Abstract
Background
Upper body subcutaneous fat is a distinct fat depot that may confer increased cardiometabolic risk. We examined the cross-sectional associations between upper body subcutaneous fat volume and cardiometabolic risk factors.
Methods
Participants were from the Framingham Heart Study who underwent multi-detector computed tomography between 2008–2011. Sex-specific multivariable-adjusted regression analyses were conducted. Covariates included age, ethnicity, smoking status, alcohol intake, postmenopausal status, and hormone replacement therapy. Additional models included adjustment for body mass index (BMI), neck circumference, or abdominal visceral adipose tissue.
Results
2306 participants (mean age 60 years, 54.4% women) were included. Mean upper body subcutaneous fat was 309.9cm3 in women and 345.6cm3 in men. Higher upper body subcutaneous fat volume was associated with adverse cardiometabolic risk factors. In women and men, each additional 50cm3 increment in upper body subcutaneous fat was associated with a 3.23 and 2.65kg/m2 increase in body mass index (BMI); 2.16 and 0.88mmHg increase in systolic blood pressure; 2.53 and 1.66mg/dL increase in fasting plasma glucose; 0.12 and 0.11mg/dL increase in log triglycerides; and 4.17 and 3.68mg/dL decrease in high-density lipoprotein cholesterol, respectively (all p≤0.008). Similar patterns were observed with prevalent cardiometabolic risk factors. These associations remained significant after additional adjustment for BMI, neck circumference, or abdominal visceral adipose tissue.
Conclusions
Higher upper body subcutaneous fat is cross-sectionally associated with adverse cardiometabolic risk factors. Our findings underscore the importance of subcutaneous adiposity in the upper body region that may provide a better understanding of the pathogenic properties of obesity in the development of cardiometabolic sequelae.
Keywords: subcutaneous adipose tissue, metabolic diseases, imaging, epidemiology
INTRODUCTION
Variation in body fat distribution, independent of generalized adiposity, is associated with differential metabolic risk factors.1–3 Abdominal visceral adipose tissue has been shown to be a pathogenic fat depot that is associated with metabolic syndrome and its risk factors.1, 4 However, abdominal visceral adipose tissue does not explain all the variation in the cardiometabolic risk models, suggesting that other factors, including other ectopic fat depots, may be contributing.
Upper body subcutaneous fat is a distinct fat depot located in an anatomic compartment separate from abdominal subcutaneous fat.5 Prior work suggests that upper body subcutaneous fat may be an important mediator of metabolic risk. Several disease states are associated with a predilection to accumulate upper body fat, including Cushing’s syndrome,6 lipodystrophy7 and human immunodeficiency virus associated lipodystrophy,7 all of which have been linked to metabolic impairments.6, 8, 9 Experimental evidence has shown that upper body subcutaneous fat is the primary source of circulating free fatty acids10 and is a strong determinant of insulin resistance.11 We have previously shown that neck circumference, used as an indirect measure of upper body subcutaneous fat, is associated with cardiometabolic risk factors12 and subclinical atherosclerosis.13 Taken together, these findings suggest upper body subcutaneous fat may be an important pathogenic fat depot that warrants further investigation. Using the actual measure of upper body subcutaneous fat, rather than the rough proxy of the body fat in the upper body region, is essential to precisely explore the pathogenic properties involved with this specific fat depot.
Therefore, the primary purpose of this research was to determine whether upper body subcutaneous fat is cross-sectionally associated with a comprehensive list of cardiometabolic risk factors. We further examined if any associations persisted after additionally accounting for generalized obesity [body mass index (BMI)], neck circumference, or abdominal visceral adipose tissue. We hypothesized that higher upper body subcutaneous fat would be associated with more adverse cardiometabolic risk factors above and beyond the contribution of BMI, neck circumference, or abdominal visceral adipose tissue.
METHODS
Study Sample
Framingham Heart Study was initiated in 1948 as a community-based longitudinal study to determine risk factors of cardiovascular disease.14 We included 2,803 participants from the Offspring cohort 9th examination, Third Generation cohort 2nd examination, and Omni cohort 2nd examination who underwent assessment of the chest and abdomen via multi-detector computed tomography (MDCT) between 2008 and 2011. Inclusion criteria for participating in the MDCT sub-study was residing in the greater New England area; an age of ≥40 years and not pregnant for women and ≥35 years of age for men; and body weight less than 450lbs due to the MDCT scanner weight restriction.
Of 2,803 participants, we excluded participants with missing upper body subcutaneous fat measurement (n=355); missing cardiometabolic risk factors (n=25); missing covariates (n=30); and/or a history of cardiovascular disease, including myocardial infarction, coronary heart disease death, stroke or congestive heart failure (n=108); resulting in a total of 2,306 participants available for analysis. This study was approved by institutional review boards of the Boston University Medical Center and Massachusetts General Hospital. All participants provided written informed consent.
MDCT-Acquired Fat Depots
Participants underwent a 64-slice MDCT scan of the chest. Study protocol was previously validated and published elsewhere with an excellent intra- and inter-reproducibility of 0.99.5 40 contiguous 0.625mm thick MDCT slices superior to the body of the sternum covering a 25mm area (40 MDCT imagesX0.625mm for each slice) were selected for the assessment of the upper body subcutaneous fat. A dedicated three-dimensional offline workstation software (Aquarius 3D Workstation, TeraRecon Inc) with a predefined image window range of −195 and −45 Hounsfield units with center attenuation of −120 Hounsfield units was used to quantify adipose tissue from the MDCT images. Total neck fat was defined as adipose tissue quantified in the entire area encompassing 25mm above the body of the sternum while excluding the adipose tissue within the mediastinum. Breast fat was specified as adipose tissue exterior to the chest wall. Upper body subcutaneous fat volume, measured in cm3, was calculated by subtracting breast fat from total neck fat.
Using an identical setting, abdominal visceral adipose tissue was assessed from the abdominal MDCT scans by manually outlining the muscular wall that differentiates abdominal subcutaneous adipose tissue and visceral adipose tissue. High inter- and intra- reproducibility of abdominal visceral adiposity has been previously reported.15
Outcomes and Covariates
Details of cardiometabolic risk factors and covariates are described in supplemental materials.
Statistical Analysis
Triglycerides were natural log-transformed to improve the normality of the distribution. All the statistical analyses were stratified by sex given results from our prior research, which found striking sex differences in adiposity measures.1, 12, 16 Interaction between sex and upper body subcutaneous fat were formally tested via multiple regression models.
Age-adjusted sex-specific Pearson correlation coefficients (r) among the adiposity measures were assessed to explore the associations among the various fat measures. Age-adjusted sex-specific Pearson correlation coefficients were computed to examine the correlations between adiposity measures and continuous cardiometabolic risk factors.
For continuous outcomes, sex-specific multivariable-adjusted linear regression models were constructed to assess the relationships between upper body subcutaneous fat and cardiometabolic risk factors. For dichotomous outcomes, sex-specific multivariable-adjusted logistic regression models were used to determine the relationship between upper body subcutaneous fat and prevalent cardiometabolic risk factors. A separate regression analysis was conducted for each cardiometabolic risk factor. β-coefficients from the linear regression models and odds ratios (ORs) from the logistic regression models describe the associations of the cardiometabolic risk factors per 50cm3 increment in upper body subcutaneous fat. Multivariable adjustment included age, ethnicity, smoking status, alcohol intake, physical activity, postmenopausal status, and hormone replacement therapy. Model-specific adjustment was applied for several linear regression models as follows: lipid-lowering treatment for the total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol models; hypertension treatment for the systolic and diastolic blood pressure models; and diabetes treatment for the fasting plasma glucose model.
Additional models assessed the associations between upper body subcutaneous fat and cardiometabolic risk factors after further adjustment for BMI, neck circumference, or abdominal visceral adipose tissue (separately). Finally, participants were stratified into tertiles based on their upper body subcutaneous fat. Logistic regression models were used to assess the association between each prevalent cardiometabolic risk factor and upper body subcutaneous fat tertile. Purpose of this study was hypothesis generating, thus significance level was not adjusted for multiple comparisons. Statistical significance was considered at the two-tailed p<0.05 level. All statistical analyses were conducted using SAS version 9.3 (SAS Institute).
RESULTS
Characteristics of Study Participants
The characteristics of participants are shown in Table 1. A final sample of 1,255 women and 1,051 men (mean age 60 years) were included in this study. The study sample consisted of 96.5% White, 1.6% Asian, 1.2% African-American, and 0.7% others (Indian, Pacific Islander, or Native American).
Table 1.
Characteristics of the study participants.
| Characteristics | Women (n=1,255) | Men (n=1,051) |
|---|---|---|
| Demographics | ||
| Age, years | 61.0 (12.0) | 57.9 (12.7) |
| Adiposity Measures | ||
| Upper Body Subcutaneous Fat, cm3 | 309.9 (72.4) | 345.6 (61.7) |
| Body Mass Index, kg/m2 | 27.5 (5.5) | 28.1 (3.8) |
| Neck Circumference, cm | 13.6 (2.7) | 15.9 (1.1) |
| Visceral Adipose Tissue, cm3 | 1,641 (1,026) | 2,936 (1,353) |
| Continuous Cardiometabolic Risk Factors | ||
| Systolic Blood Pressure, mm Hg | 120.2 (16.4) | 123.5 (13.8) |
| Diastolic Blood Pressure, mm Hg | 71.4 (8.9) | 75.7 (9.0) |
| Fasting Plasma Glucose, mg/dL | 96.5 (15.9) | 102.7 (21.0) |
| Total Cholesterol, mg/dL | 195.4 (34.2) | 182.2 (34.7) |
| Triglycerides*, mg/dL | 95.0 (71.0–130.0) | 101.0 (75.0–145.0) |
| HDL Cholesterol, mg/dL | 68.6 (18) | 53.2 (14.4) |
| LDL Cholesterol, mg/dL | 104.9 (30.8) | 105.0 (30.5) |
| Dichotomous Cardiometabolic Risk Factors | ||
| Obesity, % | 27.7 (348) | 26.8 (282) |
| Hypertension, % | 40.2 (504) | 40.4 (425) |
| Impaired Fasting Glucose, % | 28.7 (360) | 46.5 (489) |
| Diabetes Mellitus, % | 7.8 (98) | 11.4 (120) |
| Hypercholesterolemia, % | 38.5 (483) | 43.5 (457) |
| Hypertriglyceridemia, % | 39.4 (495) | 51.7 (543) |
| Low HDL Cholesterol, % | 13.3 (167) | 16.8 (177) |
| High LDL Cholesterol, % | 4.5 (56) | 3.8 (40) |
| Metabolic Syndrome, % | 34.7 (436) | 40.9 (430) |
| Current Smoking, % | 6.1 (77) | 6.6 (69) |
| Alcohol Intake, % | 3.3 (4.9) | 7.0 (8.8) |
| Hypertensive treatment, % | 34.5 (433) | 33.7 (354) |
| Lipid-Lowering Treatment, % | 31.1 (390) | 39.2 (412) |
| Postmenopausal Status, % | 70.5 (885) | – |
| Hormone Replacement Therapy, % | 7.5 (94) | – |
Unless otherwise indicated, continuous variables are described as means (standard deviations) and categorical variables are described as percentages (counts).
Described as median (25th–75th percentile) due to the skewed distribution.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Correlations among Adiposity Measures
Age-adjusted sex-specific Pearson correlation coefficients among pairs of adiposity measures are reported in Supplemental Table 1. upper body subcutaneous fat was strongly correlated with BMI and VAT in both women and men (all r≥0.72). Among women, neck circumference was moderately correlated with the other adiposity measures (r ranging from 0.33 to 0.39); Whereas in men, the correlations between neck circumference and adiposity measures were relatively higher than those observed in women (r ranging from 0.61 to 0.77).
Correlations between upper body subcutaneous fat and Cardiometabolic Risk Factors
Table 2 describes the age-adjusted sex-specific correlations between adiposity measures and continuous cardiometabolic risk factors. In general, higher upper body subcutaneous fat volumes and a larger neck circumference were associated with more adverse cardiometabolic risk factors in both women and men.
Table 2.
Age-adjusted sex-specific Pearson correlation coefficients between upper body subcutaneous fat or neck circumference and continuous cardiometabolic risk factors.
| Cardiometabolic Risk Factors | Upper Body Subcutaneous Fat | Neck Circumference | ||
|---|---|---|---|---|
|
| ||||
| Women | Men | Women | Men | |
| Systolic Blood Pressure | 0.23§ | 0.11‡ | 0.08† | 0.09† |
| Diastolic Blood Pressure | 0.23§ | 0.17§ | 0.06* | 0.15§ |
| Fasting Plasma Glucose | 0.32§ | 0.16§ | 0.23§ | 0.17§ |
| Total Cholesterol | −0.03 | −0.06* | −0.05 | −0.04 |
| Log Triglycerides | 0.42§ | 0.29§ | 0.17§ | 0.27§ |
| High-Density Lipoprotein Cholesterol | −0.35§ | −0.30§ | −0.15§ | −0.27§ |
| Low-Density Lipoprotein Cholesterol | 0.04 | −0.04 | −0.02 | −0.02 |
p<0.05,
p<0.01,
p<0.001,
p<0.0001.
Associations between upper body subcutaneous fat and Continuous Cardiometabolic Risk Factors
Results of the multivariable-adjusted regression analyses for the associations between upper body subcutaneous fat with continuous cardiometabolic risk factors are shown in Table 3. In general, higher upper body subcutaneous fat was associated with higher BMI, systolic and diastolic blood pressure, fasting plasma glucose, log triglycerides; and lower HDL cholesterol (all p≤0.008). For instance, in women and men, each additional 50cm3 increment in upper body subcutaneous fat was associated with a 3.23 and 2.65kg/m2 increase in BMI; 2.16 and 0.88mmHg increase in systolic blood pressure; 2.53 and 1.66mg/dL increase in fasting plasma glucose; and 4.17 and 3.68mg/dL decrease in HDL cholesterol, respectively (all p≤0.008, Table 3). Conversely, upper body subcutaneous fat was not significantly associated with total cholesterol in both sexes (both p=0.26); and with LDL cholesterol in men (p=0.91).
Table 3.
Multivariable-adjusted linear regression models for the associations between upper body subcutaneous fat and continuous cardiometabolic risk factors.
| Cardiometabolic Risk Factors | Models* | Women | Men | p for Sex Interaction† | ||
|---|---|---|---|---|---|---|
|
| ||||||
| β (95% CI) | p-Value | β (95% CI) | p-Value | |||
| Body Mass Index | MV | 3.23 (3.11, 3.34) | <.0001 | 2.65 (2.55, 2.76) | <.0001 | <.0001 |
| MV + Neck | 2.94 (2.81, 3.07) | <.0001 | 1.96 (1.80, 2.11) | <.0001 | ||
| MV + VAT | 2.47 (2.29, 2.66) | <.0001 | 2.32 (2.18, 2.47) | <.0001 | ||
| Systolic Blood Pressure | MV | 2.16 (1.59, 2.72) | <.0001 | 0.88 (0.24, 1.53) | 0.008 | 0.0006 |
| MV + BMI | 1.43 (0.41, 2.45) | 0.006 | 1.07 (−0.11, 2.26) | 0.08 | ||
| MV + Neck | 1.94 (1.30, 2.59) | <.0001 | 0.97 (−0.03, 1.98) | 0.06 | ||
| MV + VAT | 1.30 (0.39, 2.20) | 0.005 | −0.15 (−1.07, 0.77) | 0.75 | ||
| Diastolic Blood Pressure | MV | 1.44 (1.10, 1.78) | <.0001 | 1.15 (0.73, 1.58) | <.0001 | 0.37 |
| MV + BMI | 1.02 (0.41, 1.63) | 0.001 | 0.83 (0.05, 1.61) | 0.04 | ||
| MV + Neck | 1.33 (0.94, 1.72) | <.0001 | 1.01 (0.35, 1.67) | 0.003 | ||
| MV + VAT | 1.19 (0.64, 1.73) | <.0001 | 0.28 (−0.33, 0.88) | 0.37 | ||
| Fasting Plasma Glucose | MV | 2.53 (2.03, 3.04) | <.0001 | 1.66 (0.73, 2.60) | 0.0005 | 0.11 |
| MV + BMI | 1.53 (0.60, 2.45) | 0.001 | 1.17 (−0.55, 2.88) | 0.18 | ||
| MV + Neck | 2.19 (1.60, 2.77) | <.0001 | 0.27 (−1.18, 1.72) | 0.71 | ||
| MV + VAT | 0.20 (−0.61, 1.01) | 0.63 | −0.08 (−1.41, 1.25) | 0.90 | ||
| Total Cholesterol | MV | 0.74 (−0.55, 2.03) | 0.26 | −0.92 (−2.52, 0.68) | 0.26 | 0.051 |
| MV + BMI | 1.43 (−0.92, 3.77) | 0.23 | −1.46 (−4.40, 1.47) | 0.33 | ||
| MV + Neck | 0.80 (−0.69, 2.29) | 0.29 | −1.55 (−4.04, 0.93) | 0.22 | ||
| MV + VAT | 2.10 (0.02, 4.17) | 0.05 | −3.63 (−5.90, −1.35) | 0.002 | ||
| Log Triglycerides | MV | 0.12 (0.11, 0.14) | <.0001 | 0.11 (0.09, 0.14) | <.0001 | 0.14 |
| MV + BMI | 0.12 (0.09, 0.15) | <.0001 | 0.10 (0.05, 0.14) | <.0001 | ||
| MV + Neck | 0.11 (0.09, 0.13) | <.0001 | 0.08 (0.04, 0.12) | <.0001 | ||
| MV + VAT | 0.06 (0.03, 0.08) | <.0001 | 0.03 (−0.01, 0.06) | 0.12 | ||
| HDL Cholesterol | MV | −4.17 (−4.82, −3.52) | <.0001 | −3.68 (−4.36, −3.01) | <.0001 | 0.08 |
| MV + BMI | −3.68 (−4.87, −2.49) | <.0001 | −2.98 (−4.21, −1.76) | <.0001 | ||
| MV + Neck | −3.86 (−4.61, −3.10) | <.0001 | −2.70 (−3.74, −1.66) | <.0001 | ||
| MV + VAT | −1.18 (−2.22, −0.15) | 0.02 | −2.01 (−2.96, −1.06) | <.0001 | ||
| LDL Cholesterol | MV | 2.18 (1.01, 3.36) | 0.0003 | 0.08 (−1.30, 1.46) | 0.91 | 0.005 |
| MV + BMI | 2.33 (0.19, 4.48) | 0.03 | −0.75 (−3.27, 1.77) | 0.56 | ||
| MV + Neck | 2.34 (0.98, 3.70) | 0.0007 | −0.73 (−2.86, 1.40) | 0.50 | ||
| MV + VAT | 2.15 (0.25, 4.05) | 0.03 | −2.30 (−4.25, −0.35) | 0.02 | ||
Data are shown as β coefficients (95% CIs). The values of β coefficients describe the association with each cardiometabolic risk factor for a 50cm3 increment in upper body subcutaneous fat.
MV model adjusted for age, ethnicity, smoking status, alcohol intake, physical activity index, postmenopausal status (women only), and hormone replacement therapy (women only); MV + BMI model was additionally adjusted for BMI; MV + Neck model was additionally adjusted for neck circumference; and MV + VAT model was additionally adjusted for abdominal visceral adipose tissue volume. Model-specific adjustment was applied as follows: lipid-lowering treatment for the total cholesterol triglycerides, HDL cholesterol, and LDL cholesterol models; hypertension treatment for the systolic and diastolic blood pressure models; and diabetes treatment for the fasting plasma glucose model.
Sex interaction was tested based on MV model.
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MV, multivariable; VAT, visceral adipose tissue.
Associations between upper body subcutaneous fat and Prevalent Cardiometabolic Risk Factors
Higher upper body subcutaneous fat was associated with increased odds of prevalent cardiometabolic risk factors, except for high LDL cholesterol (Table 4). For example, each 50cm3 increment in upper body subcutaneous fat was associated with an increased prevalence of obesity [women, OR 9.93, 95% confidence interval (CI) 7.51, 13.14; men, OR 10.30, 95% CI 7.57, 14.04], diabetes (women, OR 1.88, 95% CI 1.59, 2.23; men, OR 1.56, 95% CI 1.32, 1.84), and low HDL cholesterol (women, OR 1.51, 95% CI 1.35, 1.69; men OR 1.58, 95% CI, 1.38, 1.81).
Table 4.
Multivariable-adjusted logistic regression models for the associations between upper body subcutaneous fat and dichotomous cardiometabolic risk factors.
| Cardiometabolic Risk Factors | Models* | Women | Men | p for Sex Interaction† | ||
|---|---|---|---|---|---|---|
|
| ||||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Obesity | MV | 9.93 (7.51, 13.14) | <.0001 | 10.30 (7.57, 14.04) | <.0001 | 0.93 |
| MV + Neck | 5.99 (4.42, 8.12) | <.0001 | 5.93 (4.22, 8.34) | <.0001 | ||
| MV + VAT | 6.78 (4.96, 9.26) | <.0001 | 8.14 (5.84, 11.34) | <.0001 | ||
| Hypertension | MV | 1.59 (1.43, 1.75) | <.0001 | 1.37 (1.22, 1.54) | <.0001 | 0.10 |
| MV + BMI | 1.32 (1.11, 1.57) | 0.002 | 1.15 (0.93, 1.42) | 0.20 | ||
| MV + Neck | 1.26 (1.08, 1.47) | 0.003 | 1.10 (0.92, 1.32) | 0.30 | ||
| MV + VAT | 1.25 (1.07, 1.47) | 0.006 | 1.05 (0.89, 1.24) | 0.56 | ||
| Impaired Fasting Glucose | MV | 1.81 (1.63, 2.01) | <.0001 | 1.36 (1.21, 1.51) | <.0001 | 0.0002 |
| MV + BMI | 1.46 (1.22, 1.74) | <.0001 | 1.15 (0.94, 1.40) | 0.17 | ||
| MV + Neck | 1.34 (1.15, 1.57) | 0.0003 | 1.22 (1.03, 1.44) | 0.02 | ||
| MV + VAT | 1.19 (1.01, 1.40) | 0.03 | 1.16 (0.99, 1.35) | 0.06 | ||
| Diabetes Mellitus | MV | 1.88 (1.59, 2.23) | <.0001 | 1.56 (1.32, 1.84) | <.0001 | 0.14 |
| MV + BMI | 1.38 (1.06, 1.81) | 0.02 | 1.21 (0.91, 1.62) | 0.20 | ||
| MV + Neck | 1.30 (1.01, 1.67) | 0.04 | 1.27 (0.99, 1.63) | 0.06 | ||
| MV + VAT | 1.20 (0.93, 1.54) | 0.16 | 1.36 (1.09, 1.70) | 0.006 | ||
| Hypercholesterolemia | MV | 1.41 (1.29, 1.55) | <.0001 | 1.28 (1.15, 1.43) | <.0001 | 0.18 |
| MV + BMI | 1.40 (1.19, 1.66) | <.0001 | 1.15 (0.94, 1.40) | 0.18 | ||
| MV + Neck | 1.43 (1.29, 1.60) | <.0001 | 1.21 (1.02, 1.43) | 0.03 | ||
| MV + VAT | 1.22 (1.05, 1.42) | 0.01 | 0.96 (0.82, 1.13) | 0.65 | ||
| Hypertriglyceridemia | MV | 1.70 (1.54, 1.88) | <.0001 | 1.52 (1.36, 1.70) | <.0001 | |
| MV + BMI | 1.72 (1.45, 2.05) | <.0001 | 1.21 (0.99, 1.48) | 0.06 | ||
| MV + Neck | 1.68 (1.50, 1.87) | <.0001 | 1.28 (1.08, 1.52) | 0.005 | ||
| MV + VAT | 1.28 (1.10, 1.50) | 0.002 | 1.08 (0.91, 1.26) | 0.38 | ||
| Low HDL Cholesterol | MV | 1.51 (1.35, 1.69) | <.0001 | 1.58 (1.38, 1.81) | <.0001 | 0.77 |
| MV + BMI | 1.51 (1.23, 1.86) | 0.0001 | 1.35 (1.06, 1.73) | 0.01 | ||
| MV + Neck | 1.45 (1.25, 1.68) | <.0001 | 1.29 (1.05, 1.58) | 0.02 | ||
| MV + VAT | 1.09 (0.91, 1.31) | 0.33 | 1.31 (1.09, 1.58) | 0.004 | ||
| High LDL Cholesterol | MV | 1.02 (0.84, 1.24) | 0.82 | 0.91 (0.69, 1.20) | 0.51 | 0.37 |
| MV + BMI | 1.17 (0.82, 1.67) | 0.38 | 0.90 (0.54, 1.51) | 0.70 | ||
| MV + Neck | 0.94 (0.70, 1.24) | 0.65 | 0.97 (0.63, 1.49) | 0.88 | ||
| MV + VAT | 0.93 (0.68, 1.26) | 0.63 | 0.71 (0.47, 1.06) | 0.09 | ||
| Metabolic Syndrome | MV | 2.47 (2.18, 2.80) | <.0001 | 2.30 (1.99, 2.64) | <.0001 | 0.56 |
| MV + BMI | 2.10 (1.72, 2.55) | <.0001 | 1.35 (1.08, 1.70) | 0.009 | ||
| MV + Neck | 1.69 (1.43, 2.01) | <.0001 | 1.63 (1.34, 1.98) | <.0001 | ||
| MV + VAT | 1.50 (1.26, 1.79) | <.0001 | 1.55 (1.30, 1.86) | <.0001 | ||
Data are shown as ORs (95% CIs). The ORs describe the odds of the association with cardiometabolic risk factors for a 50cm3 increment in upper body subcutaneous fat.
MV model was adjusted for age, ethnicity, smoking status, alcohol intake, physical activity index, postmenopausal status (women only), and hormone replacement therapy (women only); MV+BMI model was additionally adjusted for BMI; MV+Neck model was additionally adjusted for neck circumference; and MV+VAT model was additionally adjusted for abdominal visceral adipose tissue volume.
Sex interaction was tested based on MV model.
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MV, multivariable; OR, odds ratio; VAT, visceral adipose tissue.
Sex-Interaction, Additional Model Adjustment, and Tertile-Based Analysis
Sex-interactions were observed in the associations between upper body subcutaneous fat with BMI (p<0.0001), systolic blood pressure (p=0.0006), LDL cholesterol (p=0.005), and impaired fasting glucose (p=0.0002) (Tables 3–4). Significant interactions were reflective of the stronger associations observed among women, which was consistent with our prior fat studies where stronger associations were observed in women opposed to men.1, 3, 16 Directionality and the significance of the relationships between upper body subcutaneous fat and cardiometabolic risk factors were generally consistent in women and men. In women, most of the associations remained significant even after additional adjustment for BMI, neck circumference, or abdominal visceral adipose tissue; whereas in men, some of the associations were attenuated and no longer significant after adding these variables to the model (Tables 3–4).
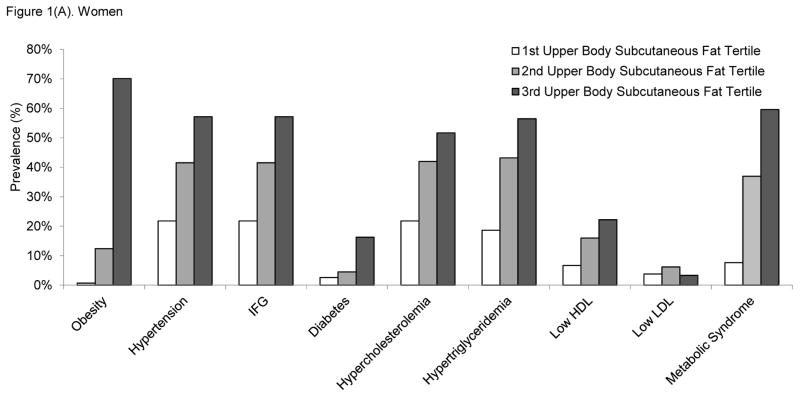
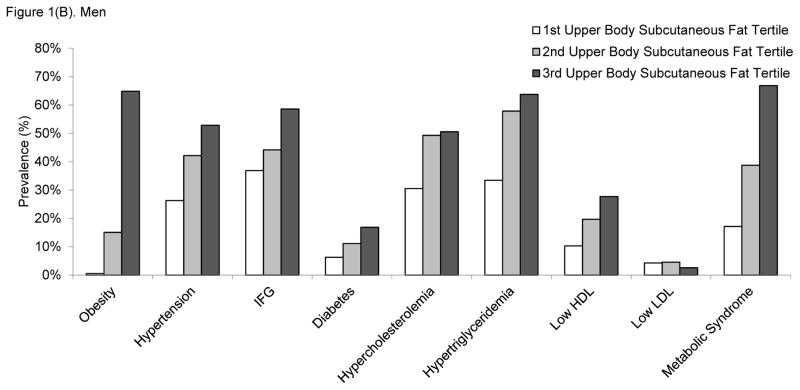
We further examined the prevalence of cardiometabolic risk factors across tertiles of upper body subcutaneous fat in women (Figure 1A) and men (Figure 1B). With the exception of high LDL cholesterol, the prevalence of cardiometabolic risk factors increased linearly in relation to increases in upper body subcutaneous fat tertile. Similar patterns of the associations were observed in both women and men.
Figure 1.
Prevalence of cardiometabolic risk factors according to the tertiles of upper body subcutaneous fat volume in A) women and B) men. Tertile 3 corresponds to the highest upper body subcutaneous fat volumes. The p for linear trend was <0.0001 for all cardiometabolic risk factor models, except for LDL cholesterol where the p-values were 0.74 for women and 0.24 for men. Abbreviations: HDL, high-density lipoprotein; IFG, impaired fasting glucose; LDL, low-density lipoprotein.
DISCUSSION
In this large, community-based cohort study, higher upper body subcutaneous fat was cross-sectionally associated with cardiometabolic risk factors. These associations remained significant even after taking into account multiple potential confounders, as well as, BMI, neck circumference, or abdominal visceral fat. Taken together, our findings support the hypothesis that upper body subcutaneous fat is a dysfunctional adipose tissue depot that is associated with a burden of cardiometabolic risk factors, independent of generalized adiposity and abdominal visceral adipose tissue. Moreover, weak to moderate correlation between upper body subcutaneous fat with neck circumference indicates that neck circumference is a poor surrogate of upper body subcutaneous fat burden.
Previous studies have relied on neck circumference as a surrogate anthropometric measure of subcutaneous fat in upper body and have identified the significant associations between neck circumference and multiple cardiometabolic risk factors, such as diabetes, hypertension, insulin resistance, and metabolic syndrome.12, 17–19 Our prior work has shown that a higher neck circumference is associated with more adverse cardiometabolic risk factors12 and a higher burden of subclinical atherosclerosis13 even after taking into account traditional risk factors and generalized adiposity. However, we have shown in this current study that neck circumference correlated only mildly with upper body subcutaneous fat, suggesting that neck circumference is merely a proxy of upper body fat and is not a sufficient measure to explore the pathogenic role of fat in the upper body region. Advances in imaging technology have led to the ability to precisely quantify the volume of upper body subcutaneous fat on MDCT with excellent reproducibility.5 To our knowledge, the present study is the first to use MDCT-measured upper body subcutaneous fat to evaluate the associations between upper body subcutaneous fat and cardiometabolic disease risk factors based on a well-organized, large, community-dwelling epidemiological study population. The current study adds to the growing body of literature by documenting the potential pathogenic role of upper body subcutaneous fat, a distinctive fat depot that is anatomically separate from abdominal adipose tissue, breast fat, or fat within the mediastinum, on the cardiometabolic abnormalities.
Conventionally, upper body obesity, such as android or apple shape obesity, has been postulated as a more metabolically pathogenic phenotype, as compared to lower body obesity, including gynoid or pear shape obesity.20 Within the boundary of the torso, considerable research has been devoted to unravel the potential pathogenic role of abdominal subcutaneous and visceral adipose tissue on cardiometabolic abnormalities1, 21, 22; in contrast, little is known regarding the fat compartment located in the upper body region. Our prior studies reported that abdominal subcutaneous adipose tissue,1, 23 as well as major ectopic fat depots, including abdominal visceral adipose tissue,1, 23 intramuscular fat,3 intrathoracic fat,24, 25 pericardial fat24, 25, thoracic periaortic fat,26 intrahepatic fat,27 and renal sinus fat28 that are considered as pathogenic fat depositions were associated with more adverse cardiometabolic risk factors. In a similar context, greater upper body subcutaneous fat within the boundary of the torso area may be viewed as an enlarged fat reservoir to store excess fatty acids in response to the chronic state of positive energy homeostasis.29 Progression to obesity is characterized as adipose tissue hyperplasia and hypertrophy, which accompanies dysfunctional changes in the characteristics of adipose tissue,30 such as hypoxia,31 altered angiogenic capacity,32 extracellular matrix overproduction,33 and macrophage infiltration.34 These dysfunctional alterations have been speculated as key mediators to the progression of cardiometabolic pathologies. Similar to other fat compartments, upper body subcutaneous fat may also serve as an active endocrine organ that release biochemical substances, including pro-inflammatory cytokines (e.g., tumor necrosis factor-α, interleukin-6, leptin, fatty acid binding protein-4) that manifest cardiometabolic disease.16, 35
Since this current report is based on a cross-sectional and observational study setting, we cannot definitively determine whether upper body subcutaneous fat acts systemically or locally (i.e., paracrine and autocrine) to impact cardiometabolic traits. In our study, we found that upper body subcutaneous fat was associated with a broad array of cardiometabolic risk factors, despite the location of upper body subcutaneous fat in a compartment that is anatomically distant from major organs (i.e., heart, liver, kidney). Additionally, we identified an association between upper body subcutaneous fat and all cardiometabolic risk factors, except for total and LDL cholesterol. Of note, total and LDL cholesterol are considered to be atherosclerotic cardiovascular disease risk factors36 and may have little effect on metabolic disease. These findings suggest that upper body subcutaneous fat may have a systemic effect rather than a local toxic effect, which is more analogous to the properties of abdominal adipose tissue, as compared to pericardial or renal sinus fat.37 More work is needed in a prospective design to precisely assess the pathogenic roles of upper body subcutaneous fat on metabolic abnormalities.
Although cross-sectional, our findings suggest that upper body subcutaneous fat may have a substantial effect on cardiometabolic risk factors independent of a number of crucial confounders, anthropometric measures of adiposity, and abdominal visceral adipose tissue. Our study is the first to use upper body subcutaneous fat volume assessed by MDCT; thus, confirmation of our findings is warranted in other population-based studies. It is important to note that no prior study has explored the associations between upper body subcutaneous fat with metabolic regulatory biomarkers. Exploring the relationship between upper body subcutaneous fat and various markers of inflammation, oxidation, fibrosis, and hypoxia may help in the understanding of the pathophysiology of upper body subcutaneous fat.38
Strengths of this study include a well-characterized community-dwelling study design based on a relatively large sample of both women and men with an extensive list of cardiometabolic risk factors assessed via a standardized protocol. A limitation of this study includes the nature of cross-sectional and observational study that limits making temporal and causal inferences of our findings. Our study population predominantly consisted of whites; thus, the generalizability of our findings is not applicable to individuals with other ethnic backgrounds. Future longitudinal studies are essential for a better understanding of the temporal association between upper body subcutaneous fat and cardiometabolic risk factors.
CONCLUSIONS
In this community-based epidemiologic cohort study, higher upper body subcutaneous fat was associated with adverse levels of cardiometabolic risk factors above and beyond the contribution of multiple confounders, easily obtainable anthropometric measures of adiposity and abdominal visceral adipose tissue. Our findings underscore the importance of subcutaneous adiposity in the upper body region that may provide a better understanding of the pathogenic properties of obesity in the development of cardiometabolic sequelae.
Supplementary Material
Clinical Significance.
Upper body subcutaneous fat is a unique fat depot located in a separate compartment from abdominal fat.
Higher upper body subcutaneous fat is associated with adverse cardiometabolic risk factors.
These associations are independent of body mass index, neck circumference, and abdominal fat.
Acknowledgments
Funding Source: This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (contract N01-HC-25195).
Footnotes
Authors’ Statement: All authors had access to the data and a role in writing the manuscript.
Conflict of Interest Disclosures: Alison Pedley is an employee of Merck & Company, Inc. There is nothing to disclose for any author other than Alison Pedley.
NHLBI Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the U.S. Department of Health and Human Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 2.Thanassoulis G, Massaro JM, O'Donnell CJ, et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3:345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Therkelsen KE, Pedley A, Speliotes EK, et al. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33:863–870. doi: 10.1161/ATVBAHA.112.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 5.Rosenquist KJ, Therkelsen KE, Massaro JM, Hoffmann U, Fox CS. Development and reproducibility of a computed tomography-based measurement for upper body subcutaneous neck fat. J Am Heart Assoc. 2014;3:e000979. doi: 10.1161/JAHA.114.000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burt MG, Gibney J, Ho KK. Characterization of the metabolic phenotypes of Cushing's syndrome and growth hormone deficiency: a study of body composition and energy metabolism. Clin Endocrinol. 2006;64:436–443. doi: 10.1111/j.1365-2265.2006.02488.x. [DOI] [PubMed] [Google Scholar]
- 7.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 8.Miller KK, Daly PA, Sentochnik D, et al. Pseudo-Cushing's syndrome in human immunodeficiency virus—infected patients. Clin Infect Dis. 1998;27:68–72. doi: 10.1086/514638. [DOI] [PubMed] [Google Scholar]
- 9.Kosmiski LA, Scherzer R, Heymsfield SB, et al. Association of increased upper trunk and decreased leg fat with 2-h glucose in control and HIV-infected persons. Diabetes care. 2011;34:2448–2453. doi: 10.2337/dc11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest. 1991;88:609. doi: 10.1172/JCI115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunfeld C, Rimland D, Gibert CL, et al. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr. 2007;46:283–290. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preis SR, Massaro JM, Hoffmann U, et al. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J Clin Endocrinol Metab. 2010;95:3701–3710. doi: 10.1210/jc.2009-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenquist KJ, Massaro JM, Pencina KM, et al. Neck circumference, carotid wall intima-media thickness, and incident stroke. Diabetes Care. 2013;36:e153–e154. doi: 10.2337/dc13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 15.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 16.Lee JJ, Britton KA, Pedley A, et al. Adipose tissue depots and their cross-sectional associations with circulating biomarkers of metabolic regulation. J Am Heart Assoc. 2016;5:e002936. doi: 10.1161/JAHA.115.002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Noun LL, Laor A. Relationship of neck circumference to cardiovascular risk factors. Obesity research. 2003;11:226–231. doi: 10.1038/oby.2003.35. [DOI] [PubMed] [Google Scholar]
- 18.Stabe C, Vasques ACJ, Lima MMO, et al. Neck circumference as a simple tool for identifying the metabolic syndrome and insulin resistance: results from the Brazilian Metabolic Syndrome Study. Clin Endocrinol. 2013;78:874–881. doi: 10.1111/j.1365-2265.2012.04487.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Ge H, Zhu M, et al. Neck circumference as an independent predictive contributor to cardio-metabolic syndrome. Cardiovasc Diabetol. 2013;12:76–82. doi: 10.1186/1475-2840-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haslam D, Sattar N, Lean M. ABC of obesity. Obesity--time to wake up BMJ. 2006;333:640–642. doi: 10.1136/bmj.333.7569.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study. Obesity (Silver Spring) 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pou KM, Massaro JM, Hoffmann U, et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32:481–485. doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 25.Thanassoulis G, Massaro JM, Hoffmann U, et al. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham Heart Study. Circ Cardiovasc Imaging. 2010;3:559–566. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britton KA, Pedley A, Massaro JM, et al. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the Framingham Heart Study. J Am Heart Assoc. 2012;1:e004200. doi: 10.1161/JAHA.112.004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58:784–790. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafontan M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014;40:16–28. doi: 10.1016/j.diabet.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 32.Gealekman O, Guseva N, Hartigan C, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 2010;88:33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- 34.Subramanian V, Ferrante AW., Jr Obesity, inflammation, and macrophages. Nestle Nutr Workshop Ser Pediatr Program. 2009;63:151–159. doi: 10.1159/000209979. discussion 159–162, 259–168. [DOI] [PubMed] [Google Scholar]
- 35.Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:T17–36. doi: 10.1530/JOE-12-0232. [DOI] [PubMed] [Google Scholar]
- 36.Expert panel on detection, evaluation and treatment of high blood cholesterol in adults. executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 37.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 38.Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab. 2013;27:163–177. doi: 10.1016/j.beem.2013.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.