Abstract
Beat-to-beat alternations in the amplitude of the cytosolic Ca2+ transient (Ca 2+ alternans) are thought to be the primary cause of cardiac alternans that can lead to cardiac arrhythmias and sudden death. Despite its important role in arrhythmogenesis, the mechanism underlying Ca 2+ alternans remains poorly understood. Here we investigated the role of cardiac ryanodine receptor (RyR2), the major Ca2+ release channel responsible forcytosolic Ca2+ transients, in cardiac alternans. Using a unique mouse model harboring a suppression -of-function (SOF) RyR2 mutation (E4872Q), we assessed the effect of genetically suppressing RyR2 function on Ca 2+ and action potential duration (APD)alternans in intact hearts , and electrocardiogram (ECG) alternans in vivo. We found that RyR2-SOF hearts displayed prolonged sarcoplasmic reticulum Ca2+ release refractoriness and enhanced propensity for Ca2+ al ternans. RyR2-SOF hearts/mice also exhibited increased propensity for APD and ECG alternans. Caffeine, which enhances RyR2 activity and the propensity for catecholaminergic polymorphic ventricular tachycardia ( CPVT), suppressed Ca2+ alternans in RyR2 -SOF hearts, whereas carvedilol, a beta-blocker that suppresses RyR2 activity and CPVT, promoted Ca2+ alternans in these hearts. Thus, RyR2 function is an important determinant of Ca2+ , APD, and ECG alternans. Our data also indicate that the activity of RyR2 influences the propensity for cardiac alternans and CPVT in an opposite manner. Therefore, overly suppressing or enhancing RyR2 function is pro-arrhythmic.
Keywords: Ventricular tachyarrhythmia, Ca2+ alternans, action potential duration alternans, ECG alternans, Ca2+ release refractoriness, sarcoplasmic reticulum, cardiac ryanodine receptor
INTRODUCTION
Cardiac alternans is a periodic, beat-to-beat alternation in the amplitude of contraction (mechanical alternans), a component of the electrocardiogram (ECG)waveform ( e.g. T-wave alternans), the action potential (AP) duration (APD alternans ), or the amplitude of the cytosolic Ca2+ transient (Ca2+ alternans). Cardiac alternans is frequently observed in various experimental settings and in patients with ischemic heart disease and heart failure [1–9], and is a well-recognized risk factor for ventricular fibrillation (VF) and sudden cardiac death[ 3,7,10–14]. Since its first description more than a century ago [15], cardiac alternans has been a subject of intensive investigation. However, despite its pivotal significance in arrhythmogenesis, the mechanisms underlying cardiac alternans remain poorly understood.
An increasing body of evidence supports the notion that among different forms of cardiac alternans, Ca2+ alternans plays a primary role. Chudin et al[ 16]observed Ca 2+ alternans in cardiomyocytes that were voltage-clamped, discarding APD alternans as a prerequisite for cardiac alternans. Furthermore, simultaneous recordings of membrane potential and Ca2+ transients in isolated cardiomyocytes revealed that APD alternans did not occur without Ca 2+ alternans, whereas Ca 2+ alternans could be triggered in the absence of APD alternans[ 17]. Thus, APD alternans is secondary to and is not required for Ca2+ alternans. An increased body of evidence indicates a primary role of Ca2+ dysregulation in cardiac alternans [4,9,16–23]. Therefore, understanding how Ca2+ alternans occurs is key to the understanding of the mechanisms of cardiac alternans.
In cardiac muscle cells, membrane depolarization triggers the release of Ca2+ from the sarcoplasmic reticulum (SR) via a mechanism known as Ca2+-induced Ca2+ release (CICR) [24]. In this process, membrane depolarization activates the L-type Ca2+ channel, resulting in a small Ca2+ influx. This Ca 2+ entry then opens the cardiac ryanodine receptor (RyR2), leading to a large SR Ca2+ release and subsequently muscle contraction. The amplitude of the Ca2+ transient (CICR ) depends on (i) the L-type Ca2+ current (ICa) -the trigger for CICR, (ii) the SR Ca2+ content, and (iii) the activity of RyR2 [24]. In ventricular myocytes, it has consistently been shown that there are no beat-to-beat alternations in the peak ICa during Ca 2+ alternans [9,25,26], and in human atrial myocytes, alternation in the peak ICa when present, is a consequence rather than a cause of Ca 2+ alternans [27,28], thus excluding ICa alternans as the cause of Ca 2+ alternans. Alternation in SR Ca 2+ content i s also unlikely to be a primary cause of Ca2+ alternans, as Ca 2+ alternans can occur with or without beat-to-beat alternations in SR Ca 2+ content [19,26]. This leaves the activity of the RyR2 channel as a primary candidate responsible for Ca2+ alternans. It has been proposed that beat -to-beat alternations in the availability/recovery of functional RyR2s determine the propensity for Ca2+ alternans [29,30]. Consistent with this view, pharmacological interventions that alter the activity of RyR2 affect the propensity for Ca2+ alternans. For instance, inhibiting RyR2 activity by tetracaine, intracellular acidification, or metabolic inhibition (reducing ATP level) promotes Ca2+ alternans in single isolated cardiomyocytes [25,31,32]. These observations led to the proposition that depressed RyR2 function underlies Ca2+ alternans [23,26,32,33]. However, these pharmacological manipulations can affect a number of cellular targets and processes. Thus, direct evidence for the link between depressed RyR2 function and Ca2+ alternans is lacking. Furthermore, whether specifically suppressing RyR2 activity can lead to APD alternans and /or electrocardiogram ( ECG) alternans has yet to be demonstrated .
To determine the effect of specifically depressing RyR2 activity on cardiac alternans, we employed a genetically engineered mouse model harboring a suppression-of-function (SOF) RyR2 mutation E4872Q [34]. We have previously shown that the E4872Q mutation abolishes luminal Ca2+ activation, inhibits cytosolic Ca2+ activation, and mar kedly reduces the opening time and open probability of single RyR2 channels[ 34]. Here we carried out in situ laser-scanning confocal Ca2+ imaging in cardiomyocytes in intact wildtype(WT) and RyR2 -SOF E4872Q mutant hearts (ex vivo) . We found that RyR2-SOF prolongs the refractoriness of SR Ca2+ release and promotes Ca2+ alternans in intact hearts . We also showed, for the first time, that RyR2-SOF enhances the propensity for APD alternans in intact hearts and ECG alternans in vivo. Furthermore, caffeine, an agonist of RyR2 that can induce catecholaminergic polymorphic ventricular tachycardia (CPVT)[ 35,36], alleviates Ca 2+ alternans in the RyR2 -SOF hearts, whereas carvedilol, a beta-blocker that suppresses RyR2 activity and CPVT[ 36], worsens it. Thus, although RyR2-SOF protects against CPVT[ 34], it enhances cardiac alternans. These results demonstrate that excessively suppressing or enhancing RyR2 activity is arrhythmogenic , and suggest that normalizing RyR2 function is key to minimize the propensity for CPVT and alternans-induced arrhythmias.
EXPERIMENTAL PROCEDURES
Animal studies
All animal studies were approved by the Institutional Animal Care and Use Committees at the University of Calgary and the University of Iowa, and performed in accordance with NIH guidelines. The RyR2 mutant mice harboring a suppression-of-function (SOF) mutation E4872Q in RyR2 (RyR2-E4872Q) were generated by using the knock-in approach as previously described [34]. Heterozygous E4872Q mice were bred with 129-E mouse strain to produce heterozygous E4872Q mice and their wildtype(WT) littermates. There are no homozygous RyR2-E4872Q mice as the homozygous RyR2-E4872Q mutation is embryonic lethal [34]. Adult heterozygous RyR2-E4872Q mutant mice and WT littermates (8 –12 weeks) were used for all experiments.
Laser scanning confocal Ca2+ imaging of intact hearts
Heterozygous RyR2-E4872Q mutant mice and their WT littermates were sacrificed by cervical dislocation. Their hearts were quickly removed and loaded with 4.4 μM Rhod-2 AM(Biotium, Inc. Hayward, CA) in Ca 2+ -free oxygenated Tyrode’s buffer (118 mM NaCl, 5.4 mM KCl, 25 mM NaHCO3, 1 mM MgCl2, 0.42 mM NaH2PO4, 11.1 mM glucose, 10 mM taurine, 5 mM creatine, pH 7.4) via retrograde Langendorff perfusion system at 25°C for 45 minutes [37]. Extracellular Ca2+ was stepwise increased to 0.25, 0.5, 1.0, and 1.8 mM and kept at 1.8 mM Ca2+ plus 5 μM blebbistatin (Toronto Research Chemicals, Toronto, ON) at 35°C throughout the experiment. The Langendorff-perfused hearts were placed on a recording chamber mounted onto the Nikon A1R microscope for in situ confocal imaging (line-scan) of Ca2+ signals from epicardial ventricular myocytes. The pixel size of the resulting line-scan images ranged between 1.8 and 2 ms in the temporal dimension and between 0.1 to 0.4 microns in the spatial dimension. Ca2+ alternans in the WT and RyR2-E4872Q mutant hearts in the absence or presence of caffeine (1 mM) or carvedilol (3 μM) was induced by rapid electrical stimulation of the hearts at increasing-frequencies (5–12 Hz, 6 V).
Determination of refractoriness of SR Ca2+ release
The refractoriness of Ca2+ induced Ca 2+ release from the sarcoplasmic reticulum (SR) was determined by using the S1S2 stimulation protocol as described previously with some modifications [23]. Briefly, Ca2+ transients in a Rhod -2 AM loaded heart were first induced at 5 Hz for 30 seconds (S1), followed by a single S2 stimulation at a specific interval. The heart was repeatedly stimulated by a series of S1S2 protocols with progressively decreased S1S2 intervals (from 200 to 40 ms). Ca2+ transients before and after S2 stimulation were continuously recorded by using the line-scan mode in the Nikon-A1R confocal microscope.
Monophasic action potential recordings in Langendorff perfused hearts
Hearts from heterozygous RyR2-E4872Q mutant mice and their wild type littermates were perfused with oxygenated Tyrode’s buffer with 95% O2 and 1.8 mM Ca2+ at 3–5°C during experiment. A monophasic action potential (MAP) electrode was placed against the left ventricles for epicardial MAP recording. Baseline MAP of sinus rhythm were recorded for 5–10 minutes, and the hearts were paced at the right atrium from 5 to 10 Hz at 4–10 mA for >20 seconds to induce action potential duration (APD) alternans. The total length of the recording was 30 minutes. MAP signal was amplified by Gould amplifiers (models 13-G 4615-58, 13-4615-50 and 13-4615-71) and acquired at 2000Hz/channel using a Data Translation (DT 2821) analog and digital input-output board. Analysis of MAP signals was performed using Acknowledgment software (BIOPAC MP System, Goleta, CA).
Telemetric ECG recordings in conscious mice
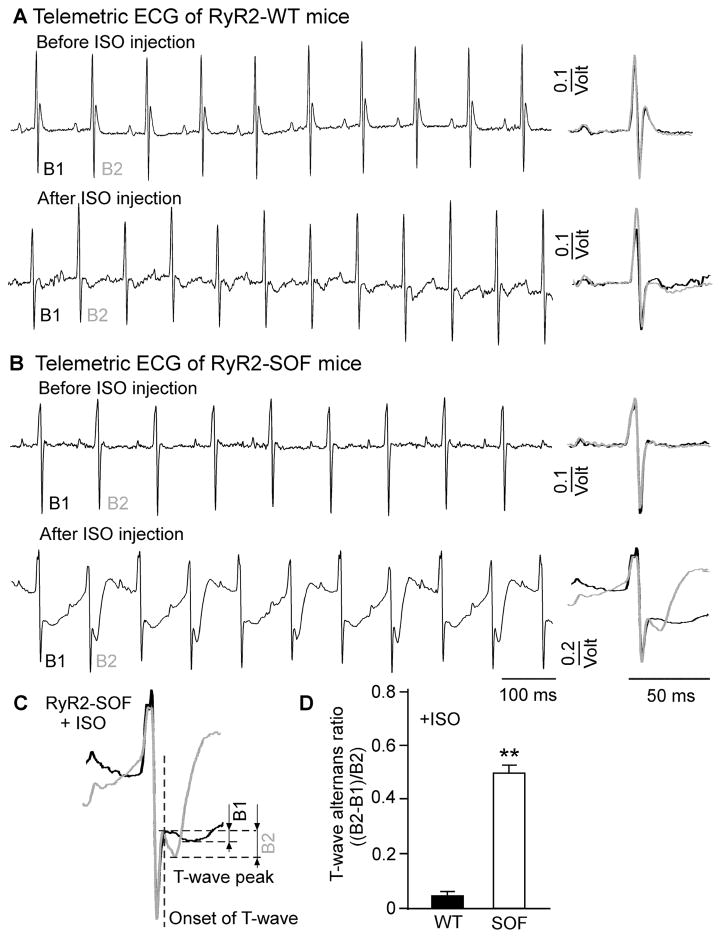
An intraperitoneal implantation of the ECG telemetric transmitter (EA-F20, Data Sciences International, USA) into the abdomen of the RyR2-WT and RyR2-SOF (heterozygous E4872Q) mice was performed under general anesthesia (2% inhaled isoflurane in O2) as described previously with some minor modifications [38]. The negative lead was placed in the right upper chest, and the positive lead in the left abdomen to form the lead II configuration. After the implantation and leads placement, the abdominal fascia and skin were sequentially closed wit h6 -0 Prolene suture. A heating pad was used throughout the surgery to maintain the mouse's temperature. Telemetric ECG recordings started 2 days after the surgical implantation. After 5 days of continuous ECG recordings, isoproterenol (ISO) (0.5 mg/kg) was administrated intraperitoneally into RyR2-WT and RyR2-SOF mice to determine the impact of adrenergic stimulation on ECG alternans. A period (2 min) of ECG recordings 5 min before ISO injection and 8 min after ISO injection were used for alternans analysis before and after adrenergic stimulation, respectively.
ECG recordings and induction of ventricular tachyarrhythmias in anesthetized mice
Heterozygous RyR2-E4872Q mice and their wild type littermates were assessed for their susceptibility to stress-induced ventricular tachyarrhythmias using ECG recordings as previously described [39]. Wildtype and mutant mice were lightly anesthetized with isoflurane vapor (0.5 –1%) and 95% O2 on a heating pad (27°C). Two subcutaneous needle electrodes were inserted into the right upper limb and left lower abdomen for ECG recordings (BIOPAC MP System, Goleta, CA). The animals’ ECG was continuously monitored under anesthesia until the heart rate became stable. Baseline ECG was recorded for 5–10 minutes. For induction of ventricular tachyarrhythmia, mutant mice and their WT littermates were subjected to intraperitoneal injection of epinephrine (3.0 mg/kg) and caffeine (150 mg/kg). ECG was continuously recorded for 30 minutes after the infusion of epinephrine and caffeine.
Image and signal processing
The following signal and image processing methods were implemented using MATLAB (The Mathworks Inc., Boston, MA). Line-scan fluorescence images were filtered using a median filter applied iteratively a number of times according to an estimation of the image noise variance. Noise variance was robustly estimated by means of a median absolute deviation of the image pixels. Identification of individual cells in the line-scan was performed by manually labeling the cell regions. Average fluorescence signals of individual cells in each line-scan were automatically obtained by spatial averaging of the pixels belonging to each marked cell. Average fluorescence signals of single cells were further filtered by applying a continuous wavelet transform of the signal with Gaussian wavelets of order 2. Zero-crossing of the derivative of the resulting wavelet transform was used to accurately locate peaks and valleys in the fluorescence signals. Peaks were then classified as either stimulated or spontaneous using cross-correlation with the stimulation pulse train. Peak amplitudes were defined as the difference between the peak and the corresponding previous valley. For each cell, alternans ratio was measured as the ratio of the absolute value of the difference in amplitude between two consecutive peaks over the amplitude of the largest peak. The presence of alternans periods was established by requiring at least six consecutive stimulated peaks presenting an alternans ratio above 0.05. For each cell, alternans duration was defined as the cumulative elapsed time of alternans periods over the total duration of the line-scan. The average alternans duration was determined by averaging alternans durations of all cells in one scan area; and the average alternans ratio was determined by averaging alternans ratios of cells that displayed alternans in the same scan area. Analysis of S1S2 refractoriness was performed using-Nikon A1R analysis system and Image J.
Statistical Analysis
All values shown are mean ± SEM unless indicated otherwise. To test for differences between groups, we used Student's t test (2-tailed). A P value <0.05 was considered to be statistically significant.
RESULTS
Genetically suppressing RyR2 activity increases the propensity for Ca2+ alternans in intact hearts
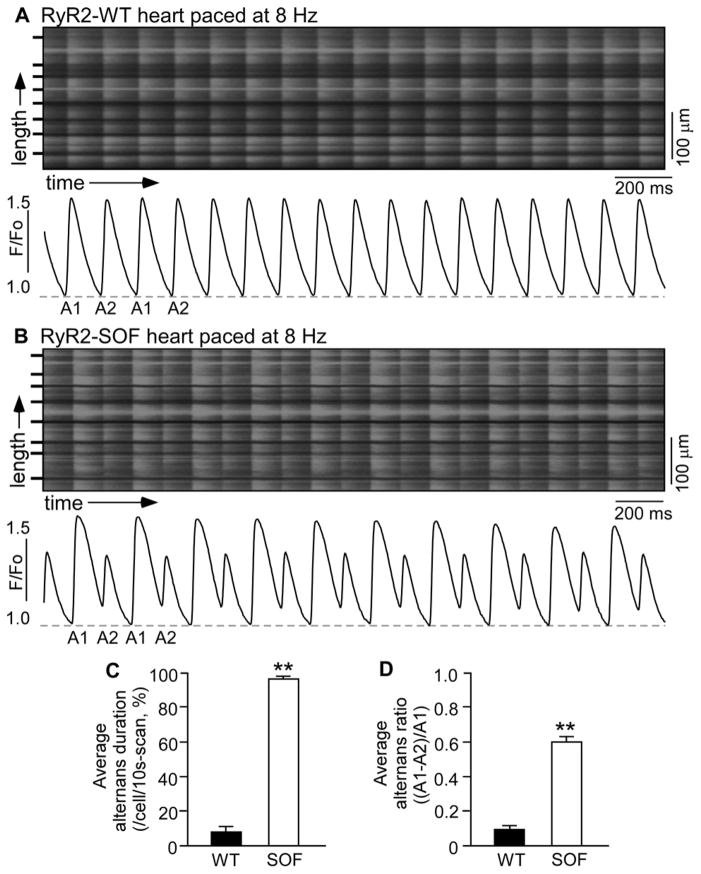
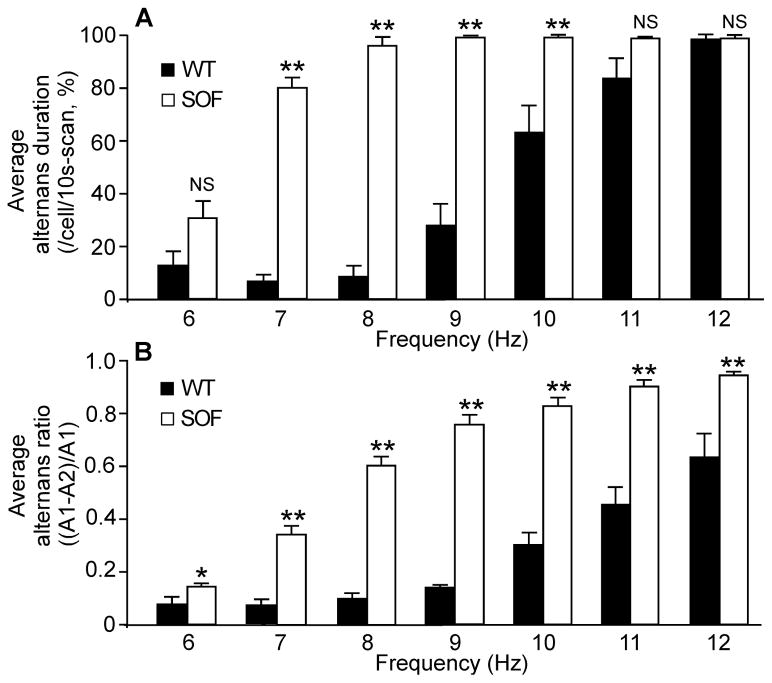
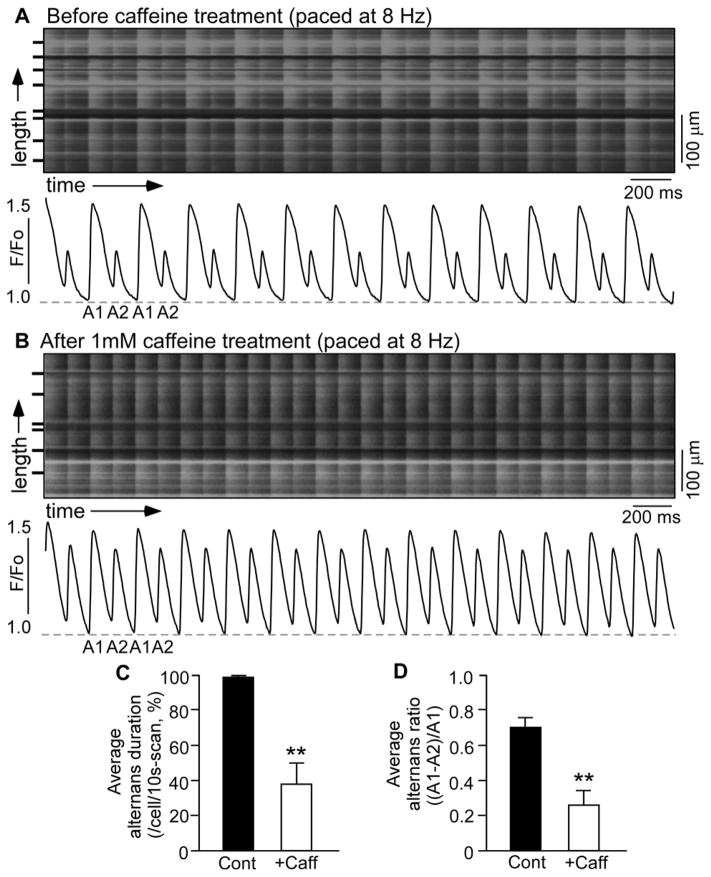
We have recently generated a knock-in mouse model harboring a suppression-of-function (SOF) RyR2 mutation (E4872Q) that markedly suppresses the activation of the RyR2 channel by luminal Ca2+ [34]. To determine the effect of genetically suppressing RyR2 function on cardiac alternans, we assessed the propensity for Ca2+ alternans in isolated Langendorff-perfused wild type (WT) and heterozygous RyR2-E4872Q (SOF)mutant hearts. As shown in Fig. 1, RyR2-WT hearts showed little or no beat-to-beat alternation in the amplitude of Ca 2+ transient s stimulated at 8 Hz (Fig. 1A and Fig. S1), whereas at the same stimulation frequency , RyR2-SOF hearts displayed large beat -to-beat variations in the amplitude of the Ca 2+ transient (Fig. 1B) . Both the average alternans duration (Fig. 1C) and average alternans ratio (Fig. 1D) were markedly increased in RyR2-SOF hearts as compared to those in the WT hearts( P<0.01). The frequency dependence of Ca2+ alternans in WT and RyR2-SOF hearts is shown in Fig. 2. Considerable Ca2+ alternans was detected in RyR2-SOF hearts at stimulation frequencies as low as 6–7 Hz(Fig. 2A) , while higher stimulation frequencies (9–10 Hz) were required to induce considerable Ca2+ alternans in WT hearts (Fig. 2B).Furthermore, RyR2-SOF hearts displayed significantly higher alternans duration at each stimulation frequency between 7–10 Hz (Fig. 2A)( P<0.01) and higher alternans ratio between 6–12 Hz compared to WT hearts(Fig. 2B) (P<0.05 ). Collectively, these data indicate that genetically suppressing RyR2 activity markedly enhances the propensity for rapid stimulation-induced Ca2+ alternans in intact hearts.
Figure 1. Ca2+ transient alternans in intact RyR2-WT and RyR2-SOF hearts.
Langendorff-perfused RyR2-WT (A) and RyR2-SOF mutant (B) hearts were loaded with Rhod-2 AM. Ca2+ transients were elicited by pacing a t 8 Hz, and recorded using line-scanning confocal imaging. Cell boundaries were indicated by black bars. The F/Fo traces depict the average fluorescence signal of the scan area . Alternans duration for each cell in the scan area and alternans ratio for each cell that displayed alternans in the same scan area were determined and averaged per cell to yield the average alternans duration (C) and average alternans ratio (D) . Alternans duration is defined as the percentage of time in alternans over the 10-s scanning period, and alternans ratio is defined as the ratio of the difference in amplitude between the large and small Ca 2+ transients over the amplitude of the large Ca2+ transient. Data shown are mean ± SEM (n=14 scan areas from 5 RyR2-WT hearts , n=30 scan areas from 12 RyR2-SOF hearts ) (**p<0.01).
Figure 2. Average alternans duration and average alternans ratio at different pacing rates in intact RyR2-WT and RyR2-SOF hearts.
Ca2+ transients in intact Rhod-2 AM loaded RyR2-WT and RyR2-SOF mutant hearts were elicited by pacing at different frequency (6 to 12 Hz), and recorded using the line-scan mode. (A) Average alternans duration (%) in cells in the scan area over the 10s scan period. (B) Average alternans ratio in cells that displayed alternans in the scan area . Data shown are mean ± SEM (n=14 scan areas from 5 RyR2-WT hearts , n=30 scan areas from 12 RyR2-SOF hearts ) (*p<0.05 and **p<0.01)
Suppressing RyR2 activity increases the refractoriness of SR Ca2+ release in intact hearts
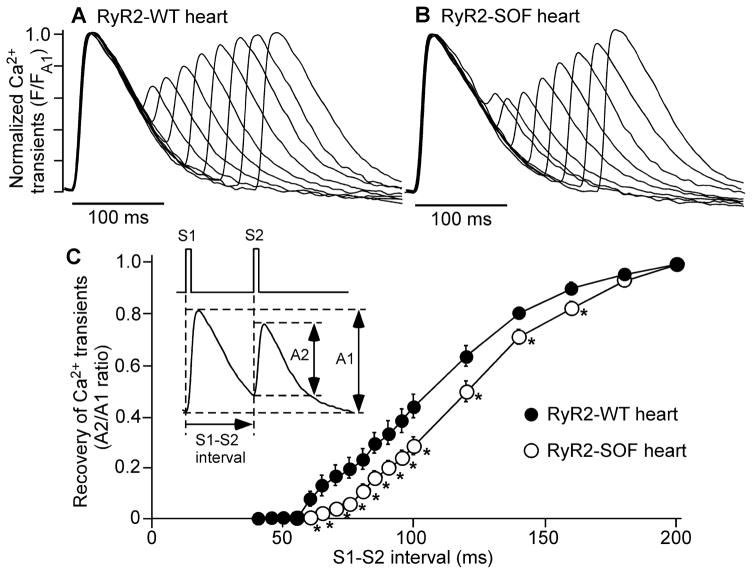
Prolonged refractoriness of SR Ca2+ release is believed to be the underlying mechanism of Ca2+ alternans [ 23,26,29,30]. To understand the mechanism by which suppressing RyR2 activity enhances Ca2+ alternans, we tested the hypothesis that suppressing RyR2 activity prolongs the refractoriness of SR Ca2+ release. To this end, we determined the refractoriness of SR Ca2+ release in isolated Langendorff-perfused WT and RyR2-SOF hearts . The heart was repeatedly stimulated by a series of S1S2 protocols with progressively decreased S1S2 intervals (from 200 to 40 ms)(Fig. 3) . The amplitude of Ca2+ transients in both the WT and RyR2-SOF hearts was reduced when the S1S2 interval was progressively decreased(Fig. 3A,B). However, the WT and RyR2-SOF hearts displayed significantly different relationship between Ca 2+ transient amplitude and S1S2 interval(Fig. 3C)(P<0.05) . The RyR2-SOF hearts required a longer S1S2 interval to recover to a given Ca2+ transient amplitude as compared to WT hearts . Therefore, these data demonstrate that suppressing RyR2 activity prolongs the refractoriness of SR Ca2+ release , which would in turn contribute to the enhanced propensity for Ca 2+ alternans.
Figure 3. RyR2-SOF mutation prolongs the refractoriness of Ca 2+ transients.
(A) Langendorff-perfused RyR2 -WT and RyR2-SOF mutant hearts were loaded with Rhod-2 AM. Hearts were first stimulated at 5 Hz for 30 seconds (S1), followed by a single S2 stimulation. A series of S1S2 stimulations are repeatedly applied with progressively reduced S1S2 interval from 200 to 40 ms. The amplitude of Ca2+ transients was recorded using the line-scan mode. (B) The relationship between S2/S1 ratio of Ca2+ transient amplitude and S1S2 interval is shown. Data shown are mean ± SEM (n=6 for RyR2-WT hearts , n=8 for RyR2-SOF hearts)(*p<0.05 and **p<0.01).
Suppressing RyR2 activity enhances action potential duration (APD) alternans in intact hearts
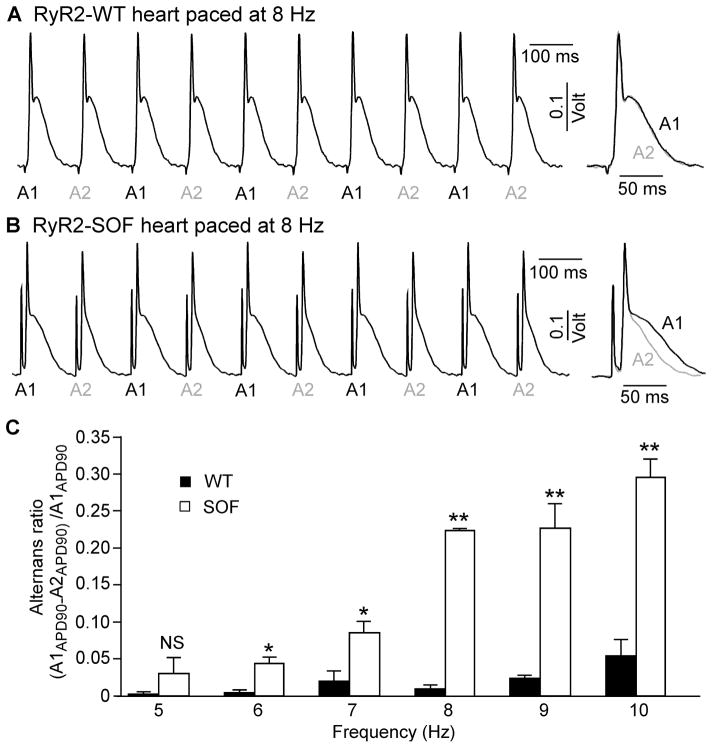
Given the close link between Ca2+ alternans and APD alternans [ 17], we next determined whether suppressing RyR2 activity also increases the propensity for APD alternans. To this end, we measured the monophasic action potential in isolated Langendorff-perfused WT and RyR2-SOF hearts stimulated at increasing frequencies(5 –10 Hz). As shown in Fig. 4, WT hearts showed no beta-to-beat alternation in the duration of action potential (APD90)at 8 Hz (Fig. 4A), whereas significant beat-to-beat variation in APD 90 was observed in RyR2 -SOF hearts at the same stimulation frequency (8 Hz)(Fig. 4B) (P<0.01). Figure 4C shows the frequency dependence of APD alternans in WT and RyR2 -SOF hearts. Considerable APD alternans was detected in RyR2-SOF hearts at as low as 6–7Hz, while higher stimulation frequencies (9–10Hz) were required to induce considerable APD alternans in WT hearts (Fig. 4C). Furthermore, RyR2-SOF hearts displayed significantly higher APD alternans ratios at each stimulation frequency between 6 –10 Hz compared to WT hearts (Fig. 4C)(P<0.05 ). These results indicate that suppressing RyR2 activity enhances the propensity for APD alternans in intact hearts.
Figure 4. RyR2-SOF mutant mice are more susceptible to APD alternans.
Hearts from the RyR2-WT and RyR2-SOF mutant mice were perfused with oxygenated Tyrode’s buffer containing 1.8 mM Ca2+ . After the heart rate became stable, rapid pacing was applied to trigger APD alternans. Representative traces of monophasic action potential (MAP) recordings from RyR2-WT (A) and RyR2-SOF heart (B) paced at 8 Hz are shown. Panel (C) shows APD alternans in RyR2-WT and RyR2-SOF hearts at different pacing frequencies (5 to 10 Hz). Data shown are mean ± SEM (n=3 hearts for each group) (*p<0.05 and **p<0.01).
RyR2-SOF mice are prone to isoproterenol-induced ECG alternans
The occurrence of APD alternans is expected to lead to ECG alternans. To test this possibility, we performed telemetric ECG recordings on conscious RyR2-WT and RyR2-SOF mutant mice at rest and after the injection of isoproterenol (ISO), which was used to induce alternans[ 40,41]. As shown in Fig. 5, RyR2-WT mice displayed little or no ECG alternans before or after the injection of ISO (0.5 mg/kg)(Fig. 5A). On the other hand, RyR2-SOF mice showed marked beat -to-beat alternations in the ECG waveform after but not before the injection of ISO (0.5 mg/kg) (Fig. 5B)in a manner similar to those described previously in mice[ 40]. In an attempt to quantify the magnitude of ECG alternans, we estimated the T-wave alternans ratio using the method described previously [40,41]. We found that the T -wave alternans ratio was significantly higher in RyR2 -SOF mice than that in RyR2-WT mice (Fig. 5C,D). However, it should be noted that due to the presence of beat-to-beat alternations in other components of ECG (Fig. 5B), it is difficult to specifically and accurately quantify the T -wave alternans ratio. Thus, the above estimation of the T-wave alternans ratio is intended to show the presence of ECG alternans, rather than the quantification of the extent of T-wave alternans. Nevertheless, these data clearly indicate that suppressing RyR2 activity enhances the propensity for ISO-induced ECG alternans in vivo.
Figure 5. RyR2-SOF mice are highly susceptible to isoproterenol-induced ECG alternans.
Telemetric ECG recordings were carried out on conscious RyR2 -WT and RyR2-SOF mice before and after the administration (i.p.) of isoproterenol (ISO) (0.5mg/kg). Representative ECG traces from RyR2-WT (A) and RyR2-SOF mice (B) 5 min before and 8 min after the injection of ISO are shown. (C) ECG waveforms of 2 consecutive beats are superimposed to depict ECG alternans. (D) Summarized data of T-wave alternans ratio defined as the ratio of the difference in the T-wave peak amplitude between the large and small T-waves over the large T-wave peak amplitude. Data shown are mean ± SEM (n=9 for RyR2-WT mice and n=9 for RyR2-SOF mice) (**p<0.001).
Caffeine suppresses Ca2+ alternans in RyR2 -SOF hearts
We have previously shown that caffeine enhances RyR2 activity by preferentially sensitizing the channel to luminal Ca2+ activation [42], an effect opposite to that of the RyR2-E4872Q mutation [34]. Thus, caffeine may be able to inhibit Ca2+ alternans in the heterozygous RyR2-SOF E4872Q mutant hearts by normalizing the effect of the RyR2-E4872Q mutation. To test this hypothesis, we determined the effect of caffeine on Ca2+ alternans in isolated Langendorff-perfused RyR2-SOF hearts . As shown in Fig. 6, caffeine (1.0 mM) significantly reduced both the alternans duration and alternans ratio in RyR2-SOF mutant hearts ( P<0.01). Caffeine also shortened the refractoriness of SR Ca2+ release (Fig. S2). These data indicate that although caffeine increases the propensity for CPVT, it can rescue Ca2+ al ternans associated with suppressed RyR2 activity.
Figure 6. Caffeine suppresses Ca2+ alternans in intact RyR2-SOF hearts.
Langendorff-perfused RyR2 -SOF mutant hearts w ere loaded with Rhod-2 AM. Ca2+ transients were elicited by pacing at 8Hz, and recorded before (A) and after (B) the application of caffeine (1 mM) using the line-scan mode. Cell boundaries were indicated by the black bars. The F/Fo traces depict the average fluorescence signal of the scan area . (C) Average alternans duration (%) in cells in the scan area over the 10s scan period. (D) Average alternans ratio in cells that displayed alternans in the scan area. Data shown are mean ± SEM (n=10 scan areas for each group from 5 RyR2-SOF hearts) (**p<0.01).
Carvedilol worsens Ca2+ alternans in RyR2 -SOF hearts
We have previously demonstrated that carvedilol, a non-selective beta-blocker, suppresses RyR2 activity, the occurrence of spontaneous Ca2+ waves, and wave-evoked VTs in a mouse model of CPVT[ 36]. These effects of carvedilol are similar to those of the RyR2-SOF mutation (E4872Q) [ 34]. This inhibitory effect of carvedilol raises an important question of whether carvedilol promotes Ca2+ alternans as the E 4872Q mutation does. To address this question, we assessed the impact of carvedilol on Ca2+ alternans in the RyR2-SOF hearts. As shown in Fig. 7 and Fig. S3, carvedilol (3 μM) increased both the alternans duration and alternans ratio ( P<0.01). Therefore, although carvedilol suppresses CPVT, it worsens Ca 2+ alternans in the setting of suppressed RyR2 activity. This observation is consistent with our previous finding that the SOF RyR2-E4872Q mutation protects against CPVT, but it enhances cardiac alternans (Figs. 2–5).
Figure 7. Carvedilol promotes Ca2+ alternans in intact RyR2-SOF hearts.
Langendorff-perfused RyR2 -SOF mutant hearts were loaded with Rhod-2 AM. Carvedilol (3 μM) was continuously applied to the heart. Ca2+ transients were elicited in intact RyR2 -SOF hearts by pacing at 5 Hz, and recorded before (A) and after (B ) the application of carvedilol using the line-scan mode. Cell boundaries were indicated by the black bars. The F/Fo traces depict the average fluorescence signal of the scan area. (C) Average alternans duration (%) in cells in the scan area over the 10s scan period. (D) Average alternans ratio in cells that displayed alternans in the scan area. Data shown are mean ± SEM (n=10 scan areas for each group from 4 RyR2-SOF hearts) (**p<0.01).
DISCUSSION
In the present study, we employed a knock -in mouse model harboring a suppression-of-function (SOF) RyR2 mutation (E4872Q)[ 34] to determine the effect of genetically suppressing RyR2 activity on cardiac alternans. We demonstrate for the first time that genetically suppressing RyR2 function promotes Ca2+ and action potential duration (APD) alternans in intact hearts and ECG alternans in vivo . We also show that agents that suppress or enhance RyR2 activity and CPVT exert an opposite impact on Ca2+ alternans. These observations suggest that the activity of RyR2 inversely determines the propensity for cardiac alternans and CPVT. These data have important implications for the treatment of RyR2-associated cardiac arrhythmias.
A large number of mutations in RyR2 have been linked to CPVT[ 43]. Most of the CPVT-associated RyR2 mutations enhance the channel activity and increase the propensity for spontaneous SR Ca2+ release, Ca2+ waves, and triggered arrhythmia [ 43]. Thus, a reasonable and logical strategy to combat CPVT is to suppress the activity of RyR2. In support of this strategy, we showed that carvedilol, a beta-blocker that is able to reduce the open duration of single RyR2 channels and inhibit the occurrence of spontaneous Ca2+ waves, effectively suppresses stress-induced VT in a CPVT mouse model harboring a gain-of-function (GOF)RyR2 mutation R4496C [36]. Similarly, we found that genetically suppressing the activity of RyR2 by knocking in a SOFRyR2 mutation E4872Q completely protects RyR2-R4496C mutant mice from CPVT[ 34]. It should be noted that the RyR2-E4872Q mutation abolishes luminal Ca2+ activation of single RyR2 channels and RyR2-mediated spontaneous Ca 2+ release in HEK293 cells and in ventricular myocytes isolated from heterozygous RyR2-E4872Q mutant mice and in intact E4872Q mutant hearts . Furthermore, homozygous RyR2-E4872Q mutation is embryonic lethal, indicating the severe impact of the E4872Q mutation on RyR2 channel function in the E4872Q mutant KI mice [34]. Therefore, pharmacologically or genetically suppressing the activity of RyR2 limits CPVT. However, loss-or suppression -of-function (LOF/SOF) RyR2 mutations have also been associated with idiopathic ventricular fibrillation[ 44,45], suggesting that overly suppressing RyR2 is also pro-arrhythmic. Indeed, we found that the SOF RyR2-E4872Q mutation enhances cardiac alternans, a well-known risk factor for re-entrant arrhythmias, VF, and sudden cardiac death. Moreover, carvedilol, which suppresses RyR2 and CPVT, exacerbates Ca2+ alternans in RyR2-SOF(E4872Q) mutant hearts. On the other hand, caffeine, which enhances RyR2 activity and induces CPVT, suppresses Ca 2+ alternans in these hearts. Suppression of Ca 2+ alternans by caffeine has also been observed in isolated cardiomyocytes [5,25,31,32]. These observations indicate that suppressing RyR2 activity, although it can protect against CPVT, could increase the propensity for cardiac alternans in already depressed hearts. Thus, although carvedilol is the beta-blocker of choice in preventing cardiac arrhythmias, using carvedilol to treat patients with LOF/SOF RyR2 function might be inappropriate or even harmful. Therefore, it is imperative to understand the exact functional impact of each disease-causing RyR2 mutation and to develop personalized treatments by normalizing the functional defect of RyR2.
Recent studies have convincingly demonstrated that rapid pacing-induced Ca2+ alternans results from beat-to-beat alternations in the refractoriness of Ca 2+ -induced Ca2+ release (CICR) from the SR[ 23,26]. This refractoriness of CICR is largely determined by (1) the recovery of the CICR trigger (i.e. the activity of the L-type Ca2+ channel), (2) the SR Ca2+ load, and (3) the activity of RyR2. While there is little evidence that ICa is the critical determinant of Ca2+ alternans [ 9,23,25,26], it has been shown that the ICa amplitude can modulate the frequency dependency of Ca2+ alternans in human cardiomyocytes [27]. Ca2+ alternans has been reported in myocytes with and without concurrent alternation in the SR Ca2+ load [19,26]. When present, alternation in the SR Ca2+ load can amplify Ca2+ alternans [26], but it may not necessarily be the cause of Ca2+ alternans [46]. Consistent with the view that recovery/refractoriness of RyR2 activity constitutes a critical determinant of Ca2+ alternans [23,26], we found that genetically suppressing RyR2 function significantly prolongs the refractoriness of CICR in intact hearts. These data suggest that suppressed RyR2 activity may delay the recovery of RyR2 from inactivation and prolong the refractoriness of SR Ca2+ release, thereby promoting Ca2+ alternans. However, despite its significance, the mechanism underlying RyR2 refractoriness remains undefined. Cytosolic Ca2+ dependent inhibition of RyR2, SR luminal Ca2+ depletion -induced desensitization of RyR2, and post-translational modulations of RyR2 have been proposed to contribute to the refractoriness of RyR2 [14]. It is of interest to point out that enhanced RyR2 activity in a mouse mode l harboring a GOF CPVT RyR2 mutation P2328S has also been shown to result in APD alternans[ 47]. However, whether the RyR2-P2328S mutation causes Ca2+ alternans in intact hearts is unclear. Nevertheless, these observations suggest that both suppressed and enhanced RyR2 activity could lead to alternans, but the exact mechanisms underlying alternans associated with suppressed or enhanced RyR2 function have yet to be determined.
It has recently been shown that Langendorff-perfused hearts isolated from a knock-in mouse model harboring a VF -associated RyR2 SOF mutation (A4860G)display VF in the presence of isoproterenol and elevated extracellular Ca2+ [ 48]. The RyR2-A4860G mutation was found to decrease the amplitude of CICR, leading to progressively increased SR Ca2+ content as a result of reduced excitation-contraction coupling gain. The resultant SR Ca2+ overload then causes bursts of prolonged SR Ca2+ release, which in turn enhances the propensity for early-after transients and early-after depolarizations (EADs)[ 48]. Hence, RyR2-SOF could lead to EADs and EAD-triggered cardiac arrhythmias. Unlike in the RyR2-SOF A4860G mutant heart, we detected little or no spontaneous SR Ca2+ release events (early -or delayed -after transients) in the RyR2-SOF E4872Q mutant heart[ 34]. Furthermore, we detected no arrhythmic events at rest or after the injection of isoproterenol or a mixture of caffeine and epinephrine in the RyR2-SOF E4872Q mice. The reasons for these differences between the A4860G and E4872Q mutant mice are unknown. One possibility is that the RyR2-A4860G mutation may suppress RyR2 activity to a smaller extent than does the RyR2-E4872Q mutation, such that the less suppressed RyR2 -A4860G mutantis more likely to be activated during SR Ca 2+ overload , leading to EADs and EAD-evoked VT/VF. On the other hand, such a SR Ca 2+ overload may not be high enough to activate the strongly suppressed RyR2-E4872Q mutant. Hence, no EADs could be triggered. Instead, the strongly suppressed RyR2-E4872Q mutation prolongs there fractoriness of SR Ca 2+ release and promotes cardiac alternans (Figs. 1–5), which would enhance the propensity for alternans-induced re-entrant arrhythmias. However, based on the critical mass theory [49], it would be difficult to induce sustained re-entrant arrhythmiasin the very small and thin mouse hearts. This may account, in part, for the lack of inducibility of ventricular arrhythmias in the RyR2-SOF E4872Q mutant hearts/mice(Fig. 5) [ 34]. Nevertheless, these findings suggest that depending on the degree of inhibition, suppressing RyR2 activity could lead to EADs or cardiac alternans.
In summary, we have demonstrated for the first time that genetically suppressing RyR2 activity prolongs the refractoriness of SR Ca2+ release and promotes Ca2+ and APD alternans in intact hearts and ECG alternans in vivo . Pharmacologically suppressing or enhancing RyR2 activity promotes or rescues Ca2+ alternans. Our data demonstrate that the RyR2 activity inversely determines the propensity for cardiac alternans and CPVT. Hence, excessively suppressing or enhancing RyR2 activity is arrhythmogenic. Therefore, normalizing RyR2 activity is key to the treatment of RyR2-associated cardiac arrhythmias.
Supplementary Material
Summary Statement.
Genetically suppressing RyR2 function prolongs Ca2+ release refractoriness and promotes cardiac alternans, but protects against catecholaminergic polymorphic ventricular tachycardia (CPVT). Thus, RyR2 activity inversely determines the propensity for cardiac alternans and CPVT.
Acknowledgments
This work was supported by research grants from the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Alberta, Northwest Territories and Nunavut, the Canada Foundation for Innovation (CFI), the Heart and Stroke Foundation/Libin Professorship in Cardiovascular Research, the Alberta Innovates-Health Solutions (to SRWC),the Spanish Ministry of Economy and Competitiveness (MINECO) (to RB, DPI2013-44584-Rand to LHM, SAF2014-58286-C2-1R), Generalitat de Catalunya (to LHM and RB, SGR2014-1465), and NHLBI (to LSS, HL090905).
THE ABBREVIATIONS USED
- SCD
sudden cardiac death
- VT
Ventricular tachyarrhythmia
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- VF
ventricular fibrillation
- ECG
electrocardiogram
- CICR
Ca2+ -induced Ca2+ release
- DAD
delayed after depolarization
- EAD
Early after depolarization
- SR
sarcoplasmic reticulum
- RyR2
cardiac ryanodine receptor
- GOF
gain-of-function
- LOF
loss-of-function
- SOF
suppression-of-function
Footnotes
CONFLICT OF INTEREST The authors declare that they have no conflicts of interest with the contents of this article.
AUTHOR CONTRIBUTIONS: XZ, BS, RW, AG, HJD, AMG, LSS, LHM, RB, SRWC designed research; XZ, BS, TM, WG, RW performed research; XZ, BS, AV, TM, WG, MN, LHM, RB, SRWC analyzed data; and XZ, BS, AV, RW, AMG, LSS, LHM, RB, SRWC wrote the paper.
References
- 1.Giudici MC, Savage MP. Transient pulsus alternans during acute myocardial ischemia and its resolution following beta-adrenergic blockade. Am Heart J. 1990;119:960–962. doi: 10.1016/s0002-8703(05)80340-0. [DOI] [PubMed] [Google Scholar]
- 2.Verrier RL, Nearing BD. Electrophysiologic basis for T wave alternans as an index of vulnerability to ventricular fibrillation. J Cardiovasc Electrophysiol. 1994;5:445–461. doi: 10.1111/j.1540-8167.1994.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 3.Armoundas AA, Tomaselli GF, Esperer HD. Pathophysiological basis and clinical application of T-wave alternans. J Am Coll Cardiol. 2002;40:207–217. doi: 10.1016/s0735-1097(02)01960-5. [DOI] [PubMed] [Google Scholar]
- 4.Eisner DA, Li Y, O'Neill SC. Alternans of intracellular calcium: Mechanism and significance. Heart Rhythm. 2006;3:743–745. doi: 10.1016/j.hrthm.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Wasserstrom JA, Kelly JE, Kadish AH, Aistrup GL. Acidosis and ischemia increase cellular Ca2+ transient alternans and repolarization alternans susceptibility in the intact rat heart. Am J Physiol Heart Circ Physiol. 2009;296:H1491–512. doi: 10.1152/ajpheart.00539.2008. [DOI] [PubMed] [Google Scholar]
- 6.Kapur S, Aistrup GL, Sharma R, Kelly JE, Arora R, Zheng J, Veramasuneni M, Kadish AH, Balke CW, Wasserstrom JA. Early development of intracellular calcium cycling defects in intact hearts of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H1843–53. doi: 10.1152/ajpheart.00623.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, Laurita KR, Rosenbaum DS. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6:251–259. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pluteanu F, Hess J, Plackic J, Nikonova Y, Preisenberger J, Bukowska A, Schotten U, Rinne A, Kienitz MC, Schafer MK, Weihe E, Goette A, Kockskamper J. Early subcellular Ca2+ remodelling and increased propensity for Ca 2+ alternans in left atrial myocytes from hypertensive rats. Cardiovasc Res. 2015;106:87–97. doi: 10.1093/cvr/cvv045. [DOI] [PubMed] [Google Scholar]
- 9.Kanaporis G, Blatter LA. The mechanisms of calcium cycling and action potential dynamics in cardiac alternans. Circ Res. 2015;116:846–856. doi: 10.1161/CIRCRESAHA.116.305404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 11.Narayan SM. T–wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–281. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 12.Verrier RL, Nieminen T. T-wave alternans as a therapeutic marker for antiarrhythmic agents. J Cardiovasc Pharmacol. 2010;55:544–554. doi: 10.1097/FJC.0b013e3181d6b781. [DOI] [PubMed] [Google Scholar]
- 13.Verrier RL, Malik M. Electrophysiology of T-wave alternans: Mechanisms and pharmacologic influences. J Electrocardiol. 2013;46:580–584. doi: 10.1016/j.jelectrocard.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Escobar AL, Valdivia HH. Cardiac alternans and ventricular fibrillation: A bad case of ryanodine receptors reneging on their duty. Circ Res. 2014;114:1369–1371. doi: 10.1161/CIRCRESAHA.114.303823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hering H. Experimentelle studien an saugertherien uber das elektrocardiogramm II. mittheilung. Z Exp Pathol Ther. 1910;7:363–378. [Google Scholar]
- 16.Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys J. 1999;77:2930–2941. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan X, Laurita KR, Pruvot EJ, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol. 2005;39:419–428. doi: 10.1016/j.yjmcc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Kihara Y, Morgan JP. Abnormal Ca2+ handling is the primary cause of mechanical alternans: Study in ferret ventricular muscles. Am J Physiol. 1991;261:H1746–55. doi: 10.1152/ajpheart.1991.261.6.H1746. [DOI] [PubMed] [Google Scholar]
- 19.Diaz ME, O'Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 20.Laurita KR, Rosenbaum DS. Cellular mechanisms of arrhythmogenic cardiac alternans. Prog Biophys Mol Biol. 2008;97:332–347. doi: 10.1016/j.pbiomolbio.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol. 2008;44:31–43. doi: 10.1016/j.yjmcc.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie LH, Sato D, Garfinkel A, Qu Z, Weiss JN. Intracellular ca alternans: Coordinated regulation by sarcoplasmic reticulum release, uptake, and leak. Biophys J. 2008;95:3100–3110. doi: 10.1529/biophysj.108.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Myles RC, De Jesus NM, Ohlendorf AK, Bers DM, Ripplinger CM. Optical mapping of sarcoplasmic reticulum Ca2+ in the intact heart: Ryanodine receptor refractoriness during alternans and fibrillation. Circ Res. 2014;114:1410–1421. doi: 10.1161/CIRCRESAHA.114.302505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 25.Huser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol. 2000;524(Pt 3):795–806. doi: 10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 27.Llach A, Molina CE, Fernandes J, Padro J, Cinca J, Hove-Madsen L. Sarcoplasmic reticulum and L-type Ca2+ channel activity regulate the beat -to-beat stability of calcium handling in human atrial myocytes. J Physiol. 2011;589:3247–3262. doi: 10.1113/jphysiol.2010.197715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molina CE, Llach A, Herraiz-Martinez A, Tarifa C, Barriga M, Wiegerinck RF, Fernandes J, Cabello N, Vallmitjana A, Benitez R, Montiel J, Cinca J, Hove-Madsen L. Prevention of adenosine A2A receptor activation diminishes beat-to-beat alternation in human atrial myocytes. Basic Res Cardiol. 2016;111:5-015-0525-2. doi: 10.1007/s00395-015-0525-2. [DOI] [PubMed] [Google Scholar]
- 29.Shkryl VM, Maxwell JT, Domeier TL, Blatter LA. Refractoriness of sarcoplasmic reticulum Ca2+ release determines Ca 2+ alternans in atrial myocytes. Am J Physiol Heart Circ Physiol. 2012;302:H2310–20. doi: 10.1152/ajpheart.00079.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lugo CA, Cantalapiedra IR, Penaranda A, Hove-Madsen L, Echebarria B. Are SR ca content fluctuations or SR refractoriness the key to atrial cardiac alternans?: Insights from a human atrial model. Am J Physiol Heart Circ Physiol. 2014;306:H1540–52. doi: 10.1152/ajpheart.00515.2013. [DOI] [PubMed] [Google Scholar]
- 31.Orchard CH, McCall E, Kirby MS, Boyett MR. Mechanical alternans during acidosis in ferret heart muscle. Circ Res. 1991;68:69–76. doi: 10.1161/01.res.68.1.69. [DOI] [PubMed] [Google Scholar]
- 32.Diaz ME, Eisner DA, O'Neill SC. Depressed ryanodine receptor activity increases variability and duration of the systolic Ca2+ transient in rat ventricular myocytes. Circ Res. 2002;91:585–593. doi: 10.1161/01.res.0000035527.53514.c2. [DOI] [PubMed] [Google Scholar]
- 33.Pieske B, Kockskamper J. Alternans goes subcellular: A "disease" of the ryanodine receptor? Circ Res. 2002;91:553–555. doi: 10.1161/01.res.0000036862.37203.f4. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, Zhou Q, Xie C, Zhang J, Guo A, Tian X, Jones PP, O'Mara ML, Liu Y, Mi T, Zhang L, Bolstad J, Semeniuk L, Cheng H, Zhang J, Chen J, Tieleman DP, Gillis AM, Duff HJ, Fill M, Song LS, Chen SR. The ryanodine receptor store–sensing gate controls Ca2+ waves and Ca 2+ -triggered arrhythmias. Nat Med. 2014;20:184–192. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, Napolitano C, Priori SG. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, Jones PP, Zhong X, Guo A, Chen H, Zhang L, Zhu W, Yang D, Li X, Chen J, Gillis AM, Duff HJ, Cheng H, Feldman AM, Song LS, Fill M, Back TG, Chen SR. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–1009. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen B, Guo A, Gao Z, Wei S, Xie YP, Chen SR, Anderson ME, Song LS. In situ confocal imaging in intact heart reveals stress-induced Ca2+ release variability in a murine catecholaminergic polymorphic ventricular tachycardia model of type 2 ryanodine receptor (R4496C+/-) mutation. Circ Arrhythm Electrophysiol. 2012;5:841–849. doi: 10.1161/CIRCEP.111.969733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fenske S, Probstle R, Auer F, Hassan S, Marks V, Pauza DH, Biel M, Wahl-Schott C. Comprehensive multilevel in vivo and in vitro analysis of heart rate fluctuations in mice by ECG telemetry and electrophysiology. Nat Protoc. 2016;11:61–86. doi: 10.1038/nprot.2015.139. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Zhou Q, Smith CD, Chen H, Tan Z, Chen B, Nani A, Wu G, Song LS, Fill M, Back TG, Chen SR. Non-beta-blocking R-carvedilol enantiomer suppresses Ca2+ waves and stress -induced ventricular tachyarrhythmia without lowering heart rate or blood pressure. Biochem J. 2015;470:233–242. doi: 10.1042/BJ20150548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shusterman V, McTiernan CF, Goldberg A, Saba S, Salama G, London B. Adrenergic stimulation promotes T-wave alternans and arrhythmia inducibility in a TNF-alpha genetic mouse model of congestive heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H440–50. doi: 10.1152/ajpheart.01024.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speerschneider T, Thomsen MB. Physiology and analysis of the electrocardiographic T wave in mice. Acta Physiol (Oxf) 2013;209:262–271. doi: 10.1111/apha.12172. [DOI] [PubMed] [Google Scholar]
- 42.Kong H, Jones PP, Koop A, Zhang L, Duff HJ, Chen SR. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J. 2008;414:441–452. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 45.Jiang D, Chen W, Wang R, Zhang L, Chen SRW. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci U S A. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez-Lacalle E, Cantalapiedra IR, Penaranda A, Cinca J, Hove-Madsen L, Echebarria B. Dependency of calcium alternans on ryanodine receptor refractoriness. PLoS One. 2013;8:e55042. doi: 10.1371/journal.pone.0055042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabir IN, Ma N, Jones VJ, Goddard CA, Zhang Y, Kalin A, Grace AA, Huang CL. Alternans in genetically modified langendorff-perfused murine hearts modeling catecholaminergic polymorphic ventricular tachycardia. Front Physiol. 2010;1:126. doi: 10.3389/fphys.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao YT, Valdivia CR, Gurrola GB, Powers PP, Willis BC, Moss RL, Jalife J, Valdivia HH. Arrhythmogenesis in a catecholaminergic polymorphic ventricular tachycardia mutation that depresses ryanodine receptor function. Proc Natl Acad Sci U S A. 2015;112:E1669–77. doi: 10.1073/pnas.1419795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garrey WE. The nature of fibrillary contraction of the heart: Its relation to tissue mass and form. Am J Physiol. 1914;33:397–414. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.