Abstract
Objectives
Although individual-level socioeconomic status is associated with poor outcomes, less is known regarding how the social context might affect cognitive outcomes. We examined the effect of neighborhood socioeconomic status (NSES) on baseline cognitive function and trajectories of decline.
Methods
The sample (N = 480) came from a longitudinal cohort recruited to study cognitive function. Mixed effects models examined the influence of NSES on baseline and rate of change in executive function, semantic memory, and episodic memory.
Results
NSES was positively associated with semantic memory scores at baseline, but not with executive function or episodic memory in adjusted models, nor was it associated with cognitive change in longitudinal analyses. In exploratory analyses, for individuals with dementia, those with higher NSES declined faster in executive function and semantic memory than those with lower NSES.
Conclusions
Results suggest that NSES has limited effects independent of personal characteristics; however, findings showed a complex relation of NSES and decline, with NSES effects observed only for individuals with dementia. Results are discussed in the context of cognitive reserve.
Clinical Implications
Clinical assessments of individuals who present with cognitive impairment might benefit from an understanding of the neighborhood context from which patients come.
Keywords: education, income, cognitive decline, aging, dementia
Dementia is a growing public health concern as the population ages and lives longer. A rich body of literature has described the various personal risk and protective factors that affect cognitive decline related to dementia, including clinical diagnosis (e.g., dementia, mild cognitive impairment, normal cognitive function), apolipoprotein E (ApoE) genotype, baseline volumetric measures of brain structure, chronic conditions such as hypertension, obesity, and diabetes, and certain individual demographic characteristics (Breteler, 2000; Carmichael et al., 2012; DeCarli et al., 2008; Evans et al., 1997; Glymour & Manly, 2008; Karlamangla et al., 2009; Mungas et al., 2010). For example, studies have shown that individual level education is associated with a reduced risk of Alzheimer’s disease (AD) (e.g., Anttila et al., 2002).
Cognitive Reserve
The idea that individual level education is protective against cognitive decline and dementia is related to the cognitive reserve hypothesis – that individuals who have high education or income have the ability to stave off the effects of brain disease longer because they have learned how to compensate against the disease process (Stern et al., 2009). This idea has been supported by studies showing a protective effect of high individual socioeconomic status (SES) on AD and dementia (Stern et al., 1994; Anttila et al., 2002). Memory clinic data from an inner city neighborhood showed that low SES was associated with a diagnosis of dementia (Fischer et al., 2009). Koster and colleagues found that low SES predicted cognitive decline in older adults (Koster et al., 2005). A more recent population-based study indicated that participants with high SES demonstrated a reduced risk of mild cognitive impairment (MCI)/AD compared to individuals who reported low SES (Sattler, Toro, Schönknecht, & Schröder, 2012). Although many studies have reported on the relation between individual level SES and cognitive decline and/or dementia, less is known about how aspects of the neighborhood environment might affect decline in cognitively and demographically diverse populations. That is, all things being equal, do individuals’ cognitive outcomes differ based on where they live?
A growing literature has demonstrated the important role the social and neighborhood environment exerts on its inhabitants. Neighborhoods are posited to affect people through its social (peers and networks), physical (exposures to violence or pollution), and cultural cues (institutional mechanisms) (see Sharkey and Faber, 2014 for a review). For example, aspects of the neighborhood such as proximity to parks or libraries and museums offer certain resources. Social psychological mechanisms, such as feeling safe or a sense of cohesion in one’s neighborhood also influence health (Bronfenbrenner, 1977; Diez Roux, 2012; Pickett & Pearl, 2001; Sampson, Morenoff, & Gannon-Rowley, 2002; Yen & Syme, 1999). One important variable is neighborhood SES. Low SES neighborhoods (typically measured by education, income, or occupation) have more stressors and fewer resources compared to high SES neighborhoods, and this might lead to cognitive impairments due to restricted opportunities for social and cognitive stimulation (Barnes, Mendes de Leon, Wilson, Bienias, & Evans, 2004; Ertel, Glymour, & Berkman, 2008; Sheffield & Peek, 2009). In contrast, high SES neighborhoods may offer people more opportunities (e.g., parks and recreation, libraries, physical activity), access to health care, and social norms that promote cognitively stimulating activities. According to the cognitive reserve hypothesis, this cognitive and mental stimulation may protect against cognitive decline either through neural reserve or neural compensation (Stern, 2009).
Neighborhood Socioeconomic Status
Neighborhood socioeconomic status (NSES) is related to a variety of poor outcomes, including health behaviors and health problems, greater morbidity and mortality (Antonovsky, 1967; Kitagawa & Hauser, 1973; Marmot, Kogevinas, & Elston, 1987; Robert, 1998) and stress and depression (Everson-Rose et al., 2011; Yen & Kaplan, 1999). Neighborhood effects on various dimensions of health may be especially important for older adults who may be more dependent on the resources in their immediate neighborhood due to financial and mobility constraints (Robert & Li, 2001). Thus, an understanding of how NSES relates to cognitive function could be an important part of targeted strategies for prevention and intervention for older adults.
Although several cross-sectional studies on NSES and cognitive outcomes exist (Clarke et al., 2012; Lang et al., 2008; Shih et al., 2011; Sisco & Marsiske, 2012; Wight et al., 2006), we are aware of only three longitudinal studies that have examined the relation between NSES and cognitive decline. Longitudinal studies have methodological advantages over cross sectional ones and are also conceptually appealing. In the study of cognitive aging, change is more important than baseline scores, and only longitudinal studies allow robust investigations of change. Sheffield & Peek (2009) examined the influence of NSES on cognitive change using a national sample of older Mexican Americans and found that odds and rate of incident cognitive decline increased as a function of poorer NSES (Sheffield & Peek, 2009). However, both Zeki Al Hazzouri et al. (2011) and Meyer et al. (2015) showed that while lower NSES was associated with poorer baseline cognitive scores, it was not associated with trajectories of decline.
The Present Study
Given the existing literature, we hypothesized that regardless of person-level covariates (e.g., education level) individuals living in high SES neighborhoods would have better scores at baseline on our cognitive outcome measures, but that rates of decline (i.e., change) would not differ by NSES. Previous longitudinal studies have included only Latinos (Al Hazzouri et al., 2011; Sheffield & Peek, 2009) or only non-Hispanic African Americans and non-Hispanic Whites (henceforth referred to as Blacks and Whites) separately (Meyer et al., 2015). Our study includes an ethnically, linguistically, and educationally diverse sample from three major racial/ethnic groups—Latinos, Blacks, and Whites, with follow-up for up to 11 years. Having more diversity in ethnicity/race also provides us more diversity and variation in NSES and allows for more generalizability across different ethnic groups. We employ psychometrically sophisticated, clinically relevant cognitive outcome measures – semantic memory, executive function, and episodic memory – that have been developed and validated for culturally and linguistically diverse groups (Mungas, Reed, Crane, Haan, & Gonzalez, 2004; Mungas, Reed, Farias, & DeCarli, 2005; Mungas, Reed, Haan, & Gonzalez, 2005). These cognitive measures were developed using modern psychometric methods based on item response theory and have psychometric characteristics that are optimized for longitudinal research (Mungas et al., 2004; Mungas et al., 2005). Lastly, our sample was also diverse in cognitive function across the full spectrum, from normal function to dementia.
Methods
Sample
The sample comprised 480 participants in an ongoing longitudinal study of cognition at the UC Davis Alzheimer’s Disease Center (ADC), located in Northern California. All participants were followed approximately annually and had at least two evaluations with a mean of 3.39 visits (SD = 2.13) and maximum of 11 visits. Participants were recruited into the study through two routes: 1) memory clinic referrals and 2) community outreach. Approximately 68% of participants were recruited through community based recruitment protocols designed to enhance both the racial and ethnic diversity and the spectrum of cognitive dysfunction of the sample with an emphasis on normal cognition and MCI. Recruiters utilized various outreach methods such as soliciting in a community hospital lobby, a community survey, health fairs or word of mouth. The other 32% of the sample initially sought a clinical evaluation at our Alzheimer’s Disease Center and subsequently were recruited for this study. These individuals predominantly had a clinical diagnosis of MCI (Hinton et al., 2010).
Inclusion criterion was ability to speak English or Spanish. Exclusion criteria included unstable major medical illness, major psychiatric disorder, and active substance abuse or dependence (excluding tobacco-related) disorders). This study was approved by the institutional review board at UC Davis and all participants provided informed consent.
Measures
Clinical evaluations
All participants received multidisciplinary diagnostic evaluations at baseline and at approximately annual intervals. All evaluations followed the same protocol and included a detailed medical history and a physical and neurological exam. A bilingual physician examined participants who only spoke Spanish. Family members or other close informants were interviewed to obtain information about levels of independent functioning for their patients. Clinical neuropsychological evaluation using standard neuropsychological tests (distinct from the outcome measures used in this study) was performed at each visit. Routine dementia work-up laboratory tests were obtained at the baseline evaluation for all participants and when clinically indicated at the time of follow-up evaluations. Diagnosis of cognitive syndrome (normal, MCI, dementia) and, for individuals with dementia, identification of underlying etiology, were made according to standardized criteria. Each case at baseline was initially diagnosed at a consensus conference by the clinical team evaluating the participant. Those appearing likely to be eligible for this study were then reviewed at a second, multidisciplinary case adjudication conference. Follow-up cases were diagnosed at a case conference of the clinical team examining the participant, and in addition, were reviewed at a case adjudication conference when the examining team identified a change in the diagnosis. All diagnoses were made blind to research neuropsychological testing. The Clinical Dementia Rating (CDR, Morris, 1993) was completed on the basis of a standardized interview with the identified participant and an informant; the sum of individual items or boxes (CDRSum) was used as a continuous measure of clinical status. The CDR was completed blind to other evaluation results including clinical and research neuropsychological test results, the physical and neurological exam, and the clinical diagnosis (DeCarli et al., 2008).
Cognitive outcomes
The cognitive outcomes in this study were from the Spanish and English Neuropsychological Assessment Scales (SENAS) and were administered at all evaluations. The SENAS has undergone extensive development as a battery of cognitive tests relevant to diseases of aging (Mungas et al., 2004; Mungas et al., 2005; Mungas, Reed, Haan, et al., 2005; Mungas, Reed, Marshall, & Gonzalez, 2000). Modern psychometric methods based on item response theory were used to create psychometrically matched measures across different scales and across English and Spanish versions and appropriate for individuals with diverse education levels. This study used a subset of SENAS tests to measure three cognitive domains affected by diseases of aging: executive function, semantic memory, and episodic memory. Executive function is a composite measure constructed from component tasks of category fluency (number of animals named in 60 seconds), phonemic (letter) fluency (words beginning with the/f/sound, words beginning with the/l/sound), and working memory (digit-span backward, visual-span backward, list sorting). Semantic memory is a composite of highly correlated verbal (object-naming) and nonverbal (picture-association) tasks. Episodic memory is a composite score derived from a multi-trial word-list-learning test (Word List Learning 1) (Mungas et al., 2004). Measure development and psychometric characteristics are described in more detail elsewhere (Crane et al., 2008; Mungas et al., 2004; Mungas, Reed, Haan, et al., 2005). SENAS scores are presented in z-score like units where a score of zero corresponds to the mean and differences from the mean are expressed in standard deviation units.
Individual-level covariates
In mixed-effects models, we controlled for variables that might confound the relation between NSES and cognitive outcomes, including race/ethnicity: Black, Latino, or White, gender, age and education in years, and diagnosis: normal, MCI, or dementia. Other covariates which influence cognitive outcomes included a practice effect: 0 if first evaluation, 1 for all other annual evaluations; recruitment source: clinic or community; language of interview; and vascular risk assessed through medical histories and medical records that assessed for presence of diabetes, hypertension, and hyperlipidemia. ApoE genotyping was carried out using the LightCycler ApoE mutation detection kit (Roche Diagnostics, Indianapolis, IN). ApoE was dichotomously coded as 1 for presence of at least one e4 allele, 0 for no e4 allele. Time was calculated as years from baseline evaluation and captures annualized rate of change.
Neighborhood socioeconomic status
Similar to prior research, neighborhoods were categorized by census tracts, an administrative boundary designated by the U.S. Census Bureau (Krieger et al., 2003). There were 365 census tracts in the present sample of 480 participants. From U.S. Census Tract 2010 data, we extracted variables that cohere conceptually and correlate empirically to capture the construct of NSES, including percentage of individuals with a high school diploma, percentage of people who owned their own home, median household income, and median number of rooms in home. Similar to prior research (Al Hazzouri et al., 2011; Clarke et al., 2012), these variables were z-score standardized, and then averaged together to create the NSES variable, with factor loadings for each item ranging from .59–.91. Coefficient alpha for this SES measure was α = 0.82.
We used ArcMap to geocode participant addresses (at baseline), and appended to these data from the 2010 U.S. Census Tract (ESRI, 2011). Geocoding was checked for quality assurance.
Data Analysis
Descriptive statistics were estimated using SPSS (IBM Corp., 2013). Mixed-effects multivariate regression models were estimated using R (R Core Development Team, 2012). For ease of interpretation, age was centered at 70 years, education centered at 12 years, and NSES centered at the sample mean. This sample was recruited from a large region that included urban as well as rural areas; thus, very few census tracts were represented by more than a single individual (census tracts - with one person: 76%, with two people: 18%, with three people: 5%, with four people: 1%). Therefore, similar to previous research (Meyer et al., 2015; Sisco & Marsiske, 2012), we treated NSES as a person-level factor in a contextual analysis rather than modeling its effects in a multilevel model.1 Random intercepts were included to account for between-person variability in level of cognitive outcomes at the baseline evaluation. Random slopes accounted for variation in rate of change between individuals. Diagnostics (using AIC and BIC criteria from a likelihood ratio test) supported using a model with autocorrelated residuals within person rather than one assuming independent residuals.
A series of hierarchical mixed-effects models were conducted for each outcome. Model 0 was a naïve model with only time as a predictor. Model 1 included NSES as a predictor along with time to examine the effect of NSES at baseline and on rate of change. To evaluate the effect of NSES above and beyond the influence of demographic covariates, Model 2 included age and gender. Model 3 included diagnosis as a predictor. The next few models included education and race/ethnicity as predictors separately and then together in the same model. Model 7 controlled for other covariates known to influence cognitive outcomes. Individual-level variables were entered in separate steps to permit an exploration of the differential contribution of these independent variables and to examine whether they might explain the effect of NSES. We included a main effect of each predictor/covariate to account for its effect on baseline score and an interaction of the predictor/covariate with the time variable to estimate the effect of the predictor/covariate on cognitive change (i.e., decline) over time (e.g., gender would indicate the baseline effect of gender on executive function, gender by time would indicate the effect of gender across time. That is, females might start off lower than males on executive function, but they may decline more slowly than males over time).
Results
Sample Characteristics
Table 1 shows characteristics of the full sample; approximately 50% were White; 27% were Black; and 23% were Latino. A majority of the sample were women (60%), had normal cognitive functioning (47%), were recruited from the community (62%), and had their assessments conducted in English (84%). The sample was highly educated on average (mean years of education = 13), but there was substantial heterogeneity of education level and the Latino sub-sample in particular had low levels of education (mean years = 9). In terms of neighborhood characteristics, a large percentage lived in neighborhoods with persons having at least a high school diploma (85%) and who owned their own homes (62%). Figure 1 illustrates the heterogeneity in NSES and individual level education.
Table 1.
Descriptive Statistics for the Entire Sample at Baseline (N = 480)
| Variable | % or Mean | Range | SDa |
|---|---|---|---|
| Individual-level (n = 480) | |||
| Age (in years) | 74.4 | 45–93 | 7.33 |
| Education (in years) | 13.2 | 0–20 | 4.35 |
| Female | 60.2 | ||
| Ethnicity | |||
| White | 49.8 | ||
| Black | 26.9 | ||
| Latino | 23.3 | ||
| Diagnosis | |||
| Normal | 46.9 | ||
| Mild Cognitive Impairment | 36.5 | ||
| Dementia | 16.7 | ||
| Recruitment Source | |||
| Clinic | 37.7 | ||
| Community | 62.3 | ||
| Executive Function | −.21 | −2.27–2.04 | .65 |
| Episodic Memory | −.34 | −3.14–2.22 | .88 |
| Semantic Memory | .08 | −2.86–1.94 | .85 |
| Neighborhood-level (n = 365) | |||
| Socioeconomic status | .66 | −6.25–7.85 | 2.70 |
| Percent High School Diploma | 84.97 | .0–99.9 | 13.32 |
| Household Income | 63,091 | 18,013–158,988 | 24,772 |
| Median # of Rooms | 5.34 | 2.4–8.10 | .92 |
| Percent Own Home | 62.40 | .0–98.40 | 20.67 |
SD = standard deviation
Figure 1.
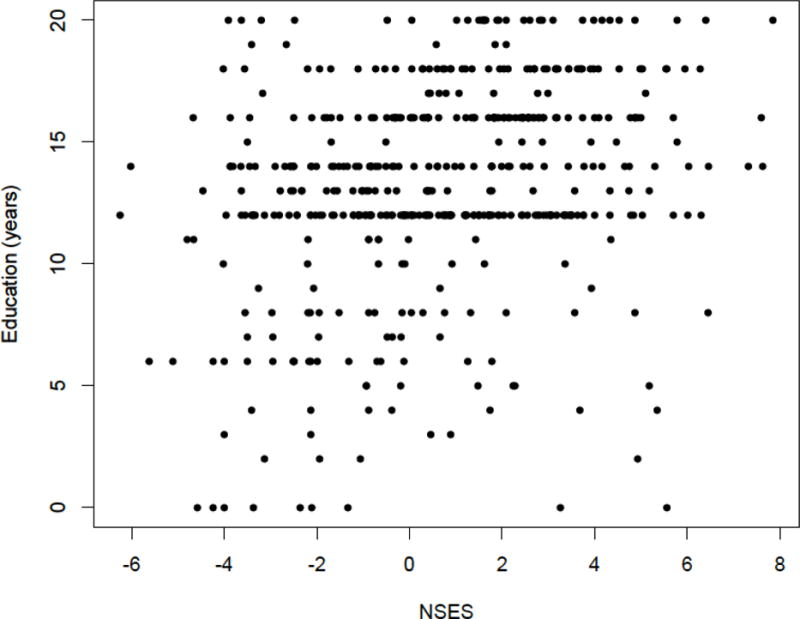
Scatterplot of neighborhood socioeconomic status and education level among full sample (N = 480).
Mixed-Effects Models
Table 2 presents the results of the mixed effects models of the associations between NSES and cognitive scores at baseline and cognitive decline.2
Table 2.
Beta Weights for Neighborhood Socioeconomic Status (NSES) on Baseline Cognitive Scores and Change in Cognition for Executive Function, Semantic Memory, and Episodic Memory.
| Executive Function | Semantic Memory | Episodic Memory | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Models | Baseline | Change | Baseline | Change | Baseline | Change |
| Model 1: Neighborhood socioeconomic status (NSES) | .04** | −.01** | .09*** | −.01* | −.0004 | −.004 |
| Model 2: NSES + age + gender | .04*** | −.01** | .08*** | −.01* | .009 | −.0004 |
| Model 3: NSES + age + gender + diagnosis | .05*** | −.004* | .08*** | −.003 | .03** | −.001 |
| Model 4: NSES + age + gender + diagnosis + education | .02* | −.004 | .05*** | −.003 | .02 | −.001 |
| Model 5: NSES + age + gender + diagnosis + race/ethnicity | .03** | −.003 | .05*** | −.002 | .02 | .0005 |
| Model 6: NSES + age + gender + diagnosis + education + race/ethnicity | .01 | −.003 | .03* | −.002 | .01 | −.0002 |
| Model 7: NSES + age + gender + diagnosis + education + race/ethnicity + vascular risk, APOE genotype, recruitment source, practice effects, and language of assessment | .01 | −.002 | .03* | −.002 | .01 | .001 |
p < .05;
p < .01;
p < .001.
Tabled results show the influence of a one standard deviation difference in NSES on the cognitive outcomes, which are expressed in standard deviation units.
Executive Function
In Model 1, individuals living in areas with higher NSES had better baseline executive function scores (β = 0.04, SE = 0.01) but faster rates of decline (β = −0.01, SE = 0.003) compared to individuals living in areas with lower NSES. After adjustment for age and gender in Model 2, and diagnosis in Model 3, NSES was still associated with better baseline scores and faster rates of decline. In Model 4, after accounting for education, NSES was associated with baseline scores, but only marginally related to decline (β = −0.004, SE = 0.002, p = 0.06). After adjustment for race/ethnicity but not education in Model 5, NSES remained associated with baseline scores but was no longer associated with decline (β = −0.003, SE = 0.002, p = 0.15). After adjusting for both education and race/ethnicity in Model 6, NSES was no longer associated with baseline or rate of change.
Semantic Memory
In Model 1, individuals with higher NSES had better baseline semantic memory scores (β = 0.09, SE = 0.01) and faster rates of decline (β = −0.01, SE = 0.002) than did individuals living in areas with lower NSES. After adjustment for age and gender in Model 2, NSES was still associated with better baseline scores and faster rates of decline. After adjustment for diagnosis in Model 3, NSES was still associated with better baseline scores, but not rates of change (β = −0.003, SE = 0.002, p = 0.11). In subsequent models, including Model 7, which accounted for all covariates, NSES remained significantly associated with semantic memory scores at baseline (β = 0.03, SE = 0.01), but not with change (β = −0.002, SE = 0.002, p = 0.44).
Episodic Memory
In Model 1, NSES was not associated with baseline episodic memory scores (β = −0.0004, SE = 0.01, p = 0.98), nor was it related to decline (β = −0.004, SE = 0.003, p = 0.21). In subsequent models – Models 2 through Model 7, including covariates in the models did not change the (lack of an) effect of NSES on baseline scores or decline.
Exploratory Analyses
Based on adjusted models (results not shown), diagnosis was a consistent predictor of cognitive decline across all outcomes. That is, individuals who had been diagnosed with dementia at baseline were more likely to decline faster than individuals with MCI, who in turn, were more likely to decline faster than normals. Thus, we added NSES to the time by diagnosis interaction, to examine if cognitive decline associated with diagnosis might vary by NSES. For executive function, the interaction of NSES, dementia, and time was significant (β = −0.01, SE = 0.004, p < 0.01), but not the interaction of NSES with MCI and time (p = 0.73). Demented individuals with higher NSES declined faster than those with dementia who had lower NSES (Figure 2).3 For semantic memory, the interaction of NSES with dementia and time (β = −0.01, SE = 0.004, p < 0.05) was significant, but not the NSES by MCI by time interaction (p = 0.86). Individuals with dementia and higher NSES declined faster than those with dementia and lower NSES (Figure 3). There was no significant moderating effect of NSES on trajectories of change by diagnosis for episodic memory.
Figure 2.
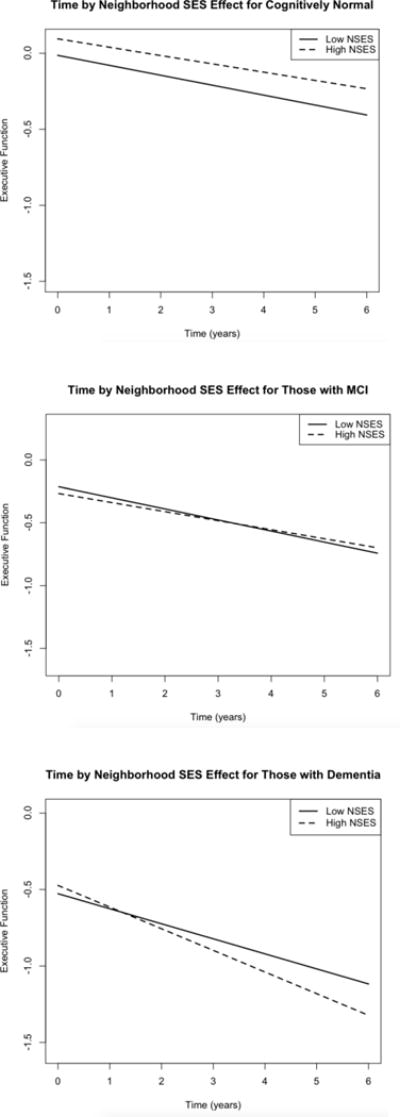
Significant three way interaction of time and diagnosis for varying levels of neighborhood socioeconomic status (SES) for Executive Function. High NSES = 1 SD above mean, low NSES = 1SD below mean.
Figure 3.
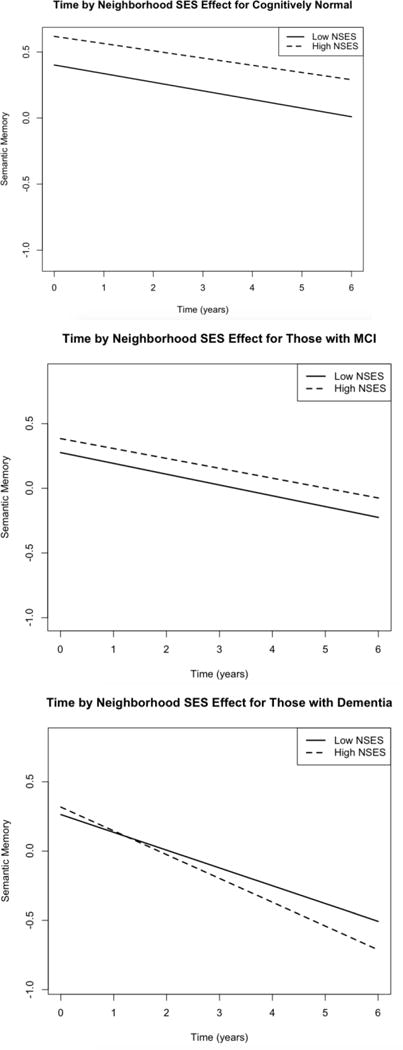
Significant three way interaction of time and diagnosis for varying levels of neighborhood socioeconomic status (SES) for Semantic Memory. High NSES = 1 SD above mean, low NSES = 1SD below mean.
Discussion
We examined whether NSES affected cognitive scores at baseline and change in a diverse sample. In unadjusted models, NSES was associated with baseline executive function and semantic memory scores, consistent with cross-sectional studies indicating that higher NSES is associated with better cognitive scores (Lang et al., 2008; Shih et al., 2011; Wight et al., 2006). Unlike previous studies however, and mostly contrary to our hypothesis, NSES was not associated with baseline executive function scores in models that adjusted for education, race/ethnicity, and diagnosis. Higher NSES was still associated with semantic memory scores at baseline in adjusted models however. This could be because NSES plays a more significant role in cognitive processes that are influenced by social contextual life experiences, which may be captured and reflected in measures of semantic memory (Mungas, Reed, Haan, et al., 2005). Indeed, in bivariate correlations, NSES was more strongly related to semantic memory than to executive function and episodic memory.
Although NSES was associated with rates of decline for executive function and semantic memory in unadjusted models, it was not in the hypothesized direction. That is, higher NSES was associated with faster rates of decline; however this effect was weak and nonsignificant after adjusting for education, race/ethnicity, and/or diagnosis, suggesting that some of the effects of NSES may be explained by these individual-level characteristics. Our findings largely corroborate longitudinal studies by Al Hazzouri et al. (2011) and Meyer et al. (2015); both did not find a significant relation between NSES and cognitive decline. However, our results differ from Sheffield and Peek (2009). These heterogeneous results may be due to differences in the sample and measures used across studies. For example, participants from both our study and those of Al Hazzouri et al. came from mostly the Sacramento, California and surrounding areas, while Sheffield and Peek’s participants came from five southwestern states in the U.S. Thus, differences in the neighborhoods and social contexts of these varying geographies may have led to different findings. Also, Sheffield and Peek operationalized their neighborhood SES variable as distinct quartiles, while we and Al Hazzouri et al. operationalized SES as a single continuous variable. Additionally, it did not appear that Sheffield and Peek (2009) specified an initial gain (practice/retest) effect in their study, which may have conflated rate of change with the initial boost that comes as a result of repeated testing.
Interestingly, for executive function and semantic memory, there was a differential effect of NSES in individuals who were noted as having dementia at the initial evaluation such that those with higher NSES declined at a faster rate than those with lower NSES. Similarly, Silva et al. (2014) studied participants in a clinical setting and found that more educated subjects declined less at early stages and more at late stages of MCI. One possible explanation involves the cognitive reserve hypothesis; that is, despite similar levels of pathology, some individuals show clinical symptoms of disease, while others do not (Stern, 2002), and this is partly due to differences in life experiences. Cognitive reserve, typically measured by proxies such as education or income, may for a time buffer the effects of brain pathology on cognition, serving to slow cognitive decline before dementia and hastening it thereafter because there is more advanced pathology at the time of diagnosis (Bennett et al., 2003; Stern, 2009; Wilson et al., 2010). This hypothesis is speculative, however and requires further study.
It is important to note several limitations of the current study. As with any neighborhood study, it is unclear whether the neighborhood factors impact the participants who move into certain areas (causation), or whether certain characteristics of residents dictate where they live (selection). People with more education and higher income over the lifespan are likely to select higher SES neighborhoods. Associations of NSES with test scores may simply reflect that better educated and more affluent people move into higher SES neighborhoods. However, Figure 1 does indicate some mismatches in person-level education and NSES. Also, we only had baseline residential addresses, and it was unclear how long people lived at these addresses or whether or not they had moved. It may be that neighborhoods affect individuals differently depending on length of time in the community. We also did not have a measure of individual income or wealth. Lastly, we believe that our finding that NSES predicts faster decline in persons with dementia provides some support for the cognitive reserve hypothesis, but without an objective measure of cognitive reserve, our explanation is speculative. Alternate explanations are possible; for example, better lifelong executive function and semantic memory (associated with better life circumstances) bias diagnosis such that individuals from more affluent neighborhoods are less likely to be diagnosed with dementia than those from less affluent neighborhoods, even after controlling for brain pathology and amount of cognitive decline preceding the initial diagnosis. Future studies should control for amount of brain pathology and rate of change in brain variables.
Despite these limitations, the current study adds new knowledge to the field of cognitive decline and neighborhood effects. This study is the first to examine the effect of NSES on cognitive decline in a diverse, longitudinal cohort of Whites, Blacks, and Latinos (thus contributing to more socioeconomically diverse neighborhoods) with follow-up data over as many as 11 years. Moreover, we used psychometrically sophisticated cognitive outcomes to examine trajectories of change. Additionally, we controlled for a number of confounders and showed that net of these individual-level covariates, NSES plays a role in baseline outcomes that are affected by social contextual life experiences (e.g., semantic memory). However, once individual level factors are taken into account (i.e., race/ethnicity, diagnosis), they seem to be more important than NSES in predicting cognitive decline.
Clinical Implications
Results of this study point to these implications:
Although the social context in which older adults live is important, individual factors, such as education level, may override the influences of the neighborhood context, supporting the idea that early experiences (e.g., amount and quality of education) are more important in predicting later cognitive function (Glymour & Manly, 2008).
Neighborhood SES appears to impact cognitive outcomes that are more influenced by exposure and access to varied learning and cultural experiences, processes such as semantic memory, as opposed to episodic memory.
An interesting finding that needs replication is the faster rate of decline for individuals with dementia who lived in high SES neighborhoods. Future research should examine the possibility that there may be some aspects at the neighborhood level, as measured by NSES, that contribute independently to building cognitive reserve (Clarke et al., 2012). If this is the case, it may benefit clinicians to know characteristics of the residential context of their patients, and for cognitive intervention programs to target certain neighborhoods.
Footnotes
Multi-level models were run and results are similar to the current analysis strategy.
All models adjust for person-level covariates on both intercepts (baseline) and slopes (decline).
High and low NSES is operationalized as 1 standard deviation above the mean and 1 standard deviation below the mean, respectively.
References
- Al Hazzouri AZ, Haan MN, Osypuk T, Abdou C, Hinton L, Aiello AE. Neighborhood socioeconomic context and cognitive decline among older Mexican Americans: results from the Sacramento Area Latino Study on Aging. American Journal of Epidemiology. 2011;174(4):423–431. doi: 10.1093/aje/kwr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonovsky A. Social class, life expectancy, and overall mortality. Milbank Memorial Fund Quarterly. 1967;45:31–73. [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Bienias JL. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Breteler MM. Vascular risk factors for Alzheimer’s disease: An epidemiologic perspective. Neurobiology of Aging. 2000;21(2):153–160. doi: 10.1016/s0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. Toward an environmental ecology of human development. American Psychologist. 1977;32:513–531. [Google Scholar]
- Carmichael O, Mungas D, Beckett L, Harvey D, Tomaszewski Farias S, Reed B, Decarli C. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiology of Aging. 2012;33(1):83–95. doi: 10.1016/j.neurobiolaging.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ, Ailshire JA, House JS, Morenoff JD, King K, Melendez R, Langa KM. Cognitive function in the community setting: the neighbourhood as a source of ‘cognitive reserve’? Journal of Epidemiology and Community Health. 2012;66(8):730–736. doi: 10.1136/jech.2010.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- Crane PK, Narasimhalu K, Gibbons LE, Pedraza O, Mehta KM, Tang Y, Mungas DM. Composite scores for executive function items: demographic heterogeneity and relationships with quantitative magnetic resonance imaging. Journal of the International Neuropsychological Society. 2008;14(5):746–759. doi: 10.1017/S1355617708081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, whites, and Hispanics. Alzheimer Disease and Associated Disorders. 2008;22(4):382–391. doi: 10.1097/wad.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV. Conceptual approaches to the study of health disparities. Annual Review of Public Health. 2012;33:41–58. doi: 10.1146/annurev-publhealth-031811-124534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel KA, Glymour MM, Berkman LF. Effects of social integration on preserving memory function in a nationally representative US elderly population. American Journal of Public health. 2008;98(7):1215–1220. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute; 2011. [Google Scholar]
- Evans DA, Hebert LE, Beckett LA, Scherr PA, Albert MS, Chown MJ, Taylor JO. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Archives of Neurology. 1997;54(11):1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Skarupski KA, Barnes LL, Beck T, Evans DA, Mendes de Leon CF. Neighborhood socioeconomic conditions are associated with psychosocial functioning in older black and white adults. Health and Place. 2011;17(3):793–800. doi: 10.1016/j.healthplace.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychological Reviews. 2008;18(3):223–254. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, Mungas D. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Disease and Associated Disorders. 2010;24(3):234–241. doi: 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. American Journal of Epidemiology. 2009;170(3):331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa E, Hauser P. Differential mortality in the United States: A study in socioeconomic epidemiology. Cambridge, MA: Harvard University Press; 1973. [Google Scholar]
- Krieger N, Zierler S, Hogan JW, Waterman P, Chen J, Lemieux K, Gjelsvik A. Geocoding and measurement of neighborhood socioeconomic position: A U.S. perspective. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. New York: Oxford University Press; 2003. pp. 147–178. [Google Scholar]
- Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Huppert FA, Melzer D. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: Analyses from the English Longitudinal Study of Ageing. Journal of the American Geriatrics Society. 2008;56(2):191–198. doi: 10.1111/j.1532-5415.2007.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, Kogevinas M, Elston M. Social/economic status and disease. Annual Review of Public Health. 1987;8:111–135. doi: 10.1146/annurev.pu.08.050187.000551. [DOI] [PubMed] [Google Scholar]
- Meyer OL, Sisco SM, Harvey D, Zahodne LB, Glymour MM, Manly JJ, Marsiske M. Neighborhood Predictors of Cognitive Training Outcomes and Trajectories in ACTIVE. Research and Aging. 2015 doi: 10.1177/0164027515618242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Beckett L, Harvey D, Farias ST, Reed B, Carmichael O, DeCarli C. Heterogeneity of cognitive trajectories in diverse older persons. Psychology of Aging. 2010;25(3):606–619. doi: 10.1037/a0019502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): Further development and psychometric characteristics. Psychological Assessment. 2004;16:347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Farias ST, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: Equivalent performance in elderly Hispanics and non-Hispanic Whites. Journal of the International Neuropsychological Society. 2005;11:620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales: Relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19:466–475. doi: 10.1037/0894-4105.19.4.466. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Marshall SC, Gonzalez HM. Development of psychometrically matched English and Spanish neuropsychological tests for older persons. Neuropsychology. 2000;19:466–475. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. Journal of Epidemiology and Community Health. 2001;55(2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Li L. Age variation in the relationship between neighborhood socioeconomic context and adult health. Research on Aging. 2001;23(2):233–258. [Google Scholar]
- Robert SA. Community-level socioeconomic status effects on adult health. Journal of Health and Social Behavior. 1998;39(1):18–37. [PubMed] [Google Scholar]
- Sampson R, Morenoff JD, Gannon-Rowley T. Assessing “neighborhood effects”: Social processes and new directions in research. Annual Review of Sociology. 2002;28:443–478. [Google Scholar]
- Sharkey P, Faber JW. Where, when, why, and for whom do residential contexts matter? moving away from the dichotomous understanding of neighborhood effects. Annual Review of Sociology. 2014;40:559–579. [Google Scholar]
- Sheffield KM, Peek MK. Neighborhood context and cognitive decline in older Mexican Americans: Results from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. American Journal of Epidemiology. 2009;169(9):1092–1101. doi: 10.1093/aje/kwp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih RA, Ghosh-Dastidar B, Margolis KL, Slaughter ME, Jewell A, Bird CE, Espeland MA. Neighborhood socioeconomic status and cognitive function in women. American Journal of Public Health. 2011;101(9):1721–1728. doi: 10.2105/AJPH.2011.300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D, Guerreiro M, Faria C, Maroco J, Schmand BA, de Mendonça A. Significance of subjective memory complaints in the clinical setting. Journal of Geriatric Psychiatry and Neurology. 2014;27(4):259–265. doi: 10.1177/0891988714532018. [DOI] [PubMed] [Google Scholar]
- Sisco SM, Marsiske M. Neighborhood influences on late life cognition in the ACTIVE Study. Journal of Aging Research. 2012;2012:1–11. doi: 10.1155/2012/435826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. Retrieved from http://www.R-project.org/ [Google Scholar]
- Wight RG, Aneshensel CS, Miller-Martinez D, Botticello AL, Cummings JR, Karlamangla AS, Seeman TE. Urban neighborhood context, educational attainment, and cognitive function among older adults. American Journal of Epidemiology. 2006;163(12):1071–1078. doi: 10.1093/aje/kwj176. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Aggarwal NT, Boyle PA, Hebert LE, Mendes de Leon CF, Evans DA. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology. 2010;75(11):990–996. doi: 10.1212/WNL.0b013e3181f25b5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen I, Syme S. The social environment and health: A discussion of the epidemiologic literature. Annual Review of Public Health. 1999;20:287–308. doi: 10.1146/annurev.publhealth.20.1.287. [DOI] [PubMed] [Google Scholar]
- Yen IH, Kaplan GA. Poverty area residence and changes in depression and perceived health status: evidence from the Alameda County Study. International Journal of Epidemiology. 1999;28(1):90–94. doi: 10.1093/ije/28.1.90. [DOI] [PubMed] [Google Scholar]


