Abstract
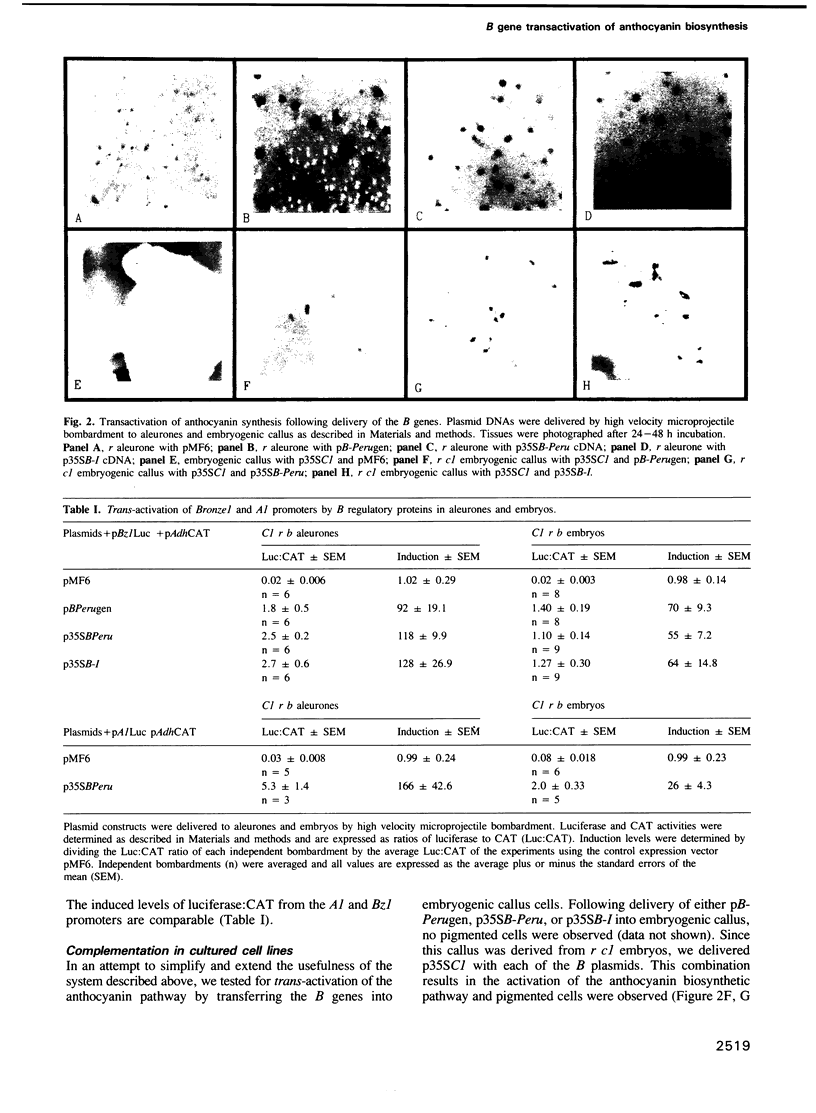
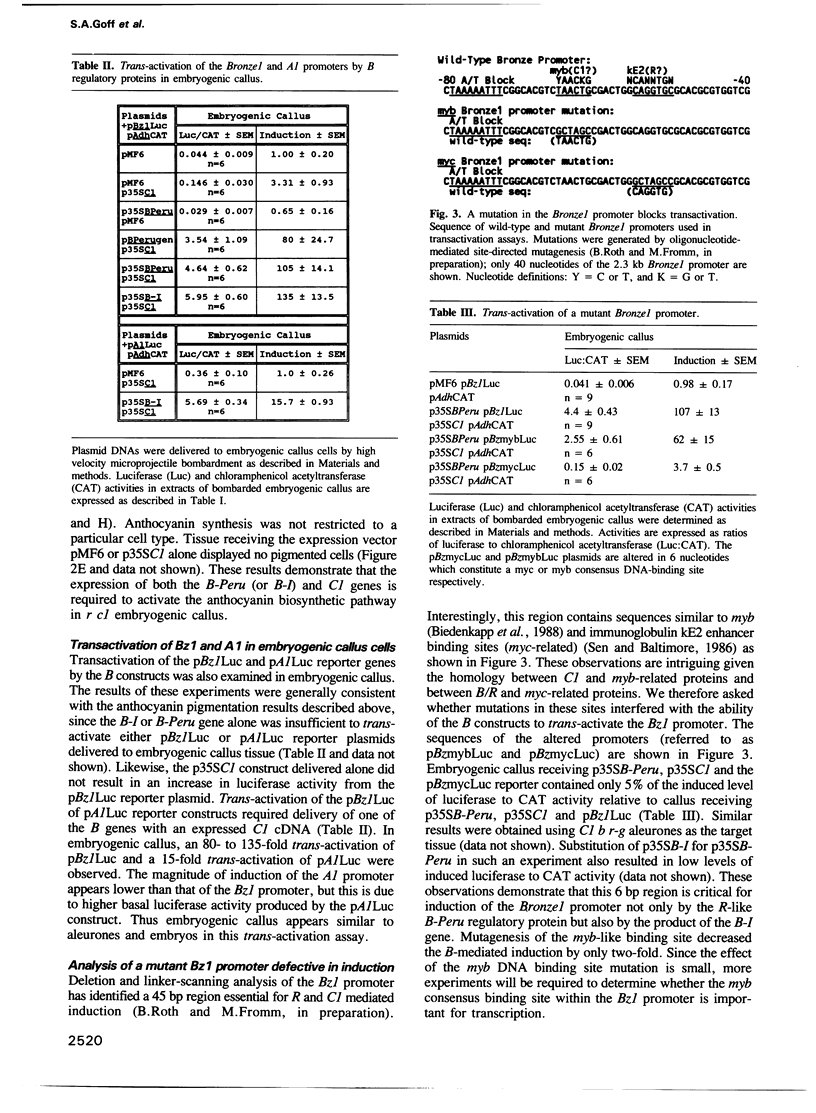
The C1, B and R genes regulating the maize anthocyanin biosynthetic pathway encode tissue-specific regulatory proteins with similarities to transcriptional activators. The C1 and R regulatory genes are usually responsible for pigmentation of seed tissues, and the B-Peru allele of B, but not the B-I allele, can substitute for R function in the seed. In this study, members of the B family of regulatory genes were delivered to intact maize tissues by high velocity microprojectiles. In colorless r aleurones or embryos, the introduction of the B-Peru genomic clone or the expressed cDNAs of B-Peru or B-I resulted in anthocyanin-producing cells. Luciferase produced from the Bronze1 anthocyanin structural gene promoter was induced 100-fold when co-introduced with the expressed B-Peru or B-I cDNAs. This quantitative transactivation assay demonstrates that the proteins encoded by these two B alleles are equally able to transactivate the Bronze1 promoter. Analogous results were obtained using embryogenic callus cells. These observations suggest that one major contribution towards tissue-specific anthocyanin synthesis controlled by the various alleles of the B and R genes is the differential expression of functionally similar proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedenkapp H., Borgmeyer U., Sippel A. E., Klempnauer K. H. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988 Oct 27;335(6193):835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- Bruce W. B., Christensen A. H., Klein T., Fromm M., Quail P. H. Photoregulation of a phytochrome gene promoter from oat transferred into rice by particle bombardment. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9692–9696. doi: 10.1073/pnas.86.24.9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J., Fromm M., Walbot V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987 Dec;1(10):1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- Chandler V. L., Radicella J. P., Robbins T. P., Chen J., Turks D. Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. Plant Cell. 1989 Dec;1(12):1175–1183. doi: 10.1105/tpc.1.12.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. M., Coe E. H., Jr Control of anthocyanin synthesis by the C locus in maize. Biochem Genet. 1977 Apr;15(3-4):333–346. doi: 10.1007/BF00484464. [DOI] [PubMed] [Google Scholar]
- Cone K. C., Burr F. A., Burr B. Molecular analysis of the maize anthocyanin regulatory locus C1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9631–9635. doi: 10.1073/pnas.83.24.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner H. K., Nelson O. E. Interaction among C, R and Vp in the Control of the Bz Glucosyltransferase during Endosperm Development in Maize. Genetics. 1979 Feb;91(2):309–315. doi: 10.1093/genetics/91.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerats A. G., Bussard J., Coe E. H., Jr, Larson R. Influence of B and Pl on UDPG:Flavonoid-3-O-glucosyltransferase in Zea mays L. Biochem Genet. 1984 Dec;22(11-12):1161–1169. doi: 10.1007/BF00499639. [DOI] [PubMed] [Google Scholar]
- Klein T. M., Roth B. A., Fromm M. E. Regulation of anthocyanin biosynthetic genes introduced into intact maize tissues by microprojectiles. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6681–6685. doi: 10.1073/pnas.86.17.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G., Lau A., Klein J., Golas C., Bologa-Campeanu M., Soldin S., MacLeod S. M., Prober C. Pharmacokinetics and adverse effects of amphotericin B in infants and children. J Pediatr. 1988 Sep;113(3):559–563. doi: 10.1016/s0022-3476(88)80653-x. [DOI] [PubMed] [Google Scholar]
- Ludwig S. R., Bowen B., Beach L., Wessler S. R. A regulatory gene as a novel visible marker for maize transformation. Science. 1990 Jan 26;247(4941):449–450. doi: 10.1126/science.247.4941.449. [DOI] [PubMed] [Google Scholar]
- Ludwig S. R., Habera L. F., Dellaporta S. L., Wessler S. R. Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7092–7096. doi: 10.1073/pnas.86.18.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Paz-Ares J., Ghosal D., Wienand U., Peterson P. A., Saedler H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987 Dec 1;6(12):3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J., Wienand U., Peterson P. A., Saedler H. Molecular cloning of the c locus of Zea mays: a locus regulating the anthocyanin pathway. EMBO J. 1986 May;5(5):829–833. doi: 10.1002/j.1460-2075.1986.tb04291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot G. H., Cone K. C. Nucleotide sequence of the maize R-S gene. Nucleic Acids Res. 1989 Oct 11;17(19):8003–8003. doi: 10.1093/nar/17.19.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Twell D., Klein T. M., Fromm M. E., McCormick S. Transient expression of chimeric genes delivered into pollen by microprojectile bombardment. Plant Physiol. 1989 Dec;91(4):1270–1274. doi: 10.1104/pp.91.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]



