Abstract
Although the establishment of trophic ecomorphology in living crocodylians can contribute to estimating feeding habits of extinct large aquatic reptiles, assessment of ecomorphological traits other than the snout shape has scarcely been conducted in crocodylians. Here, I tested the validity of the proposed trophic ecomorphological traits in crocodylians by examining the correlation between those traits and the snout shape (an established trophic ecomorphology), using 10 non‐alligatoroid crocodylian species with a wide range of snout shape. I then compared the ontogenetic scaling of trophic ecomorphology to discuss its adaptive and taxonomic significance. The results demonstrated that degree of heterodonty, tooth spacing, size of supratemporal fenestra (STF), ventral extension of pterygoid flange and length of lower jaw symphysis are significantly correlated with snout shape by both non‐phylogenetic and phylogenetic regression analyses. Gavialis gangeticus falls outside of 95% prediction intervals for the relationships of some traits and the snout shape, suggesting that piscivorous specialization involves the deviation from the typical transformation axis of skull characters. The comparative snout shape ontogeny revealed a universal trend of snout widening through growth in the sampled crocodylians, implying the existence of a shared size‐dependent biomechanical constraint in non‐alligatoroid crocodylians. Growth patterns of other traits indicated that G. gangeticus shows atypical trends for degree of heterodonty, size of STF, and symphysis length, whereas the same trends are shared for tooth spacing and ventral extension of pterygoid flange among non‐alligatoroid crocodylians. These suggest that some characters are ontogenetically labile in response to prey preference shifts through growth, but other characters are in keeping with the conserved biomechanics among non‐alligatoroid crocodylians. Some important taxonomic characters such as the occlusal pattern are likely correlated with ontogeny and trophic ecomorphology rather than are constrained by phylogenetic relationships, and careful reassessment of such characters might be necessary for better reconstructing the morphological phylogeny of crocodylians.
Keywords: allometry, crocodylian, occlusal pattern, piscivory, trophic ecomorphology
Introduction
Ecomorphology, in its broad sense, is the study of the interaction between the organismal design and environment (Bock, 1990; Wainwright & Reilly, 1994; Motta et al. 1995), and the correspondence between the two is made by linking the morphology with function and performance, and by measuring the effects of the latter on fitness (Arnold, 1983; Garland & Losos, 1994; Ricklefs & Miles, 1994; Wainwright, 1994). Because morphology can be rigorously measured based on the homologous structures and has a high repeatability of measurement, it has been used to derive ecological inferences including resource partitioning and niche relationships among taxa, community structure, and diversity within taxa (Ricklefs & Miles, 1994). Probably, the utility of ecomorphological analysis is most appreciated in paleontological researches, because direct measurements of performance and fitness are not possible in extinct animals, but the morphology of hard tissues is often available in fossils. By using morphology, we can estimate important biological aspects of extinct organisms such as feeding and locomotor habits and body size (Kubo & Benton, 2009; Zanno & Makovicky, 2011; Campione & Evans, 2012), and can get insights into the ecological diversity pattern, species or higher level interactions, and history of adaptation in an evolutionary time scale (Brusatte et al. 2008; Sookias et al. 2012; Larson et al. 2016).
Crocodylians are opportunistic ambush predators, feeding on a wide range of invertebrates and vertebrates, depending on the availability of their habitat (Pooley, 1989; Webb & Manolis, 1989). Generally, their feeding habits are thought to reflect the skull shapes, and most of its variation is explained by broad to slender‐snouted shape axis in extant as well as extinct forms (Brochu, 2001; Pierce et al. 2008; Okamoto et al. 2015). The extreme end‐member in the slender‐snouted taxa is Indian gharial (Gavialis gangeticus), which is considered as the most predominant fish‐eater in extant crocodylians (Whitaker & Basu, 1982; Trutnau & Sommerlad, 2006). Its long and narrow snout accounts for the high angular velocity of the jaw tip while lateral head sweeping (Thorbjarnarson, 1990) and jaw closure, thus favorable for catching small agile prey. However, this type of snout is mechanically weak for stress during biting (Busbey, 1995; McHenry et al. 2006; Pierce et al. 2008; Walmsley et al. 2013), and hydrodynamically costly as compared with the cranial morphology of generalist‐type taxa (e.g. Crocodylus porosus and Crocodylus niloticus; McHenry et al. 2006). On the other hand, experimental studies revealed that interspecific variation in crocodylian bite force is primarily explained by body mass if G. gangeticus is excluded, and slender‐snouted forms can generate equivalent bite forces with broader‐snouted forms, suggesting that slender‐snouted species may reduce jaw stresses by targeting on smaller prey such as fish and crustaceans (Erickson et al. 2012).
Although the snout shape has been particularly focused in the previous ecomorphological analyses of crocodylians, there are a number of other characters that have also been proposed as ecomorphological characters (Iordansky, 1973; Langston, 1973; Brochu, 2001). However, quantitative assessments of such potential ecomorphological traits have rarely been conducted (Walmsley et al. 2013; Gignac & Erickson, 2015). Crocodylians are known as the sole remnants of Pseudosuchia (the most inclusive clade containing C. niloticus but not Passer domesticus: Nesbitt, 2011), a diverse and successful group of reptilians that rivaled dinosaurs in the early Mesozoic (Brusatte et al. 2008), and are often regarded as modern analogs of extinct large‐bodied aquatic predators. Hence, establishment of trophic ecomorphology in living crocodylians can contribute to estimating feeding habits in extinct pseudosuchians and non‐pseudosuchian reptiles such as marine crocodyliforms, dinosaurs, phytosaurs and choristoderans (Erickson, 1972; Massare, 1987; Hunt, 1989; Sereno et al. 1998, 2001), and helps answer various ecological questions by integrating space, time and phylogenetic perspectives. Moreover, the finding of the ecological characters and their correlation (Sadleir & Makovicky, 2008) may help better understand the crocodylian phylogeny. It should be noted that a suite of potential feeding ecomorphological traits in rostrum is indeed consistent with molecular tree rather than supporting the morphological tree (Gatesy et al. 2003). However, removal or proper weighting of such non‐independent characters are necessary for rigorously comparing the fit of morphological data on morphological and molecular trees, and thus resolving the long‐standing discussion on the placement of G. gangeticus in the crocodylian phylogeny (Densmore, 1983; Norell, 1989; Poe, 1996; Brochu, 1997; Gatesy et al. 2003).
Meanwhile, as most of the animals do, crocodylian skulls change remarkably during the postnatal development (Mook, 1921b; Müller, 1927; Kälin, 1933; Dodson, 1975; Monteiro & Soares, 1997; Monteiro et al. 1997; Sadleir, 2009; Piras et al. 2010; Bona & Desojo, 2011; Watanabe & Slice, 2014; Fernandez‐Blanco et al. 2015; Foth et al. 2015). Previous studies demonstrated that growth trajectories of Crocodylus skulls can fall into some stages, and hypothesized that the breakpoints between the stages correspond to changes in diets (Hall & Portier, 1994; Radloff et al. 2012), whereas an experimental study suggested that positive allometry of bite force is conserved among crocodylians regardless of snout shape variation (Erickson et al. 2014). Comparisons of the developmental pathways of trophic ecomorphology among crocodylians would tell us whether dietary adaptation during growth occurs through certain morphological changes, and the role of developmental process in shaping the morphological and ecological diversity of crocodylians.
Here, using the postnatal growth series of extant crocodylians, I first assess the validity of proposed trophic ecomorphology of non‐alligatoroid crocodylians, with the special focus on piscivorous ecomorphology. I then compare the growth patterns of trophic ecomorphology among non‐alligatoroid crocodylians for finding differential and shared scaling patterns, and discuss their adaptive and taxonomic significance. Although the current study restricts the sample to non‐alligatoroid crocodylians, additional sampling of alligatoroid species may permit the generalization about the observed trends and patterns among crocodylians.
Materials and methods
Specimen sampling
For conducting species comparisons of snout shape and the associated skull characters among Crocodylia, multiple taxa with a wide range of snout shape are required. I sampled the postnatal growth series of skulls from 10 crocodylians (n = 236; seven species of Crocodylus, Mecistops cataphractus, Tomistoma schlegelii and G. gangeticus; Fig. 1). Alligatoroid species were not included in the sample because the current study specifically focuses on piscivorous specialization. Crocodylus is the most diverse and species‐rich genus in extant crocodylians, including 12 known species (Grigg & Kirshner, 2015) from blunt‐snouted type to slender‐snouted type. A recent molecular study by Oaks (2011) revealed the late Miocene divergence of this group, suggesting that Crocodylus snout shape is ecologically plastic and is not strongly constrained by the phylogenetic relationships. Three remaining non‐Crocodylus species are all slender‐snouted, yet they differ in the degree. In crocodylian systematics, the relationships among Crocodylus, and placement of T. schlegelii and G. gangeticus are still not fully settled (Densmore, 1983; Densmore & White, 1991; Brochu, 1997, 1999; Gatesy et al. 2003; McAliley et al. 2006; Roos et al. 2007; Oaks, 2011). However, in the current study, I chose to use the most recent and comprehensive time‐calibrated molecular tree by Oaks (2011) as a phylogenetic framework for statistical analyses because, contrary to the molecular analyses, previous morphological analyses (Brochu, 2000; Brochu et al. 2010; Brochu & Storrs, 2012) have not resolved the phylogenetic relationships among Crocodylus. Nearly all (232/236) samples used in this study are wild‐caught with locality information (Table S1). In 10 crocodylians used in this study, some species are known to have morphologically distinct cryptic populations [C. niloticus (Hekkala et al. 2011; Nestler, 2012); Crocodylus novaeguineae (Hall, 1989); Crocodylus palustris (Trutnau & Sommerlad, 2006) and M. cataphractus (Shirley et al. 2014)]. With this in mind, I sampled individuals from a limited geographical area for C. niloticus (Congo basin: Hekkala et al. 2011; Nestler, 2012) and for M. cataphractus (Congolian zone: Shirley et al. 2014), but other species are from all available localities to increase the sample size.
Figure 1.
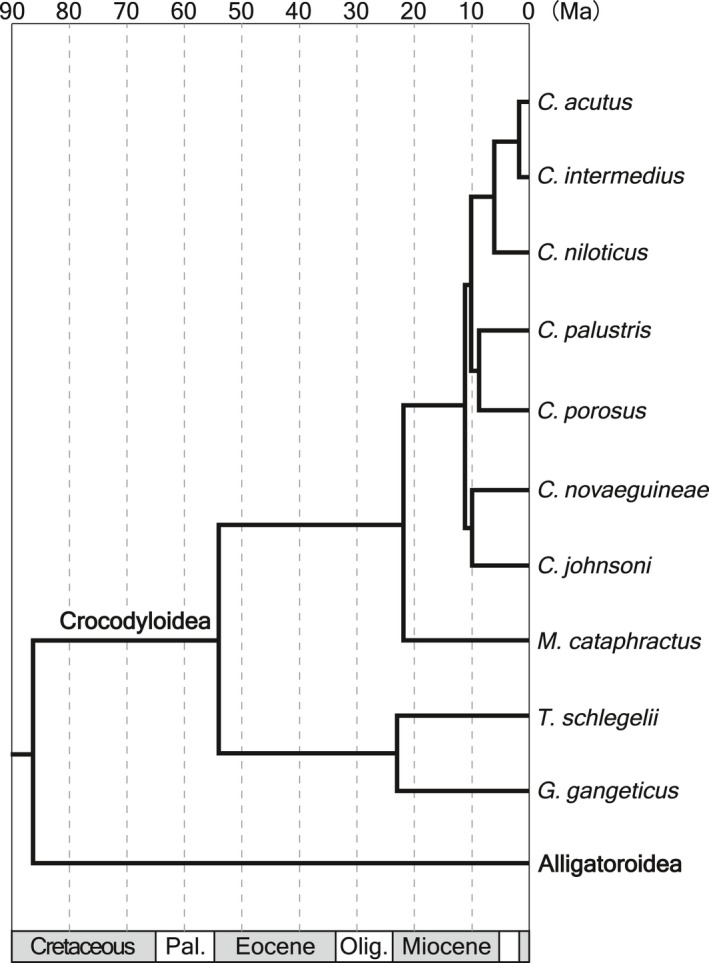
A time‐calibrated molecular phylogeny of 10 sampled crocodylian species (based on Oaks, 2011).
Data acquisition
A list of previously proposed trophic ecomorphology in crocodylians that are functionally explained in relation with feeding behavior is shown in Table 1. Among the variables, I did not consider the tooth shape because teeth are often shed and their original positions are not available in the specimens. To assess validity of the proposed ecomorphological characters in crocodylians, I conducted regression analyses of the proposed ecomorphological characters against the established ecomorphological character (snout shape) to examine their correlations. In crocodylians, snout shape is considered as being correlated with their diet (Brochu, 2001), and is known to reflect the feeding function and performance (Taylor, 1987; Thorbjarnarson, 1990; McHenry et al. 2006). Therefore, if a character correlates with snout shape, then it can also be regarded as a trophic ecomorphological character.
Table 1.
Proposed trophic ecomorphological variables and their piscivorous states in crocodylians
| Variable | Piscivorous state | Function and performance | Reference |
|---|---|---|---|
| Snout shape | Long and slender | Higher angular velocity of jaw tip during lateral head sweeping and jaw closing, thus favorable for catching small agile prey | Taylor, 1987; Thorbjarnarson, 1990; McHenry et al. 2006 |
| Degree of heterodonty (tooth size disparity) | Less degree of heterodonty (coupled with less undulated jaw margin) | Efficient for detaining relatively docile prey, but inefficient for holding larger prey firmly | Iordansky, 1973; Busbey, 1995 |
| Spacing of teeth | Widely spaced | Striking and impaling prey | Langston, 1973 |
| Tooth count | Increased in number | Increase the chance of catching prey and facilitates transport of the food items towards the pharynx (while inertial feeding) | Busbey, 1995 |
| Tooth shape | Long, slender and recurved | Striking and impaling prey, and maneuvering fish back toward gullet | Langston, 1973; Massare, 1987; Busbey, 1995 |
| Size of STF | Increased | Increased mass of M. pseudotemporalis, which is assciated with rapid but weak jaw adduction | Iordansky, 1964; Endo et al. 2002 |
| Ventral extension of pterygoid flange | Reduced | Reduced mass and moment arm of Mm. pterygoidei dorsalis et ventralis (strong jaw adductors), and reduction in maximal mouth opening | Iordansky, 1964; Endo et al. 2002; Piras et al. 2014 |
| Development of basioccipital tubera | Highly developed and pendulous | Modified neck musculature (M. longissimus capitis profundus and M. rectus capitis anticus major; Tsuihiji 2007) advantageous for head motions during fish eating (e.g. lateral head sweeping) | Langston, 1973 |
| Length of lower jaw symphysis | Elongated | Incur higher strain under loads used for feeding upon large prey | Walmsley et al. 2013 |
STF, supratemporal fenestra.
For the quantification of the snout shape, I performed two‐dimensional geometric morphometrics with seven landmarks (type 1 and 2 of Bookstein, 1991) and 38 sliding semi‐landmarks that were digitized from palates (Fig. 2A), using tpsdig v. 2.17 (Rohlf, 2013a). The current method is probably better in capturing snout shapes than previous studies that used the ratio of linear measurements from dorsal snouts (Hall & Portier, 1994; Erickson et al. 2012), which did not take into account subtle differences in snout outlines. Landmark and semi‐landmark coordinates of the pooled samples (n = 236) were superimposed by a generalized Procrustes analysis (Gower, 1975; Rohlf & Slice, 1990), and subjected to relative warp (RW) analysis using tpsrelw v. 1.53 (Rohlf, 2013b). Because much of the snout shape variation would be accounted for by the first axis of RWs, RW1 scores were extracted as the snout shape proxy.
Figure 2.
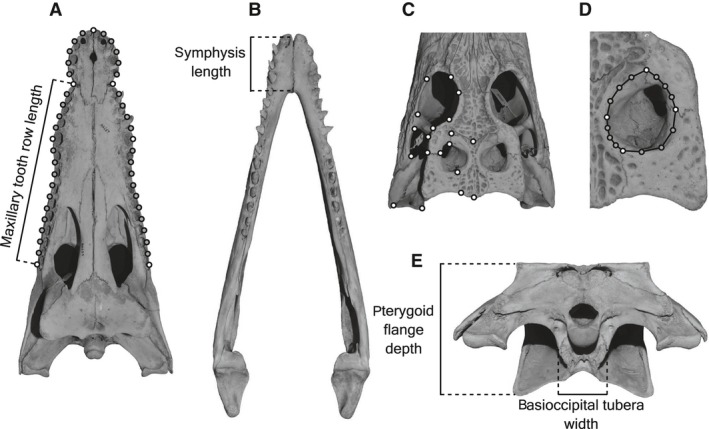
(A) Rostral landmarks and semi‐landmarks used in this analysis. Seven landmarks (white markers) are taken at: 1: anterior end of rostrum; 2, 3: both lateral edges of premaxillary–maxillary contact; 4, 5: both lateral edges of maxillary alveolus #5; 6, 7: both lateral edges of rostrum at the level of posterior end of last maxillary alveolus. A total of 38 semi‐landmarks (gray markers) are taken on the outline of six palatal outline segments. (B) Measurement of lower jaw symphysis length. (C) Nineteen post‐rostral landmarks taken at: 1: orbit‐prefrontal‐lacrimal contact; 2: orbit‐lacrimal‐jugal contact; 3: orbit‐prefrontal‐frontal contact; 4: orbit‐frontal‐postorbital contact; 5: anteroventral end of postorbital bar; 6: posteroventral end of postorbital bar; 7: anterodorsal end of postorbital bar; 8: posterodorsal end of postorbital bar; 9: infratemporal fenestra‐jugal‐quadratojugal contact; 10: lateral edge of skull table at postorbital‐squamosal contact; 11: supratemporal fenestra (STF)‐postorbital‐squamosal contact; 12: triple junction of frontal‐parietal‐postorbital; 13: STF‐parietal‐postorbital contact; 14: STF‐parietal‐squamosal contact; 15: frontal‐parietal contact on midline; 16: supraoccipital‐parietal contact on midline; 17: posterior edge of skull table at parietal‐squamosal contact; 18: posteromedial corner of quadrate jaw joint; 19; posterior end of quadrate‐quadratojugal contact. (D) Landmarks and semi‐landmarks for calculating area of STF. Four landmarks (white markers) are taken at: 1: anterior edge of STF at postorbital‐parietal contact; 2: lateral edge of STF at postorbital‐squamosal contact; 3: medial‐most point of STF along parietal; 4: posterior edge of STF at parietal‐squamosal contact. A total of 12 semi‐landmarks (gray markers) are taken on the outline of STF to calculate the STF area including its smooth floor. (E) Measurements of pterygoid flange depth (PFD) and basioccipital tubera width.
The degree of heterodonty (tooth size disparity) was calculated as the coefficient of variation (ratio of standard deviation to mean) of mesiodistal diameters of all right/left maxillary alveoli (Table S2). The spacing of teeth was represented as the ratio of the sum of mesiodistal diameters of all right/left maxillary alveoli to the maxillary tooth row length (Fig. 2A), which was measured in juvenile–adult specimens showing interalveolar bones. The tooth count was taken from left and right maxillae, because tooth counts in maxillae are more variable than those of premaxillae among crocodylians (Iordansky, 1973). These tooth size, tooth spacing and tooth count patterns in the upper jaw can almost certainly be generalized for the lower jaw (Brown et al. 2015). The size of supratemporal fenestra (STF) was measured as an area enclosed by landmarks and semi‐landmarks (Fig. 2D) using tpsutil v. 1.58 (Rohlf, 2013c), based on the photos of posterior skulls taken from dorsal view, with the scale on the flat part of skull table. The measurements of other metrics are shown in Fig. 2B,E. As the body size proxy, I used the centroid size (CS) of superimposed dorsal post‐rostrum landmarks (Fig. 2C) of each species obtained from tpsrelw, because the post‐rostrum shape is more conservative compared with the rostrum (Piras et al. 2014). To obtain the size information for the digitized landmark data, samples were scaled with reference to maximum skull width at jaw joint in tpsdig. Linear measurements were taken by Tresna point digital caliper (Series SC02, ID 111–203, resolution = 0.01 mm). Photos of palatal and dorsal aspects of the skulls for geometric morphometrics were taken by positioning Nikon D3100 camera (AF‐S DX NIKKOR 18–55 mm f/3.5–5.6G VR, 14.2 Mp resolution) perpendicular to the palatal and skull table surface, respectively, consistently centering specimens in the field of view, and keeping enough distance between the lens and specimens.
Assessment of the proposed trophic ecomorphology in non‐alligatoroid crocodylians
To size‐normalize the dimensioned variables, linear measurements were divided by CS, and area measurements were square rooted and then divided by CS. However, even after the size‐removal, various allometric trends in ecomorphological variables need to be controlled. A common approach for correcting allometry is to regress variables on size metrics using least squares regression and then to take the residuals (Piras et al. 2014; Watanabe & Slice, 2014; Foth et al. 2015), but crocodylian snout shapes are known to show non‐linear growth trajectories (Hall & Portier, 1994; Radloff et al. 2012; this study), thus use of such an approach would be inappropriate. Therefore, I selected individuals within the arbitrarily set thresholds of log10 transformed CS [log(CS)], which correspond to subadult–adult stages for all species. For examining the correlations between proposed ecomorphological variables and snout shape (RW1 score) within the set size range, I first conducted ordinary least squares (OLS) regression with whole samples. Maxillary tooth count was not analyzed in the whole‐sample OLS. Secondly, species comparison was performed by phylogenetic generalized least squares (PGLS) regression, in which average species ecomorphological variable values were regressed on average species snout shape (RW1 score) within the set size range, on an assumption of Brownian motion model of trait evolution. For maxillary tooth count, mode instead of mean was used for the regression. Because PGLS regression requires a time‐calibrated tree, a phylogenetic tree of 10 sampled crocodylian species (Fig. 1) was constructed with reference to a molecular study by Oaks (2011), and divergence time estimates were adopted from the species tree of the original study with a 90 myr upper limit on the root age. Additionally, a morphological tree (Fig. S1; Appendix S1) was used for testing the sensitivity of the PGLS results to tree shape. PGLS regression was conducted using software r with caper package (Orme et al. 2013), and a phylogenetic signal parameter (Pagel's λ) was optimized and took into account in the analysis.
Comparative growth trajectories of trophic ecomorphology in non‐alligatoroid crocodylians
Prior to analyses, linear and area measurements were log10 transformed, yet dimensionless variables (snout shape, degree of heterodonty and spacing of teeth) were left untransformed. The growth trajectories of ecomorphological variables were visualized using a bivariate plot of log(CS) of the post‐rostrum and each variable. Because the trajectories of snout shape were non‐linear with breakpoint(s), piecewise linear models, where two straight lines are joined at an unknown knot (breakpoint), were fit to find the breakpoint. The analysis was conducted using r with the sizer package (Sonderegger, 2012), and the breakpoint estimate was reported with 95% confidence intervals. In the model fitting, very small individuals were excluded by setting an arbitrary size threshold for all species so as not to include individuals from relative skull growth phase I (Hall & Portier, 1994). Therefore, the first and second segments in the model correspond to the skull growth phases II and III (Hall & Portier, 1994). Because some species do not grow larger than the assumed breakpoint or have small sample size beyond the breakpoint, the analysis was performed only in large‐bodied well‐sampled taxa (six species: Crocodylus acutus, C. niloticus, C. palustris, C. porosus, G. gangeticus and T. schlegelii). After the mean snout shape breakpoint of six species was obtained, I additionally tested if snout shapes differ between growth phase II (smaller than mean breakpoint) and phase III (larger than mean breakpoint) in the six species using Welch's t‐test.
For the remaining variables, I performed reduced major axis (RMA) regression of ecomorphological variables against log(CS) with whole samples using r with smatr package (Warton et al. 2012). Dimensionless variables were examined if there are significant relationships between the variables and size [log(CS)], and dimensioned variables were examined if RMA slopes for the relationships of variables (log10 transformed) and size [log(CS)] are different from the expected isometric slopes (1.0 and 2.0 for linear and area measurement variables, respectively). The growth of the variables was considered either positively or negatively allometric if 95% confidence intervals of the slopes do not overlap the expected isometric slopes. Because maxillary tooth count is different in its data type, it was separately subjected to Spearman rank correlation to test the correlation between tooth count and size. All statistical tests were carried out with r language and environment (R Core Team, 2014).
Results
Assessment of the proposed trophic ecomorphology
Because body size [log(CS)] of sampled crocodylians (10 species, n = 236) ranges from ~1.6 to 2.6, and snout shape (RW1 score) largely changes beyond log(CS) of ~ 2.4 for all taxa (Fig. 4), I selected individuals from the arbitrary set size range [2.1 ≤ log(CS) ≤ 2.4], which covers a relatively stable size range of the snout shape, and secures 110–123 individuals for each variable. A summary of the whole‐sample OLS regression and species PGLS regression between proposed trophic ecomorphological variables and snout shape (RW1 score) are found in Fig. 3 and Table 2. Among the proposed ecomorphological variables, CV of maxillary alveolus diameter, maxillary tooth spacing, relative STF area, relative pterygoid flange depth (PFD) and relative lower jaw symphysis length are significantly correlated with snout shape in both whole‐sample OLS and species PGLS regressions, thus verified as trophic ecomorphology. The use of an alternative tree (morphological tree) for PGLS did not significantly change the results (Table S3). These indicate that generally, tooth size variability becomes smaller, maxillary teeth are more spaciously positioned, STF size increases, PFD decreases and lower jaw symphysis length extends in accord with the snout narrowing in the sampled crocodylians. On the contrary, relative basioccipital tubera width and mode species maxillary tooth count were not significantly correlated with snout shape in either whole‐sample OLS or species PGLS regression.
Figure 4.
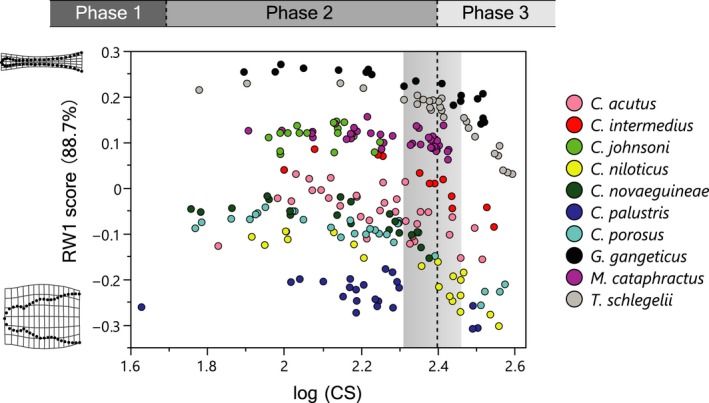
Bivariate plot of log(CS) and snout shape (RW1 scores). Area with gray gradient represents the estimated range of log(CS) breakpoints of snout shape for six species (C. acutus, C. niloticus, C. palustris, C. porosus, G. gangeticus and T. schlegelii), and the dashed line is their mean.
Figure 3.

(A,B,D–G) Bivariate plots of snout shape (RW1 score) and proposed ecomorphological variables for individuals within 2.1 ≤ log(CS) ≤ 2.4. Ordinary least squares (OLS) regression lines are fit on the plots. (C) Bivariate plot of mean species RW1 score and mode species maxillary tooth count for individuals within 2.1 ≤ log(CS) ≤ 2.4. CV, coefficient of variation. Dashed lines are 95% prediction intervals (PIs).
Table 2.
Whole‐sample and species regressions of proposed ecomorphological variables against snout shape (RW1 score). Individuals with 2.1 ≤ log(CS) ≤ 2.4 were used for the analyses
| Variable by snout shape (RW1 score) | n | R 2 | P | Slope | Intercept | Pagel's λ |
|---|---|---|---|---|---|---|
| Whole‐sample analysis with ordinary least squares (OLS) regression | ||||||
| CV of maxillary alveolus diameter | 111 | 0.668 | < 0.001 | −0.242 | 0.151 | NA |
| Maxillary tooth spacing | 111 | 0.860 | < 0.001 | −0.676 | 0.593 | NA |
| Relative STF area [(STF area)1/2/CS] | 123 | 0.315 | < 0.001 | 0.069 | 0.134 | NA |
| Relative PFD (PFD/CS) | 120 | 0.376 | < 0.001 | −0.247 | 0.564 | NA |
| Relative basioccipital tubera width (BTW/CS) | 122 | 0.009 | 0.311 | 0.015 | 0.184 | NA |
| Relative symphysis length (SL/CS) | 110 | 0.667 | < 0.001 | 2.233 | 0.567 | NA |
| Species analysis with phylogenetic generalized least squares (PGLS) regression | ||||||
| CV of maxillary alveolus diameter | 10 | 0.863 | < 0.001 | −0.232 | 0.155 | 0.000 |
| Maxillary tooth spacing | 10 | 0.972 | < 0.001 | −0.646 | 0.591 | 0.000 |
| Mode maxillary tooth count | 10 | 0.054 | 0.519 | 3.154 | 16.352 | 1.000 |
| Relative STF area [(STF area)1/2/CS] | 10 | 0.509 | 0.021 | 0.098 | 0.137 | 0.000 |
| Relative PFD (PFD/CS) | 10 | 0.634 | 0.006 | −0.237 | 0.541 | 1.000 |
| Relative basioccipital tubera width (BTW/CS) | 10 | 0.130 | 0.306 | 0.053 | 0.187 | 0.000 |
| Relative symphysis length (SL/CS) | 10 | 0.676 | 0.004 | 1.476 | 0.835 | 1.000 |
CS, centroid size; CV, coefficient of variation; PFD, pterygoid flange depth; STF, supratemporal fenestra. Mean species RW1 scores and mean variable values were used for species analysis (PGLS regression). The molecular tree of Oaks (2011) was used for PGLS regression.
Growth trajectories of trophic ecomorphology
An observation of the bivariate plot of snout shape (RW1 score) and log(CS) (Fig. 4) revealed a universal trend of snout widening through growth. In most of the species, the snout shape is isometric or somewhat positively allometric in its width until reaching log(CS) of ~2.4, but beyond this size the snout grows increasingly wider. Although a rigorous comparison of species trajectories was not attempted because of the difficulty in comparing non‐linear trajectories, this pattern seems global and unrelated with interspecific snout shape differences. The result of the snout shape breakpoint estimation by piecewise linear model fitting is shown in Fig. 5 (Table S4). In this analysis, individuals with log(CS) ≥ 2.0 were used for excluding very small forms that might belong to relative skull growth phase I (Hall & Portier, 1994). The estimated log(CS) breakpoints of snout shape growth trajectories are 2.31–2.46 in six species (C. acutus, C. niloticus, C. palustris, C. porosus, G. gangeticus and T. schlegelii), and their mean is 2.40. Individuals with log(CS) smaller and larger than the mean snout shape breakpoint were considered as members of growth phase II and III (Hall & Portier, 1994), respectively, and Welch's t‐test confirmed that snout shapes are significantly different between two phases (Table 3).
Figure 5.
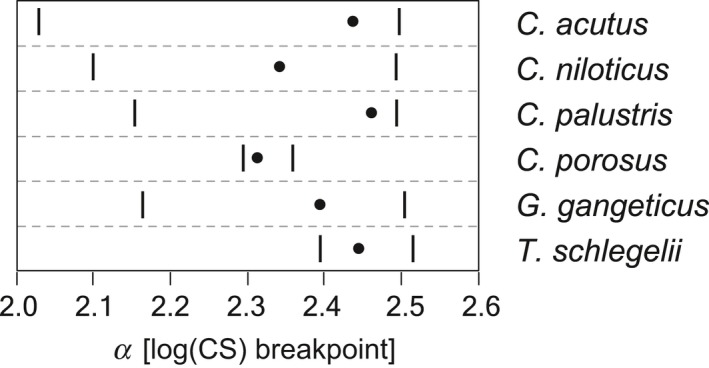
Log(CS) breakpoints of snout shape growth trajectories in six species estimated by piecewise linear model fitting. Individuals with log(CS) ≥ 2.0 were used for this analysis. Dots are the estimated values, and bars represent their 95% confidence intervals (CIs).
Table 3.
Welch's t‐test of snout shape (RW1 score) between skull growth phases II and III. Individuals with log(CS) ≥ 2.0 were used for the analysis
| Taxon | n | t | df | P |
|---|---|---|---|---|
| C. acutus | 31 | −3.591 | 5.958 | 0.012 |
| C. niloticus | 17 | −5.777 | 13.607 | < 0.001 |
| C. palustris | 21 | −3.819 | 2.665 | 0.039 |
| C. porosus | 23 | −12.821 | 5.068 | < 0.001 |
| G. gangeticus | 16 | −5.696 | 12.045 | < 0.001 |
| T. schlegelii | 25 | −4.821 | 17.774 | < 0.001 |
Bivariate plots of log(CS) and trophic ecomorphological variables are provided in Fig. 6, and a summary of the RMA regression between ecomorphological variables and log(CS) for whole samples is found in Table 4. Maxillary tooth size variability is significantly (P < 0.05) and near significantly (P < 0.1) correlated with size in seven species, excluding G. gangeticus, Crocodylus johnsoni and M. cataphractus, all showing a positive trend through growth. Maxillary tooth spacing is significantly and near significantly correlated with size in six species, and all of which show a positive trend. A strong positive correlation (R 2 > 0.6) is found in two most longirostral taxa (G. gangeticus and T. schlegelii), indicating a significant decrease in the relative spacing of maxillary teeth through growth. The relationship of relative STF area is negatively allometric (95% CI < 2) in four species, isometric (95% CI includes 2) in five species, and positively allometric (95% CI > 2) in G. gangeticus. The slopes for relative PFD are positively allometric for all taxa, and those for relative basioccipital tubera widths vary among 10 species. The relationship of relative symphysis length is positively allometric and isometric in nine species, but negatively allometric in G. gangeticus.
Figure 6.
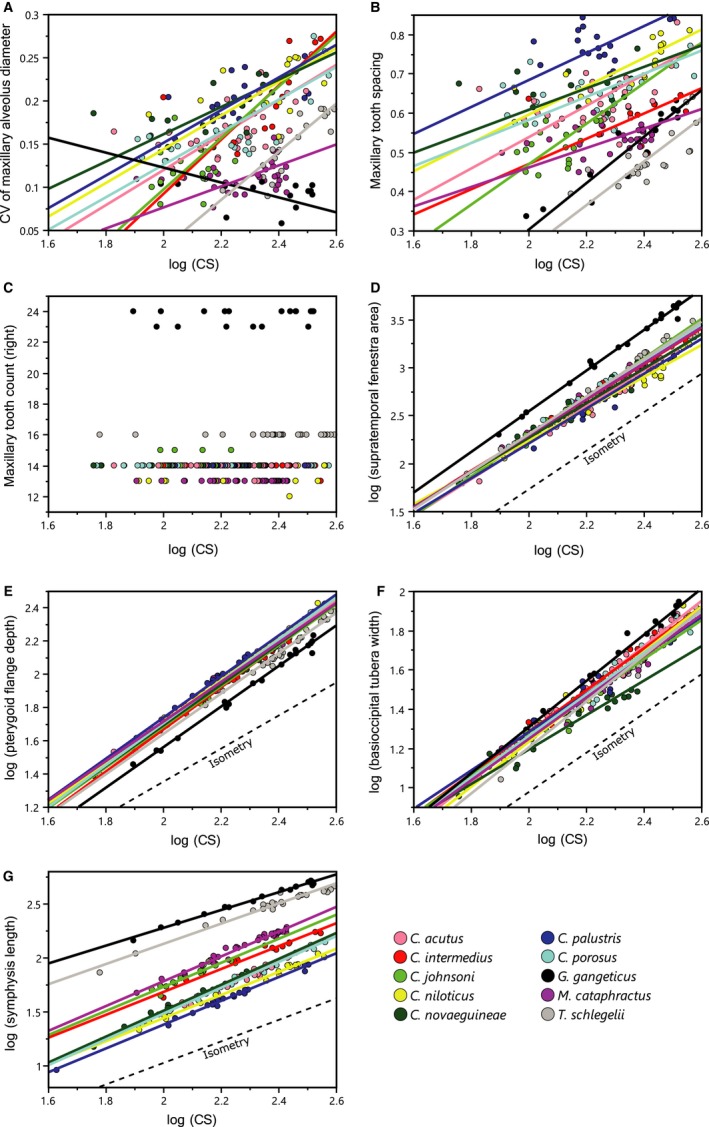
(A–G) Bivariate plots of log(CS) and ecomorphological variables. Reduced major axis (RMA) regression lines are fit on the plots (A,B,D–G).
Table 4.
RMA regressions of ecomorphological variables against size [log(CS)]
| Taxon | n | R 2 | P | Slope | Lower 95% CI | Upper 95% CI | Allometric trend |
|---|---|---|---|---|---|---|---|
| CV of maxillary alveolous diameter | |||||||
| C. acutus | 29 | 0.401 | < 0.001 | 0.203 | NA | NA | NA |
| C. intermedius | 11 | 0.329 | 0.065 | 0.313 | NA | NA | NA |
| C. johnsoni | 17 | 0.082 | 0.264 | 0.296 | NA | NA | NA |
| C. niloticus | 15 | 0.221 | 0.077 | 0.195 | NA | NA | NA |
| C. novaeguineae | 17 | 0.303 | 0.022 | 0.158 | NA | NA | NA |
| C. palustris | 19 | 0.296 | 0.016 | 0.189 | NA | NA | NA |
| C. porosus | 23 | 0.503 | < 0.001 | 0.188 | NA | NA | NA |
| G. gangeticus | 14 | 0.030 | 0.554 | −0.086 | NA | NA | NA |
| M. cataphractus | 31 | 0.079 | 0.127 | 0.122 | NA | NA | NA |
| T. schlegelii | 22 | 0.690 | < 0.001 | 0.279 | NA | NA | NA |
| Maxillary tooth spacing | |||||||
| C. acutus | 29 | 0.198 | 0.015 | 0.399 | NA | NA | NA |
| C. intermedius | 11 | 0.000 | 0.982 | 0.322 | NA | NA | NA |
| C. johnsoni | 17 | 0.078 | 0.279 | 0.524 | NA | NA | NA |
| C. niloticus | 15 | 0.551 | 0.002 | 0.361 | NA | NA | NA |
| C. novaeguineae | 17 | 0.000 | 0.993 | 0.273 | NA | NA | NA |
| C. palustris | 19 | 0.018 | 0.584 | 0.343 | NA | NA | NA |
| C. porosus | 23 | 0.211 | 0.027 | 0.294 | NA | NA | NA |
| G. gangeticus | 14 | 0.875 | < 0.001 | 0.592 | NA | NA | NA |
| M. cataphractus | 31 | 0.103 | 0.079 | 0.248 | NA | NA | NA |
| T. schlegelii | 22 | 0.606 | < 0.001 | 0.557 | NA | NA | NA |
| log(STF area) | |||||||
| C. acutus | 33 | 0.958 | < 0.001 | 1.895 | 1.758 | 2.043 | = |
| C. intermedius | 12 | 0.981 | < 0.001 | 1.852 | 1.682 | 2.039 | = |
| C. johnsoni | 16 | 0.947 | < 0.001 | 2.044 | 1.792 | 2.332 | = |
| C. niloticus | 19 | 0.964 | < 0.001 | 1.659 | 1.506 | 1.828 | − |
| C. novaeguineae | 19 | 0.954 | < 0.001 | 1.799 | 1.613 | 2.007 | = |
| C. palustris | 22 | 0.969 | < 0.001 | 1.816 | 1.673 | 1.971 | − |
| C. porosus | 30 | 0.992 | < 0.001 | 1.916 | 1.850 | 1.984 | − |
| G. gangeticus | 18 | 0.991 | < 0.001 | 2.118 | 2.015 | 2.225 | + |
| M. cataphractus | 34 | 0.978 | < 0.001 | 1.877 | 1.779 | 1.981 | − |
| T. schlegelii | 24 | 0.977 | < 0.001 | 1.955 | 1.828 | 2.090 | = |
| log(PFD) | |||||||
| C. acutus | 31 | 0.993 | < 0.001 | 1.231 | 1.193 | 1.270 | + |
| C. intermedius | 12 | 0.996 | < 0.001 | 1.267 | 1.209 | 1.328 | + |
| C. johnsoni | 17 | 0.977 | < 0.001 | 1.222 | 1.125 | 1.327 | + |
| C. niloticus | 18 | 0.994 | < 0.001 | 1.241 | 1.193 | 1.292 | + |
| C. novaeguineae | 18 | 0.998 | < 0.001 | 1.225 | 1.193 | 1.258 | + |
| C. palustris | 20 | 0.994 | < 0.001 | 1.236 | 1.190 | 1.283 | + |
| C. porosus | 30 | 0.997 | < 0.001 | 1.261 | 1.236 | 1.286 | + |
| G. gangeticus | 18 | 0.992 | < 0.001 | 1.221 | 1.164 | 1.281 | + |
| M. cataphractus | 38 | 0.989 | < 0.001 | 1.191 | 1.149 | 1.234 | + |
| T. schlegelii | 27 | 0.994 | < 0.001 | 1.215 | 1.175 | 1.255 | + |
| log(basioccipital tubera width) | |||||||
| C. acutus | 31 | 0.980 | < 0.001 | 1.151 | 1.091 | 1.215 | + |
| C. intermedius | 12 | 0.962 | < 0.001 | 1.063 | 0.927 | 1.220 | = |
| C. johnsoni | 17 | 0.840 | < 0.001 | 0.990 | 0.796 | 1.231 | = |
| C. niloticus | 18 | 0.973 | < 0.001 | 1.147 | 1.052 | 1.251 | + |
| C. novaeguineae | 19 | 0.950 | < 0.001 | 0.878 | 0.783 | 0.985 | − |
| C. palustris | 20 | 0.920 | < 0.001 | 0.977 | 0.850 | 1.123 | = |
| C. porosus | 30 | 0.978 | < 0.001 | 1.060 | 1.001 | 1.122 | + |
| G. gangeticus | 17 | 0.986 | < 0.001 | 1.176 | 1.102 | 1.256 | + |
| M. cataphractus | 38 | 0.954 | < 0.001 | 1.055 | 0.981 | 1.134 | = |
| T. schlegelii | 27 | 0.982 | < 0.001 | 1.173 | 1.111 | 1.239 | + |
| log(symphysis length) | |||||||
| C. acutus | 30 | 0.985 | < 0.001 | 1.186 | 1.130 | 1.244 | + |
| C. intermedius | 12 | 0.970 | < 0.001 | 1.061 | 0.939 | 1.198 | = |
| C. johnsoni | 17 | 0.897 | < 0.001 | 1.112 | 0.933 | 1.325 | = |
| C. niloticus | 18 | 0.992 | < 0.001 | 1.070 | 1.022 | 1.121 | + |
| C. novaeguineae | 13 | 0.989 | < 0.001 | 1.200 | 1.118 | 1.287 | + |
| C. palustris | 20 | 0.992 | < 0.001 | 1.102 | 1.053 | 1.153 | + |
| C. porosus | 24 | 0.995 | < 0.001 | 1.195 | 1.157 | 1.234 | + |
| G. gangeticus | 17 | 0.991 | < 0.001 | 0.825 | 0.784 | 0.868 | − |
| M. cataphractus | 37 | 0.976 | < 0.001 | 1.149 | 1.089 | 1.212 | + |
| T. schlegelii | 24 | 0.968 | < 0.001 | 0.936 | 0.864 | 1.013 | = |
CV, coefficient of variation; PFD, pterygoid flange depth; STF, supratemporal fenestra.
+, positive allometry; −, negative allometry; =, isometry.
The maxillary tooth count is stable through growth in all sampled taxa (Fig. 6). In nine out of 10 species, maxillary tooth count varies within each species, but it is not significantly correlated with size, as confirmed by Spearman rank correlation (Table 5).
Table 5.
Spearman rank correlation of maxillary tooth count and size [log(CS)]
| Taxon | n (right) | n (left) | Mode | Mean | Deviation from mode (total) | Mode tooth count frequency (%) (total) | Spearman ρ (right) | P (right) | Spearman ρ (left) | P (left) |
|---|---|---|---|---|---|---|---|---|---|---|
| C. acutus | 33 | 33 | 14 | 13.94 | 4 | 93.9 | −0.147 | 0.415 | −0.187 | 0.298 |
| C. intermedius | 12 | 12 | 14 | 13.92 | 2 | 91.7 | −0.194 | 0.545 | NA | NA |
| C. johnsoni | 17 | 17 | 14 | 14.18 | 6 | 82.4 | 0.085 | 0.746 | 0.112 | 0.669 |
| C. niloticus | 19 | 19 | 13 | 13.21 | 10 | 73.7 | 0.358 | 0.133 | 0.589 | 0.008 |
| C. novaeguineae | 19 | 19 | 14 | 14.00 | 0 | 100.0 | NA | NA | NA | NA |
| C. palustris | 20 | 20 | 14 | 13.98 | 1 | 97.5 | NA | NA | −0.099 | 0.677 |
| C. porosus | 30 | 30 | 14 | 13.98 | 1 | 98.3 | −0.225 | 0.231 | NA | NA |
| G. gangeticus | 19 | 19 | 24 | 23.74 | 10 | 73.7 | 0.269 | 0.266 | 0.165 | 0.500 |
| M. cataphractus | 38 | 38 | 13 | 13.01 | 1 | 98.7 | −0.037 | 0.823 | NA | NA |
| T. schlegelii | 27 | 27 | 16 | 15.98 | 1 | 98.1 | NA | NA | −0.302 | 0.126 |
Discussion
Non‐alligatoroid crocodylian trophic ecomorphology and the uniqueness of G. gangeticus
The crocodylian skulls are featured by the high level of character convergence that is thought to reflect trophic convergence (Brochu, 2001; Sadleir & Makovicky, 2008). The non‐phylogenetic and phylogenetic methods in testing the correlations between the proposed trophic ecomorphology and snout shape indicated that with narrowing of the snout, maxillary tooth size disparity increases (lateral maxillary margin more festooned), maxillary teeth less packed, STF enlarged, pterygoid flange becomes shallow and lower jaw symphysis extended. These characters are intercorrelated with each other, and supposedly transformed concurrently in the trophic specialization of non‐alligatoroid crocodylians. Other variables (tooth count and relative basioccipital tubera width) yielded non‐significant correlation with snout shape. However, it is possible that the character transformation of those two variables is stepwise, and increase in tooth count and enlargement of basioccipital tubera occur once snout is narrowed to a certain degree, perhaps as much as G. gangeticus, the most predominant fish‐eater in extant crocodylians. Previous studies demonstrated that skull of G. gangeticus is known to occupy unique morphospace among extant adult crocodylians (Pierce et al. 2008; Sadleir, 2009), and its developmental pathway begins and ends in a distinct region of the morphospace (Piras et al. 2010). Its bite force performance is also different from other species, because G. gangeticus falls outside the 95% confidence interval of the OLS regression of bite force and size (Erickson et al. 2012). The current result is consistent with the previous notion that G. gangeticus is morphologically and functionally atypical. In the whole‐sample OLS regressions of ecomorphological variables against snout shape (RW1 score), G. gangeticus is placed outside of the 95% prediction interval for relative STF area, relative symphysis length and relative PFD among the verified trophic ecomorphology (Fig. 3). These suggest that specialization for true piscivory involves the deviation from the conserved transformation axis of crocodylian skull characters. However, the results of the current analyses should be interpreted carefully. Inclusion of extant alligatoroids and some fossil forms, especially with relatively narrow snouts such as Caiman crocodilus apaporiensis, Orthogenysuchus (Mook, 1924) and Procaimanoidea (Gilmore, 1946) in the analyses may have a significant impact on the results.
Until now, adaptation for feeding on small aquatic prey has been suggested for many taxa of large aquatic reptiles, including thalattosuchian (Langston, 1973; Massare, 1987; Hua, 1994; Young et al. 2010) and tethysuchian (Denton et al. 1997; Sereno et al. 2001; Hastings et al. 2011; Schwarz‐Wings, 2014), crocodyliforms, phytosaurs (Hunt, 1989; Renesto & Paganoni, 1998) and choristoderans (Erickson, 1972; Evans & Hecht, 1993), mostly on the grounds of long and narrow snouts as well as slender and pointed teeth. Establishment of trophic ecomorphology in crocodylians, and a close comparison of above‐listed forms with crocodylians will reveal a degree to which the adaptation to similar ecology is driven by convergence among large aquatic reptiles.
Comparative snout shape ontogeny
Using 2D geometric morphometrics of palate shapes, I demonstrated that the snout widening is a universal trend in non‐alligatoroid crocodylians, regardless of the interspecific snout shape differences. Moreover, growth trajectories of sampled crocodylians are non‐linear, and beyond some size, the snout grows increasingly wider. This is evident when the trajectories of extremely slender‐snouted taxa (G. gangeticus and T. schlegelii), moderately slender‐snouted taxa (e.g. Crocodylus intermedius) and generalist‐type taxa (e.g. C. porosus and C. niloticus) are compared (Fig. 4). Statistical tests supported that the mean snout shape threshold in six species is log(CS) of 2.40, and snout shape is significantly wider beyond the threshold in all six species (Fig. 7). The log(CS) of post‐rostrum landmarks is strongly correlated with log(skull width) for all taxa [n = 236, log(skull width) = 1.185 log(CS) − 0.459, R 2 = 0.993], and the skull width predicted from the mean snout shape threshold [2.40 log(CS)] is 242 mm, with the lowest and highest estimate values of six species range 191–287 mm. In other words, extant non‐alligatoroid crocodylians begin significant snout shape changes when their skull widths are about 242 mm during growth. Meanwhile, larger variance in the snout shape of C. acutus might be derived from greater geographical variation, because C. acutus samples were collected from Florida (USA), Mexico, and Caribbean, Central American and Northern South American countries.
Figure 7.
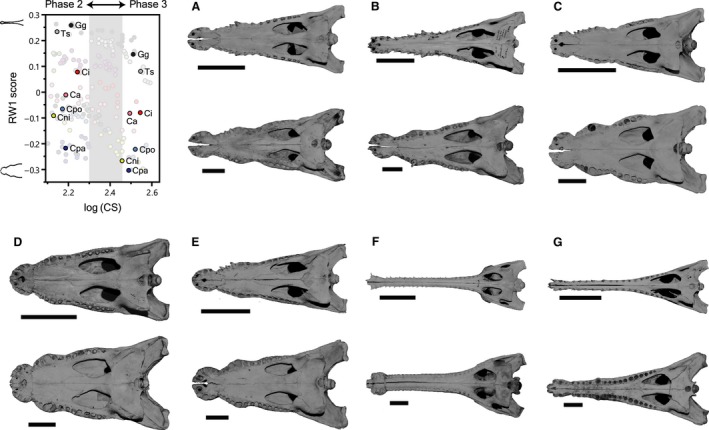
Significant snout shape changes across the skull growth phases II and III. (A) C. acutus (from top: FMNH 20159; FMNH 59070). (B) C. intermedius (from top: FMNH 75659; SMF 28139). (C) C. niloticus (from top: IRSNB 5007; IRSNB 5012). (D) C. palustris (from top: BMNH 1848.2.5.9; BMNH 1868.4.9.11). (E) C. porosus (from top: FMNH 63744; FMNH 14071). (F) G. gangeticus (from top: YPM R10514; ZSM 28.1912). (G) T. schlegelii (from top: BMNH 1899.1.31.1; ZSM 395.1907). Ca, C. acutus; Ci, C. intermedius; Cni, C. niloticus; Cpa, C. palustris; Cpo, C. porosus; Gg, G. gangeticus; Ts, T. schlegelii. All scale bars are 10 cm.
The results are partly congruent with previous studies on comparative skull shape allometry (Piras et al. 2010; Watanabe & Slice, 2014), yet differ in showing a universal trend of snout widening in non‐alligatoroid crocodylians. A non‐linearity of the snout growth in some species of crocodylians was mentioned by Hall & Portier (1994) and Watanabe & Slice (2014). Both studies described U‐shaped trajectories, in which snout narrowing occurs in early stages, followed by snout widening in late stages. Hall & Portier (1994) defined three general growth phases of skulls in C. novaeguineae, each characterized by snout narrowing (phase I: ≤ 60–70 mm skull length), stationary snout shape (phase II: 60–70 mm ≤ skull length ≤ 350 mm) and snout widening (phase III: ≥ 350 mm skull length), respectively. Although the boundaries of the three growth phases are ambiguous, they hypothesized that the phase transitions are concomitant with dietary shifts during ontogeny. This idea was examined in C. niloticus using stable carbon and nitrogen isotope analyses of scute keratin, finding some support for the correlated changes of skull morphometrics and the isotopic ratios (Radloff et al. 2012). The estimated snout shape breakpoint in the current samples (191 mm ≤ skull width ≤ 287 mm) is close to Hall & Portier's (1994) phase II–III boundary (estimated skull width of 188 mm), which corresponds 350 mm skull length in the current C. novaeguineae samples. However, because the pattern of snout shape change seems homogeneous among sampled taxa that display various dietary patterns, distinct growth phases of the skull imply the existence of a shared size‐dependent biomechanical constraint, rather than the morphological response to the dietary shift in non‐alligatoroid crocodylians.
The significant skull shape change in very large size classes of crocodylians has been known (Mook, 1921b; Müller, 1927; Kälin, 1933), and previous studies on extant crocodylians have been concerned with the effect of ontogenetic allometry in species or larger group comparisons of skull shapes (Pierce et al. 2008; Sadleir & Makovicky, 2008; Piras et al. 2009, 2014). However, perhaps more susceptible to allometry are analyses of fossil forms, because most of the species are represented by single individuals, and their growth patterns and maximum sizes are not known. If the size‐related shape change is large and samples from different size classes are used simultaneously without size correction, ecological diversity inferences such as morphological disparity metrics are spuriously calculated, and higher level analyses of disparity integrating time, phylogeny and geography are confounded with various allometric effects. Figure 8 illustrates the effect of size range on the rostrum shape disparity of crocodylians using the empirical dataset, by randomly sampling 10 individuals of each 10 species from a series of size ranges. While the mean variance seems unrelated with size range, 95% CI of variance is more than doubled with the increase in the size range. A significant widening of the 95% CI of snout shape variance occurs where log(CS) > 2.4, which corresponds to the skull growth phase III. Given that most of the skull shape variation is related with rostrum shape in extant crocodylians (Pierce et al. 2008), analysis of skull shape disparity of fossil taxa (Young et al. 2010; Wilberg, 2012; Hastings & Hellmund, 2017) may need to limit the size range of samples.
Figure 8.
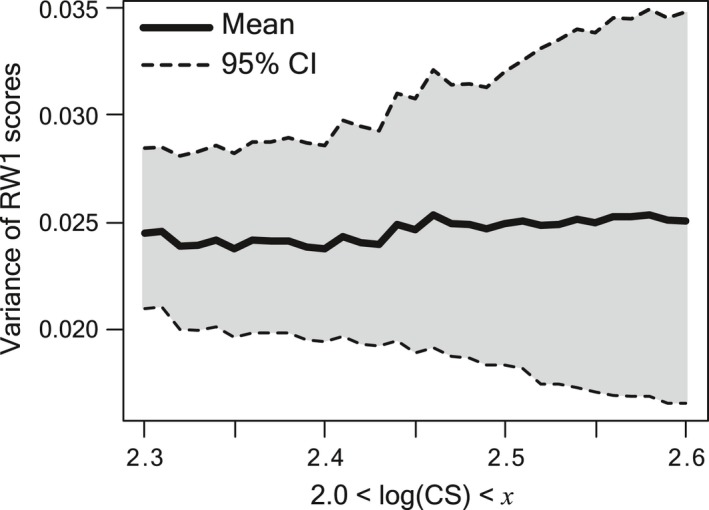
Effect of size ranges on snout shape disparity tested by the empirical dataset. Ten individuals from each 10 species were randomly subsampled (10 000 replicates) without replacement, and the snout shape (RW1 scores) variance of 10 species was calculated for series of size ranges (2.0 ≤ log(CS) ≤ x, 2.30 ≤ x ≤ 2.60), where x sequentially changes by 0.01. The means and 95% confidence intervals of the variance for the series of size ranges were shown by a line chart. The subsampling and variance calculation were performed using the software r.
Allometry of trophic ecomorphology in non‐alligatoroid crocodylians
As indicated by regression slopes of ecomorphological variables against log(CS), the allometric patterns of the variables differ among species, possibly related to trophic specialization during growth. The relative maxillary tooth size variation increases with size in seven species, yet the variation remains smaller throughout life in G. gangeticus, and possibly in C. johnsoni and M. cataphractus (Fig. 6; Table 4). Accordingly, the lateral maxillary margin is more ‘festooned’ in adults of non‐Gavialis taxa, including T. schlegelii (Fig. 9). The increased degree of tooth size disparity in large adults of non‐Gavialis taxa is most likely advantageous for holding larger prey firmly (Iordansky, 1973). On the other hand, G. gangeticus is arguably a predominant fish‐eater, although they occasionally feed on prey other than fish (Whitaker & Basu, 1982; Trutnau & Sommerlad, 2006). Thus, it is intuitive that they maintain near‐homodont dentition through growth. While most of the sampled taxa acquire more heterodont dentition toward adults, interalveolar space of maxillary dentition decreases during growth in six species, in which two longirostrine taxa (G. gangeticus and T. schlegelii) exhibit the most significant trends (Fig. 6; Table 4). These, combined with constant tooth counts through growth, imply that at least some of the maxillary teeth exhibit positive allometric growth relative to the maxillary tooth row length in non‐alligatoroid crocodylians. As expected, a qualitative observation revealed that teeth positioned near the top of the convexities of the maxillary margin grow with positive allometry (Fig. 9). A previous study agrees with this observation by showing positive allometry in the diameter of ‘caniniform’ tooth relative to tooth row length (maxillary tooth #5 in C. porosus and #4 in Alligator mississippiensis), which is positioned at the apex of the ‘first wave’ of maxillary tooth row (Brown et al. 2015).
Figure 9.

Comparative growth of snout shape (right palate) in sampled crocodylians. (A) C. palustris (from top: ZSM 523.1911; ZSM 542.1911; BMNH 1897.12.31.1). (B) C. acutus (from top: USNM 52336; FMNH 11038; USNM 268760). (C) T. schlegelii (from top: ZSM 202.1907; SMF 28135; ZSM 375.1907). (D) G. gangeticus (from top: BMNH 46.1.7.3; ZSM 29.1912; BMNH 1974.3009). All scale bars are 5 cm.
Relative STF area and PFD are associated with the relative mass of jaw adductor muscles. Growth trends for relative STF area are isometric or negatively allometric in most of the sampled taxa, which agree with the STF scaling in A. mississippiensis (Dodson, 1975), but only G. gangeticus exhibits a positive trend (Fig. 6; Table 4). Because enlarged STF area is correlated with the hypertrophied Musculus pseudotemporalis, which helps weak but rapid jaw closure (Iordansky, 1964; Endo et al. 2002) and hence is advantageous for catching agile prey, G. gangeticus might be specialized for fish eating through growth. By contrast, all taxa show a positive allometric trend for relative PFD (Figs 6 and 10; Table 4), as suggested in A. mississippiensis (Dodson, 1975), which indicates that relative mass of Mm. pterygoidei dorsalis et ventralis, strong jaw adductor muscles (Iordansky, 1964), increases through growth in all sampled species. This is consistent with the study of adductor muscle mass and physiological cross‐sectional areas (PCSA) scaling in A. mississippiensis (Gignac & Erickson, 2016). This study demonstrated that the mass and PCSA of both dorsal and ventral pterygoid muscles are positively allometric, in which the ventral one shows greatest relative growth of all adductor muscles. Moreover, experimental studies of bite force scaling among Crocodylia showed that a positive allometry of bite force is conserved among extant crocodylians regardless of phylogenetic relatedness and snout shape (Erickson et al. 2003, 2014). The other aspect of relative ventral extension of pterygoid flange is its correlation with maximal mouth opening (Piras et al. 2014). It could be possible that crocodylians, regardless of their snout shapes, achieve greater maximal mouth opening during growth.
Figure 10.

Comparison of occipital part of skulls in sampled crocodylians. (A) C. palustris (from left: ZSM 523.1911; ZSM 517.1911; BMNH 1897.12.31.1). (B) C. acutus (from left: AMNH 15182; AMNH 43299; USNM 268760). (C) T. schlegelii (from left: ZSM 202.1907; SMF 28135; ZSM 375.1907). (D) G. gangeticus (from left: BMNH 1896.7.7.4; ZSM 29.1912; AMNH 7138). All scale bars are 5 cm.
Growth patterns of relative lower jaw symphysis length show ontogenetic convergence through growth (Fig. 6; Table 4), where patterns of G. gangeticus and T. schlegelii are negatively allometric (95% CI < 1) or almost negatively allometric (upper 95% CI slightly exceeds 1), respectively, whereas that of other species are isometric or positively allometric. Because shorter symphysis incurs lower strain under equivalent loads (Walmsley et al. 2013), slender‐snouted taxa such as G. gangeticus and T. schlegelii might be able to tolerate higher relative loads through growth. Overall, it is conceivable that non‐alligatoroid crocodylian skull characters react differently towards the trophic specialization: some of the characters respond to the change in prey preference during growth, yet other characters are in keeping with the conserved relationship of maximum bite force and body size among crocodylians (Erickson et al. 2012, 2014).
Taxonomic implication
The occlusal pattern has been traditionally used for the classification of two large groups of crocodylians, Alligatoroidea and Crocodyloidea: maxillary teeth of living alligatoroids occlude buccal to dentary teeth, but maxillary and dentary teeth of living crocodyloids interfinger. While fossils suggested the presence of the intermediate character state, and the character transformation history has been understood (Brochu, 1999, 2003), the occlusal pattern is still recognized as a popular identification key for crocodylians in academic and public scenes. The observation of the ontogenetic series of non‐alligatoroid crocodylians revealed that the occlusal pattern is continuous in nature rather than discrete, and is strongly associated with growth stages and snout shape.
As the consequence of increased tooth size disparity and positive allometry of teeth at maxillary margin convexity through ontogeny, the first five maxillary teeth and last five–six teeth are densely packed in large adults of generalist‐type taxa (e.g. C. acutus; C. niloticus; C. palustris). This is also a characteristic of large adults of slender‐snouted taxa, such as C. intermedius and T. schlegelii (Fig. 9). By contrast, in all taxa maxillary teeth #6–8 are widely spaced even in large adults, to occlude with large dentary teeth that are positioned at a convex margin of the dentary. The ‘packed’ anterior and posterior maxillary teeth lead to an unavoidable result: overbite occlusion at anterior and posterior maxilla. This is clear from the observation of live individuals (M. I. personal observation), and occlusal pits occasionally left on palates, that are placed lingual to maxillary alveoli, even in slender‐snouted taxa like T. schegelii (Fig. 11A). Yet still, in‐line occlusion is maintained at the concavity of maxillary margin (maxillary tooth # 6–8). The current study also suggested that degree of heterodonty and spacing of maxillary teeth are strongly correlated with snout shape: in taxa with wider snout, maxillary teeth are more heterodont and more packed. Considering these, it can be supposed that short‐snouted taxa like C. palustris are prone to exhibit ‘semi‐overbite’ (overbite at anterior and posterior maxilla) occlusion, and achieve it in younger age (with smaller size) compared with slender‐snouted taxa. It might also be worthwhile to mention that a few specimens of P. palustris (e.g. BMNH 1848.2.5.9; BMNH 1852.5.9.45; ZSM 565/1911) examined here bear a pit between premaxilla and maxilla for reception of the fourth dentary tooth (Fig. 12), although its lateral wall is not fully formed. Formation of such pit is often associated with overbite occlusion in crocodylians (Brochu, 2003). When fossils are taken into account, the acquisition of ‘semi’ and ‘full’ overbite occlusion of maxillary and dentary teeth are common in stem Crocodylidae (Brochu, 1997, 1999), also occurred repeatedly among Crocodylidae, mainly in large specimens. In subfossil remains of osteolaemine Voay robustus from Madagascar (Mook, 1921a; Brochu, 2007), and the Pleistocene remain of Crocodylus rhombifer from Cuba (Varona, 1984), the interalveolar space of maxillary teeth # 6–8 is much narrower than that in C. palustris. As a result, occlusal pits are placed lingually to maxillary teeth # 6–8, achieving a near full overbite occlusion (Fig. 11B,C), while in smaller individuals of V. robustus, more in‐line occlusion is recognized (Mook, 1921a; Brochu, 2007). Likewise, a various degree of overbite occlusion is observed in mekosuchine crocodiles (Willis et al. 1990; Megirian et al. 1991; Willis, 1993), probably caused by densely packed teeth. Although further studies are necessary to understand the interaction of the occlusal pattern with snout shape, tooth spacing and tooth count, there seem to be a few barriers in the transition of ‘interfingering’ and ‘overbite’ patterns in crocodyloids, mostly in short‐snouted forms.
Figure 11.
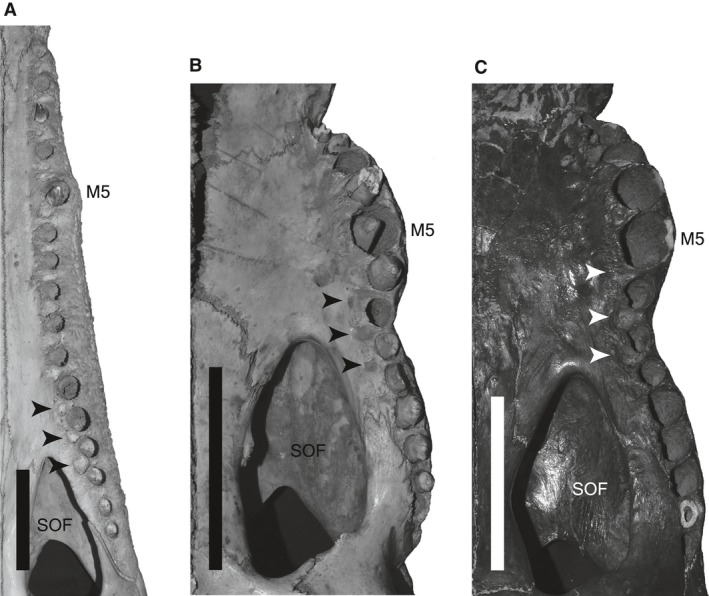
Occlusal pits left on palates. (A) T. schlegelii (BMNH 94.2.21.1). (B) Voay robustus (AMNH 3101). (C) Crocodylus rhombifer (AMNH 6181). Arrows indicate occlusal pits placed medial to maxillary tooth row. M5, maxillary alveolus #5; SOF, suborbital fenestra. All scale bars are 10 cm.
Figure 12.
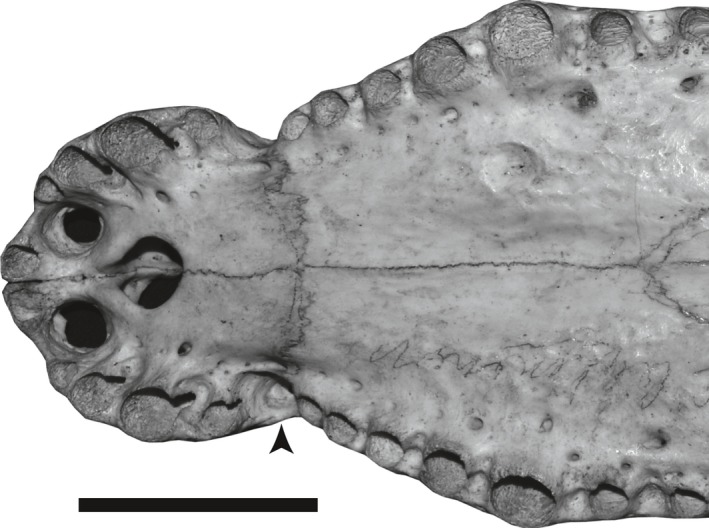
A pit between premaxilla and maxilla for reception of the fourth dentary tooth (C. palustris: BMNH 1852.5.9.45). The arrow indicates the pit. Scale bar: 5 cm.
Taxonomic characters that are related with trophic ecomorphology can be found in other parts of the skull. Associated with the ventral extension of the pterygoid flange is the extent to which basisphenoid exposes below median Eustachian opening. In the sampled taxa, it is longest in C. palustris (Fig. 13), the most wide‐snouted taxon in genus Crocodylus, and shortest in slender‐snouted G. gangeticus and T. schlegelii. Consequently, the posterior pterygoid process is also tallest in C. palustris (Fig. 13), as opposed to the shortest condition in G. gangeticus and T. schlegelii. These characters (chars. #98 and #119 in Brochu, 1999) are highly variable even within the identical character states, and rather correlated with the PFD, and hence with the snout shape. The problem of ecomorphological character correlation has been debated in morphological phylogenetic analyses of crocodylians (Gatesy et al. 2003; Sadleir & Makovicky, 2008). Although improved taxon and character sampling is key for the better phylogenetic reconstruction, and rooting and the choice of outgroups have a great impact on the placement of ingroup taxa (Wilberg, 2015), recognition of ecomorphological characters and their inter‐correlations, and evaluation of such characters in datasets will be of further help in accurately reconstructing the phylogenetic relationships of crocodylians.
Figure 13.
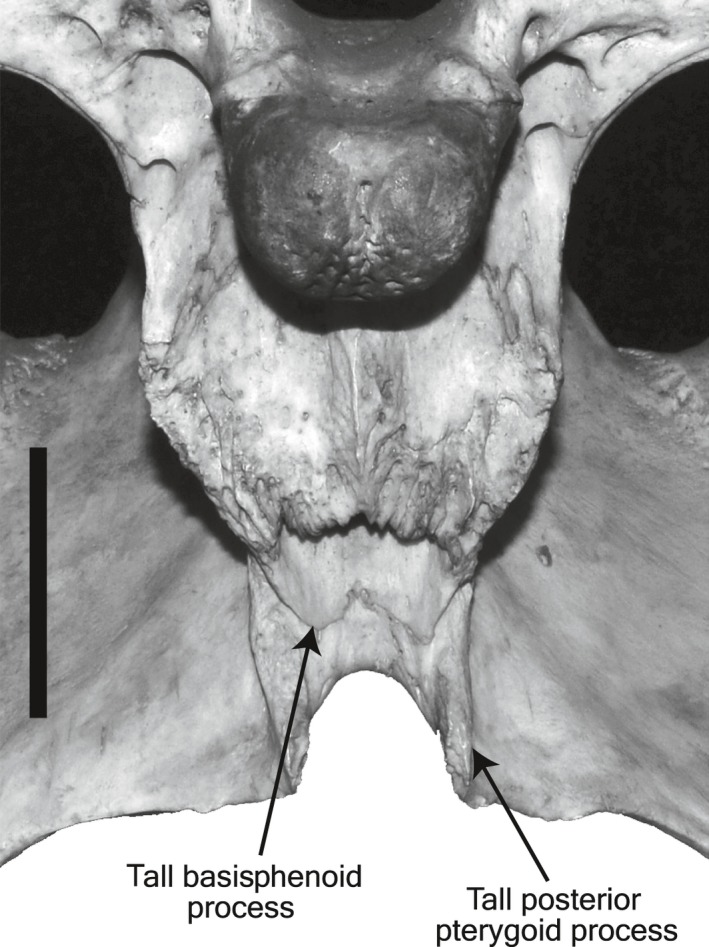
Basioccipital region of the skull in C. palustris (BMNH 1897.12.31.1). Scale bar: 3 cm.
Supporting information
Fig. S1. A time‐calibrated morphological phylogeny of 10 sampled crocodylian species.
Table S1. Specimens, locality information and raw data used in the current study.
Table S2. Measurements of mesiodistal diameters of all right/left maxillary alveoli.
Table S3. Phylogenetic generalized least squares (PGLS) regressions of proposed ecomorphological variables against snout shape (RW1 score), using an alternative (morphological) tree (Fig. S1).
Table S4. Complete results of piecewise linear model fitting for the relationship of snout shape (RW1 score) and size [log(CS)].
Appendix S1. Literature cited.
Acknowledgements
M. I. is grateful to members of Hokkaido Univ. Paleobiology Group for discussion, and M. Mizuta (HU) for the statistical advice. M. I. is thankful to the following people for their assistance during the collection visits: A. Gishlick, D. Dicky and D. Kizirian (AMNH), K. Krysko and M. Nickerson (FLMNH), A. Resetar (FMNH), J. Jacobs, A. Wynn and K. Tighe (USNM), P. Campbell (BMNH), G. Köhler and L. Acker (SMF), E. Weber (ZIT), M.‐O. Rödel and F. Tillack (ZMB), F. Glaw and M. Franzen (ZSM), S. Bruaux (IRSNB), L. Pierre and S. Bailon (MNHN Paris), G. Dally (MAGNT), and P. Couper and A. Amey (QM). This work was supported by International Travel Grant from the Florida Museum of Natural History, Hokkaido University Grant for Research Activities Abroad, Hokkaido University Clark Memorial Foundation, and JSPS Kakenhi Grant Number 15J02626 to M. I.
References
- Arnold SJ (1983) Morphology, performance and fitness. Am Zool 23, 347–361. [Google Scholar]
- Bock WJ (1990) From biologische anatomie to ecomorphology. Netherl J Zool 40, 254–277. [Google Scholar]
- Bona P, Desojo JB (2011) Osteology and cranial musculature of Caiman latirostris (Crocodylia: Alligatoridae). J Morphol 272, 780–795. [DOI] [PubMed] [Google Scholar]
- Bookstein FL (1991) Morphometric Tools for Landmark Data. Cambridge: Cambridge University Press. [Google Scholar]
- Brochu CA (1997) Morphology, fossils, divergence timing, and the phylogenetic relationships of Gavialis . Syst Biol 46, 479–522. [DOI] [PubMed] [Google Scholar]
- Brochu CA (1999) Phylogenetics, taxonomy, and historical biogeography of Alligatoroidea. J Vertebr Paleontol 19, 9–100. [Google Scholar]
- Brochu CA (2000) Phylogenetic relationships and divergence timing of Crocodylus based on morphology and the fossil record. Copeia 3, 657–673. [Google Scholar]
- Brochu CA (2001) Crocodylian snouts in space and time: phylogenetic approaches toward adaptive radiation. Am Zool 41, 564–585. [Google Scholar]
- Brochu CA (2003) Phylogenetic approaches toward crocodylian history. Annu Rev Earth Planet Sci 31, 357–397. [Google Scholar]
- Brochu CA (2007) Morphology, relationships, and biogeographical significance of an extinct horned crocodile (Crocodylia, Crocodylidae) from the Quaternary of Madagascar. Zool J Linn Soc 150, 835–863. [Google Scholar]
- Brochu CA, Storrs GW (2012) A giant crocodile from the Plio‐Pleistocene of Kenya, the phylogenetic relationships of Neogene African crocodylines, and the antiquity of Crocodylus in Africa. J Vertebr Paleontol 32, 587–602. [Google Scholar]
- Brochu CA, Njau J, Blumenschine RJ, et al. (2010) A new horned crocodile from the Plio‐Pleistocene hominid sites at Olduvai Gorge, Tanzania. PLoS One 5, e9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, VanBuren CS, Larson DW, et al. (2015) Tooth counts through growth in diapsid reptiles: implications for interpreting individual and size‐related variation in the fossil record. J Anat 226, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusatte SL, Benton MJ, Ruta M, et al. (2008) Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science 321, 1485–1488. [DOI] [PubMed] [Google Scholar]
- Busbey AB (1995) The structural consequences of skull flattening in crocodilians In: Functional Morphology in Vertebrate Paleontology. (ed. Thomason JJ.), pp. 173–192. Cambridge: Cambridge University Press. [Google Scholar]
- Campione NE, Evans DC (2012) A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. BMC Biol 10. doi:10.1186/1741‐7007‐10‐60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densmore LD (1983) Biochemical and immunological systematics of the order Crocodilia In: Evolutionary Biology. (eds Hechet MK, Wallace B, Prance GH.), pp. 397–465. New York: Plenum. [Google Scholar]
- Densmore LD, White PS (1991) The systematics and evolution of the Crocodilia as suggested by restriction endonuclease analysis of mitochondrial and nuclear ribosomal DNA. Copeia 3, 602–615. [Google Scholar]
- Denton RK, Dobie JL, Parris DC (1997) The marine croocdilian Hyposaurus in North America In: Ancient Marine Reptiles. (eds Callaway JM, Nicholls EL.), pp. 375–397. London: Academic Press. [Google Scholar]
- Dodson P (1975) Functional and ecological significance of relative growth in Alligator . J Zool 175, 315–355. [Google Scholar]
- Endo H, Aoki R, Taru H, et al. (2002) Comparative functional morphology of the masticatory apparatus in the long‐snouted crocodiles. Anat Histol Embryol 31, 206–213. [DOI] [PubMed] [Google Scholar]
- Erickson BR (1972) The lepidosaurian reptile Champsosaurus in North America. Sci Museum Minnesota Monogr 1, 1–91. [Google Scholar]
- Erickson GM, Lappin AK, Vliet KA (2003) The ontogeny of bite‐force performance in American alligator (Alligator mississippiensis). J Zool 260, 317–327. [Google Scholar]
- Erickson GM, Gignac PM, Steppan SJ, et al. (2012) Insights into the ecology and evolutionary success of crocodilians revealed through bite‐force and tooth‐pressure experimentation. PLoS One 7, e31781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson GM, Gignac PM, Lappin AK, et al. (2014) A comparative analysis of ontogenetic bite‐force scaling among Crocodylia. J Zool 292, 48–55. [Google Scholar]
- Evans SE, Hecht MK (1993) A history of an extinct reptilian clade, the Choristodera: longevity, Lazarus‐taxa, and the fossil record. Evol Biol 27, 323–338. [Google Scholar]
- Fernandez‐Blanco MV, Bona P, Olivares AI, et al. (2015) Ontogenetic variation in the skulls of Caiman: the case of Caiman latirostris and Caiman yacare (Alligatoridae, Caimaninae). Herpetol J 25, 65–73. [Google Scholar]
- Foth C, Bona P, Desojo JB (2015) Intraspecific variation in the skull morphology of the black caiman Melanosuchus niger (Alligatoridae, Caimaninae). Acta Zool 96, 1–13. [Google Scholar]
- Garland T Jr, Losos JB (1994) Ecological morphology of locomotor performance in squamate reptiles In: Ecological Morphology. (eds Wainwright PC, Reilly SM.), pp. 240–302. Chicago: The University of Chicago Press. [Google Scholar]
- Gatesy J, Amato G, Norell M, et al. (2003) Combined support for wholesale taxic atavism in gavialine crocodylians. Syst Biol 52, 403–422. [DOI] [PubMed] [Google Scholar]
- Gignac PM, Erickson GM (2015) Ontogenetic changes in dental form and tooth pressures facilitate developmental niche shifts in American alligators. J Zool 295, 132–142. [Google Scholar]
- Gignac PM, Erickson GM (2016) Ontogenetic bite‐force modeling of Alligator mississippiensis: implications for dietary transitions in a large‐bodied vertebrate and the evolution of crocodylian feeding. J Zool 299, 229–238. [Google Scholar]
- Gilmore CW (1946) A new crocodilian from the Eocene of Utah. J Paleontol 20, 62–67. [Google Scholar]
- Gower JC (1975) Generalized Procrustes analysis. Psychometrika 40, 33–51. [Google Scholar]
- Grigg G, Kirshner D (2015) Biology and Evolution of Crocodylians. Clayton South, Vic., Australia: CSIRO Publishing. [Google Scholar]
- Hall PM (1989) Variation in geographic isolates of the New Guinea crocodile (Crocodylus novaeguineae Schmidt) compared with the similar, allopatric, Philippine crocodile (C. mindorensis Schmidt). Copeia 1, 71–80. [Google Scholar]
- Hall PM, Portier KM (1994) Cranial morphometry of New Guinea crocodiles (Crocodylus novaeguineae): ontogenetic variation in relative growth of the skull and an assessment of its utility as a predictor of the sex and size of individuals. Herpetol Monogr 8, 203–225. [Google Scholar]
- Hastings AK, Hellmund M (2017) Evidence for prey preference partitioning in the middle Eocene high‐diversity crocodylian assemblage of the Geiseltal‐Fossillagerstätte, Germany utilizing skull shape analysis. Geol Mag 154, 119–146. doi:10.1017/S0016756815001041 [Google Scholar]
- Hastings AK, Bloch JI, Jaramillo CA (2011) A new longirostrine dyrosaurid (Crocodylomorpha, Mesoeucrocodylia) from the Paleocene of north‐eastern Colombia: biogeographic and behavioural implications for New‐World Dyrosauridae. Palaeontology 54, 1095–1116. [Google Scholar]
- Hekkala E, Shirley MH, Amato G, et al. (2011) An ancient icon reveals new mysteries: mummy DNA resurrects a cryptic species within the Nile crocodile. Mol Ecol 20, 4199–4215. [DOI] [PubMed] [Google Scholar]
- Hua S (1994) Hydrodynamique et modalitée d'allègement chez Metrorhynchus supercilosus (Crocodylia, Thalattosuchia): implications paléologique. Neues Jahrb Geol Palaontol Abhandlungen 193, 1–19. [Google Scholar]
- Hunt AP (1989) Cranial morphology and ecology among phytosaurs In: Dawn of the Age of Dinosaurs in the American Southwest. (eds Lucas SG, Hunt AP.), pp. 349–354. Albuquerque: New Mexico Museum of Natural History. [Google Scholar]
- Iordansky NN (1964) The jaw muscles of the crocodiles and some relating structures of the crocodilian skull. Anat Anz 115, 256–280. [PubMed] [Google Scholar]
- Iordansky NN (1973) The skull of the Crocodilia In: Biology of the Reptilia, Vol. 4 (eds Gans C, Parson TS.), pp. 201–262. New York: Academic Press. [Google Scholar]
- Kälin JA (1933) Beiträge zur vergleichenden Osteologie des Crocodilidenschädels. Zool Jahrbücher Abteilung Anat Ontog der Tiere 57, 535–714. [Google Scholar]
- Kubo T, Benton MJ (2009) Tetrapod postural shift estimated from Permian and Triassic trackways. Palaeontology 52, 1029–1037. [Google Scholar]
- Langston WJ (1973) The crocodilian skull in historical perspective In: Biology of the Reptilia, Vol. 4 (eds Gans C, Parson TS.), pp. 263–289. New York: Academic Press. [Google Scholar]
- Larson DW, Brown CM, Evans DC (2016) Dental disparity and ecological stability in bird‐like dinosaurs prior to the end‐Cretaceous mass extinction. Curr Biol 26, 1325–1333. [DOI] [PubMed] [Google Scholar]
- Massare JA (1987) Tooth morphology and prey preference of Mesozoic marine reptiles. J Vertebr Paleontol 7, 121–137. [Google Scholar]
- McAliley LR, Willis RE, Ray DA, et al. (2006) Are crocodiles really monophyletic? – Evidence for subdivisions from sequence and morphological data. Mol Phylogenet Evol 39, 16–32. [DOI] [PubMed] [Google Scholar]
- McHenry CR, Clausen PD, Daniel WJT, et al. (2006) Biomechanics of the rostrum in crocodilians: a comparative analysis using finite‐element modeling. Anat Rec Part A 288, 827–849. [DOI] [PubMed] [Google Scholar]
- Megirian D, Murray PF, Willis P (1991) A new crocodile of the gavial ecomorph morphology from the Miocene of northern Australia. Beagle Rec North Territ Museum Arts Sci 8, 135–158. [Google Scholar]
- Monteiro LR, Soares M (1997) Allometric analysis of the ontogenetic variation and evolution of the skull in Caiman Spix, 1825 (Crocodylia: Alligatoridae). Herpetologica 53, 62–69. [Google Scholar]
- Monteiro LR, Cavalcanti MJ, Sommer HJS (1997) Comparative ontogenic shape changes in the skull of Caiman species (Crocodylia, Alligatoridae). J Morphol 231, 53–62. [DOI] [PubMed] [Google Scholar]
- Mook CC (1921a) Description of a skull of the extinct Madagascar crocodile, Crocodilus robustus Vaillant and Grandidier. Bull Am Mus Nat Hist 44, 25–33. [Google Scholar]
- Mook CC (1921b) Individual and age variations in the skulls of Recent Crocodilia. Bull Am Mus Nat Hist 44, 51–66. [Google Scholar]
- Mook CC (1924) A new crocodilian from the Wasatch beds. Am Mus Novit 137, 1–4. [Google Scholar]
- Motta PJ, Norton SF, Luczkovich JJ (1995) Perspectives on the ecomorphology of bony fishes. Environ Biol Fishes 44, 11–20. [Google Scholar]
- Müller L (1927) Ergebnisse der Forschungsreisen Prof. E. Stromers in den Wüsten Ägyptens. V. Tertiäre Wirbeltiere: 1. Beiträge zur Kenntnis der Krokodilier des ägyptischen Tertiärs. Abhandlungen der Bayer Akad der Wissenschaften 31, 1–97. [Google Scholar]
- Nesbitt SJ (2011) The early evolution of archosaurs: relationships and the origin of major clades. Bull Am Mus Nat Hist 352, 1–292. [Google Scholar]
- Nestler JH (2012) A geometric morphometric analysis of Crocodylus niloticus: evidence for a cryptic species complex. MSc thesis, Iowa: University of Iowa. [Google Scholar]
- Norell MA (1989) The higher level relationships of the extant Crocodylia. J Herpetol 23, 325–335. [Google Scholar]
- Oaks JR (2011) A time‐calibrated species tree of Crocodylia reveals a recent radiation of the true crocodiles. Evolution 65, 1–13. [DOI] [PubMed] [Google Scholar]
- Okamoto KW, Langerhans RB, Rashid R, et al. (2015) Microevolutionary patterns in the common caiman predict macroevolutionary trends across extant crocodilians. Biol J Linn Soc 116, 834–846. [Google Scholar]
- Orme D, Freckleton R, Thomas G, et al. (2013) caper: Comparative Analyses of Phylogenetics and Evolution in R. Available at: http://cran.r-project.org/package=caper
- Pierce SE, Angielczyk KD, Rayfield EJ (2008) Patterns of morphospace occupation and mechanical performance in extant crocodilian skulls: a combined geometric morphometric and finite element modeling approach. J Morphol 269, 840–864. [DOI] [PubMed] [Google Scholar]
- Piras P, Teresi L, Buscalioni AD, et al. (2009) The shadow of forgotten ancestors differently constrains the fate of Alligatoroidea and Crocodyloidea. Glob Ecol Biogeogr 18, 30–40. [Google Scholar]
- Piras P, Colangelo P, Adams DC, et al. (2010) The Gavialis‐Tomistoma debate: the contribution of skull ontogenetic allometry and growth trajectories to the study of crocodylian relationships. Evol Dev 12, 568–579. [DOI] [PubMed] [Google Scholar]
- Piras P, Buscalioni ÁD, Teresi L, et al. (2014) Morphological integration and functional modularity in the crocodilian skull. Integr Zool 9, 498–516. [DOI] [PubMed] [Google Scholar]
- Poe S (1996) Data set incongruence and the phylogeny of crocodilians. Syst Biol 45, 393–414. [Google Scholar]
- Pooley AC (1989) Food and feeding habits In: Crocodiles and Alligators. (ed. Ross CA.), pp. 76–91. New York: Facts on File. [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation of Statistical Computing. [Google Scholar]
- Radloff FGT, Hobson KA, Leslie AJ (2012) Characterising ontogenetic niche shifts in Nile crocodile using stable isotope (δ13C, δ15N) analyses of scute keratin. Isotopes Environ Health Stud 48, 439–456. [DOI] [PubMed] [Google Scholar]
- Renesto S, Paganoni A (1998) A phytosaur skull from the Norian (Late Triassic) of Lombardy (northern Italy). Riv Ital di Paleontol e Stratigr 104, 115–122. [Google Scholar]
- Ricklefs RE, Miles DB (1994) Ecological and evolutionary inferences from morphology: an ecological perspective In: Ecological Morphology. (eds Wainwright PC, Reilly SM.), pp. 13–41. Chicago: The University of Chicago Press. [Google Scholar]
- Rohlf FJ (2013a) TpsDig Version 2.17. Stony Brook, NY: Department of Ecology and Evolution, State University of New York. [Google Scholar]
- Rohlf FJ (2013b) TpsRelw Version 1.53. Stony Brook, NY: Department of Ecology and Evolution, State University of New York. [Google Scholar]
- Rohlf FJ (2013c) TpsUtil Version 1.58. Stony Brook, NY: Department of Ecology and Evolution, State University of New York. [Google Scholar]
- Rohlf FJ, Slice D (1990) Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool 39, 40–59. [Google Scholar]
- Roos J, Aggarwal RK, Janke A (2007) Extended mitogenomic phylogenetic analyses yield new insight into crocodylian evolution and their survival of the Cretaceous‐Tertiary boundary. Mol Phylogenet Evol 45, 663–673. [DOI] [PubMed] [Google Scholar]
- Sadleir RW (2009) A morphometric study of crocodylian ecomorphology through ontogeny and phylogeny. PhD dissertation, Chicago: The University of Chicago. [Google Scholar]
- Sadleir RW, Makovicky PJ (2008) Cranial shape and correlated characters in crocodilian evolution. J Evol Biol 21, 1578–1596. [DOI] [PubMed] [Google Scholar]
- Schwarz‐Wings D (2014) The feeding apparatus of dyrosaurids (Crocodyliformes). Geol Mag 151, 144–166. [Google Scholar]
- Sereno PC, Beck AL, Dutheil DB, et al. (1998) A long‐snouted predatory dinosaur from Africa and the evolution of spinosaurids. Science 282, 1298–1302. [DOI] [PubMed] [Google Scholar]
- Sereno PC, Larsson HCE, Sidor CA, et al. (2001) The giant crocodyliform Sarcosuchus from the Cretaceous of Africa. Science 294, 1516–1519. [DOI] [PubMed] [Google Scholar]
- Shirley MH, Vliet KA, Carr AN, et al. (2014) Rigorous approaches to species delimitation have significant implications for African crocodilian systematics and conservation. Proc R Soc B 281. doi:10.1098/rspb.2013.2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger D (2012) SiZer: Significant Zero Crossings. Available at: http://cran.r-project.org/package=SiZer.
- Sookias RB, Butler RJ, Benson RBJ (2012) Rise of dinosaurs reveals major body‐size transitions are driven by passive processes of trait evolution. Proc R Soc B 279. doi:10.1098/rspb.2011.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MA (1987) How tetrapods feed in water: a functional analysis by paradigm. Zool J Linn Soc 91, 171–195. [Google Scholar]
- Thorbjarnarson JB (1990) Notes on the feeding behavior of the gharial (Gavialis gangeticus) under semi‐natural conditions. J Herpetol 24, 99–100. [Google Scholar]
- Trutnau L, Sommerlad R (2006) Crocodilians: their Natural History & Captive Husbandry. Frankfurt: Edition Chimaira. [Google Scholar]
- Tsuihiji T (2007) Homologies of the longissimus, iliocostalis, and hypaxial muscles in the anterior presacral region of extant Dapsida. J Morphol 268, 986–1020. [DOI] [PubMed] [Google Scholar]
- Varona LS (1984) Los cocodrilos fosiles de Cuba (Reptilia: Crocodylidae). Caribb J Sci 20, 13–18. [Google Scholar]
- Wainwright PC (1994) Functional morphology as a tool in ecological research In: Ecological Morphology. (eds Wainwright PC, Reilly SM.), pp. 42–59. Chicago: The University of Chicago Press. [Google Scholar]
- Wainwright PC, Reilly SM (1994) Introduction In: Ecological Morphology. (eds Wainwright PC, Reilly SM.), pp. 42–59. Chicago: The University of Chicago Press. [Google Scholar]
- Walmsley CW, Smits PD, Quayle MR, et al. (2013) Why the long face? The mechanics of mandibular symphysis proportions in crocodiles. PLoS One 8, e53873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warton DI, Duursma RA, Falster DS, et al. (2012) SMATR 3 – an R package for estimation and inference about allometric lines. Methods Ecol Evol 3, 257–259. [Google Scholar]
- Watanabe A, Slice DE (2014) The utility of cranial ontogeny for phylogenetic inference: a case study in crocodylians using geometric morphometrics. J Evol Biol 27, 1078–1092. [DOI] [PubMed] [Google Scholar]
- Webb G, Manolis C (1989) Australian Crocodiles: A Natural History. Sydney: Reed Books. [Google Scholar]
- Whitaker R, Basu D (1982) The gharial (Gavialis gangeticus): a review. J Bombay Nat Hist Soc 79, 531–548. [Google Scholar]
- Wilberg EW (2012) Phylogenetic and morphometric assessment of the evolution of the longirostrine crocodylomorphs. PhD dissertation, Iowa: University of Iowa. [Google Scholar]
- Wilberg EW (2015) What's in an outgroup? The impact of outgroup choice on the phylogenetic position of Thalattosuchia (Crocodylomorpha) and the origin of Crocodyliformes. Syst Biol 64, 621–637. [DOI] [PubMed] [Google Scholar]
- Willis P (1993) Trilophosuchus rackhami gen. et sp. nov., a new crocodilian from the early Miocene limestones of Riversleigh, northwestern Queensland. J Vertebr Paleontol 13, 90–98. [Google Scholar]
- Willis P, Murray P, Megirian D (1990) Baru Darrowi gen. et sp. nov., a large broad‐snouted crocodyline (Eusuchia: Crocodylidae) from mid‐Tertiary freshwater limestones in northern Australia. Mem Queensl Museum 29, 521–540. [Google Scholar]
- Young MT, Brusatte SL, Ruta M, et al. (2010) The evolution of Metriorhynchoidea (Mesoeucrocodylia, Thalattosuchia): an integrated approach using geometric morphometrics, analysis of disparity, and biomechanics. Zool J Linn Soc 158, 801–859. [Google Scholar]
- Zanno LE, Makovicky PJ (2011) Herbivorous ecomorphology and specialization patterns in theropod dinosaur evolution. Proc Natl Acad Sci USA 108, 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. A time‐calibrated morphological phylogeny of 10 sampled crocodylian species.
Table S1. Specimens, locality information and raw data used in the current study.
Table S2. Measurements of mesiodistal diameters of all right/left maxillary alveoli.
Table S3. Phylogenetic generalized least squares (PGLS) regressions of proposed ecomorphological variables against snout shape (RW1 score), using an alternative (morphological) tree (Fig. S1).
Table S4. Complete results of piecewise linear model fitting for the relationship of snout shape (RW1 score) and size [log(CS)].
Appendix S1. Literature cited.


