-2697(82)90133-6. [DOI] [PubMed] [Google Scholar]
- Trapp B. D., Hauer P., Lemke G. Axonal regulation of myelin protein mRNA levels in actively myelinating Schwann cells. J Neurosci. 1988 Sep;8(9):3515–3521. doi: 10.1523/JNEUROSCI.08-09-03515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. L., Raine C. S. Reinnervation of peripheral nerve segments implanted into the rat central nervous system. Brain Res. 1980 Sep 29;198(1):1–11. doi: 10.1016/0006-8993(80)90339-x. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J. K., Dyck P. J. Cholesterol esterifying enzyme in normal and degenerating peripheral nerve. J Neurochem. 1981 Jul;37(1):156–163. doi: 10.1111/j.1471-4159.1981.tb05303.x. [DOI] [PubMed] [Google Scholar]