Abstract
The continuous culture of Plasmodium falciparum is often seen as a means to an end, that end being to probe the biology of the parasite in question, and ultimately for many in the malaria drug discovery arena, to identify means of killing the parasite in order to treat malaria. In vitro continuous culture of Plasmodium falciparum is a fundamental requirement when undertaking malaria research where the primary objectives utilise viable parasites of a desired lifecycle stage. This investigation, and resulting data, compared the impact culturing Plasmodium falciparum long term (4 months) in different environmental conditions had on experimental outcomes and thus conclusions. The example presented here focused specifically on the effect culture conditions had on the in vitro tolerance of Plasmodium falciparum to standard anti-malarial drugs, including artemisinin and lumefantrine. Historical data from an independent experiment for 3D7-ALB (5% O2) was also compared with that obtained from this study. We concluded that parasites cultured for several months in media supplemented with a serum substitute such as Albumax II® or within hyperoxic conditions (21% O2), demonstrate highly variable responses to artemisinin and lumefantrine but not all anti-malarial drugs, when compared to those cultured in human serum in combination with Albumax II® under normoxic conditions (5% O2) for the parasite.
Keywords: Malaria, Plasmodium falciparum, Drug tolerance, Intra-erythrocytic, Artemisinin, Lumefantrine, In vitro culture, Transcriptional variation
Graphical abstract

1. Introduction
Forty years ago, Trager and Jensen (1976) first published the continuous culture of Plasmodium falciparum (Pf) in vitro, pushing the door wide open for research into the malaria parasite and subsequently for the testing of compounds for in vitro anti-malarial activity. Many manuscripts cite this reference as the basis of their culturing protocol (6734 citations according to google scholar -6th July 2017) but employ variations in media components, % haematocrit (%H) and parasitaemia (P), plus incubation gas mixtures. The consequences of variations in continuous culturing parameters of Pf in vitro is an under represented area of malaria research, especially with respect to parasite tolerance and sensitivity to anti-malarial drugs and compounds.
We have identified that the sole use of Albumax II®, a lipid rich bovine serum albumin for the culture of Pf laboratory stains, post 2012, can be problematic (Duffy et al., 2016). However, this has not always been the case, with the use of Albumax II® as a total serum replacement previously being common practice within our culturing protocols and those of other researchers (Cranmer et al., 1997, Saliba and Kirk, 1999, Srivastava et al., 2007, Yeh and DeRisi, 2011, Reader et al., 2015). When comparing a serum plus Albumax II® combination media (S/A) with one containing only Albumax II® as a complete serum substitute (ALB), two phenotypic observations were made, one a decreased invasion rate of red blood cells (RBC) and two, an increased in vitro asexual lifecycle duration for those parasites cultured in ALB media (Duffy et al., 2016). As a laboratory specializing in High Throughput Screening (HTS) and profiling of compound activities, we investigated whether changes in the parasite growth phenotype resulted in any alterations in our standard HTS asexual assays (Duffy and Avery, 2012) and found no major differences. However, when performing evaluations of compound activity against highly synchronous age defined parasites (hrs post RBC invasion), certain compounds demonstrated variations in their activity. We postulated that this could be related to the parasite culturing conditions employed at the time of testing over numerous years. With the provisional observation of an extended asexual lifecycle duration for the 3D7 parasite cultured in ALB, (3D7-ALB) in relation to 3D7 parasites cultured in S/A (3D7-S/A) with standard O2 levels of 5%, we considered if other aspects of Pf culturing could also influence parasite growth characteristics. An obvious alternative condition to compare was elevated levels of O2 (21% O2: Hyperoxia) with standard CO2 levels of 5% maintained, conditions a number of publications describe for Pf culture (Van Huyssen and Rieckmann, 1993, Witkowski et al., 2010, Ariey et al., 2014). The gas mixture condition is easily maintainable in a standard tissue culture incubator. We have previously found that 3D7 grows well within a hyperoxic environment (21% O2), although growth of other strains stall after several lifecycles (unpublished observation SD). The 3D7 parasites cultured in Albumax II® and incubated in 21% O2 conditions is referred to as 3D7-ALB-CO2 throughout this manuscript.
To compare the influence of long term continuous culturing conditions on parasite growth characteristics, and parasite tolerance and susceptibility to anti-malarial drugs, we employed optimized culturing and magnetic column isolation methods for obtaining ring stage parasites of 0–1 h (or 0–2 h) post RBC invasion. The influence of culture conditions on asexual lifecycle duration, parasite stage of arrest and sensitivities to five anti-malarial drugs (pyrimethamine, artemisinin, dihydroartemisinin (DHA), chloroquine and lumefantrine) and puromycin throughout the parasite asexual lifecycle are described.
The singular aim of this experiment, performed as one biological replicate in triplicate point, was to compare how alternative long term continuous in vitro culturing conditions for Pf may influence experimental results, specifically in relation to parasite tolerance to anti-malarial drugs throughout their intra-erythrocytic asexual lifecycle. The approach was kept as simple as possible in order to limit other complex parameters, for example, differential kinetics for each compound potentially coming into play when completing wash out evaluations. Maintaining the parasites in a controlled culturing programme and subsequent isolation, preparation and testing within strict time constraints, not only for culture throughout the four months but also within the experimental processing, was considered a variable parameter to be controlled for accurate comparisons to be made. Time was considered at many other levels inclusive of compound preparation, length of time compounds were kept in the water dilution plate (potential solubility or stability effects), the order of assay plate processing (instrument variation such as tip wash number and repeated tip use) and numerous other considerations in order to perform the comparison.
2. Materials and methods
To undertake detailed comparisons of the effect media composition had on asexual lifecycle duration and parasite sensitivities to anti-malarial drugs in vitro, the same stock of 3D7 parasite was maintained in three alternative conditions (3D7-S/A, 3D7-ALB, and 3D7-ALB-CO2) for four months. Each culture was maintained in duplicate throughout the identical culturing programme of daily parasitaemia adjustments by the addition of non-infected RBC to maintain parasitaemia levels between 1 and 6%, and single sorbitol treatments of one of the duplicate cultures at midday for every second intra-erythrocytic lifecycle.
Details of all reagents used in the culturing of Pf within this laboratory are provided in detail elsewhere (Duffy et al., 2016). The base media consisted of RPMI-1640 containing Phenol Red, L-glutamine and sodium bicarbonate, which was further supplemented with 25 mM HEPES buffer and 50 μg/mL hypoxanthine. To prepare the S/A media, 2.5 mg/mL Albumax II® and 5% pooled Human serum was added to the base media. To prepare the ALB Media 5 mg/mL Albumax II® was added to the base media. All complete media was made from the same base media in order to eliminate other media stock effects on the experimental outcome. All media was made fresh each fortnight.
The 3D7-S/A, and 3D7-ALB cultures were incubated at 37 °C in a tri-gas incubator where gas balance was maintained at 5% O2, 5% CO2 and 90% N2. The 3D7-ALB–CO2 culture was maintained in a standard tissue culture incubator at 37 °C in 21% O2, 5% CO2 and 74% N2. Time outside of the incubator was kept to a minimum.
2.1. Age defined parasite synchronization and isolation
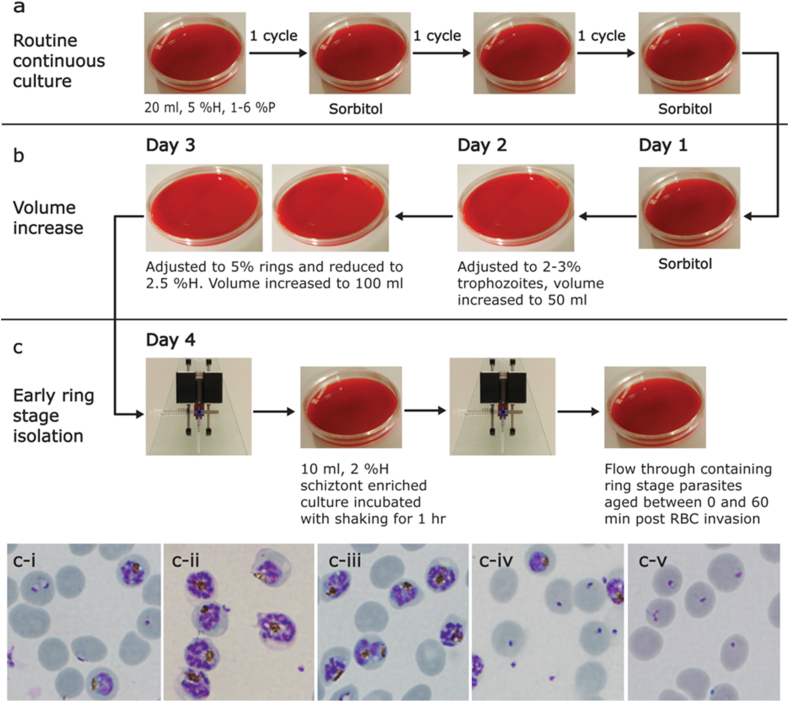
Fig. 1 provides details for the continuous culture and age defined ring stage parasite isolation. All parasite cultures were maintained in duplicate and sorbitol synchronized on alternating days, to enable a single parasite culture to be isolated for each culturing condition on the same day, irrespective of the cell cycle duration of each parasite culture. The 3D7-S/A cultured parasites, due to their short asexual lifecycle duration, would often “switch” from expected ring stage to trophozoite stage after two to three cycles of proliferation. However, the duplicate batches for each culture condition provided the necessary parasite state required each day for the maintenance of the long term culturing programme.
Fig. 1.
Schematic representation of the age defined parasite synchronization and isolation method. (a) Continuous in vitro P. falciparum culture. Twenty millilitres of parasite culture were maintained between 1 and 6%P at 5%H with daily adjustments of parasitaemia and media exchange. A single noon sorbitol treatment (Lambros and Vanderberg, 1979) was performed every second full lifecycle to maintain the cultures in a synchronous cycle of rings one day and trophozoites/schizonts the next. (b) Expansion of parasite culture. Day one consists of a single afternoon sorbitol treatment of 20 ml of predominantly ring stage culture at 5%H. The culture was incubated overnight without shaking in 10 cm petri dishes. Day 2 involved the adjustment of parasitaemia to 2–3% trophozoites and the total culture volume increased to 50 mL in 15 cm petri dishes. On Day 3, ring stage parasitaemia was adjusted to 5% and the haematocrit reduced to 2.5% with an increase in volume to 100 mL. The culture was then incubated overnight in static conditions. (c) Early ring stage parasite isolation. On Day 4, the 100 mL of culture were centrifuged, supernatant removed and the cell pellet suspended in 10 mL of media. The suspended culture was then loaded onto the MAC column, which was washed through with 4 reservoir volumes of media. The column was removed from the magnet and the schizonts, retained on the magnetic column, gently pulled into the syringe and deposited in a 10 cm petri dish. Two hundred μL of non-infected RBC were added to the schizonts and the culture incubated for 60 or 120 min. Addition of non-infected RBC was designated as time 0 for the beginning of the intra-erythrocytic lifecycle. After incubation, the culture was passed once more through the column and the flow through containing the ring stage parasites collected. The ring stage parasites were 0–60 or 0–120 min post erythrocyte invasion. (ci) Culture prior to schizont isolation. (cii) Culture post schizont isolation before erythrocyte addition. (ciii) Schizont enriched culture after RBC addition. (civ) Culture after 1 h incubation prior to isolation showing merozoite release. (cv) Isolated 0–60 min post RBC invasion rings.
2.2. Determination of asexual lifecycle duration
One week prior to undertaking the experimental comparison to evaluate the impact of long term continuous culture conditions on parasite susceptibility and tolerance to anti-malarial compounds, parasite isolations were performed for all three culture conditions within the same working day, obtaining 0–60 min ring stage parasites post RBC invasion. An advantage of the magnetic schizont isolation procedure (Fig. 1) was the ability to remove the majority of non-infected RBC and replace with those stored in the same conditions of 4 °C in RPMI 1640 with no supplements. By processing the parasites in this manner all the newly parasite infected RBC's have an identical prior storage history thus minimizing the influence of culture conditions on the host RBC. To determine the duration of the asexual lifecycle each isolated culture (10 mL) was adjusted to 5%P and 1%H. The culture was dispensed as1.5 mL volumes/well into 12-well tissue culture microtiter plates, and incubated using conditions appropriate for each parasite culture. The media was exchanged after 25 h to minimize any nutritional stress placed on the parasites. At 25 h post ring isolation a single well from each 12-well plate was harvested by aspiration, centrifuged and supernatant removed. Thin blood smears of the remaining culture pellet were made and Giemsa stained. The percent parasitaemia was accurately determined by counting the number of parasitized RBC in a total of more than 1000 erythrocytes to provide the percentage parasitaemia prior to schizont rupture. This process was repeated hourly for all strains from 40 h onwards. The lifecycle duration was determined as the time at which a 50% reduction in the initial schizont number was achieved.
2.3. Parasite tolerance to artemisinin, DHA, puromycin, pyrimethamine and lumefantrine throughout the asexual intra-erythrocytic lifecycle
The assay used to determine compound sensitivity employed high content confocal image analysis of 4′,6-diamidino-2-phenylindole (DAPI) stained parasites (Guiguemde et al., 2010, Duffy and Avery, 2012). Confocal microscopy allows for a thin slice of the multiple layers of cells contained within the assay wells (approximately 33 thousand infected RBC in a total of approximately 1.12 million RBC) to be imaged with minimal interference from fluorescent/quenching compound attributes or excess fluorescent stain. The number of parasites present after proliferation, in comparison to those which are prevented from proliferating by the action of the compounds, is the quantifiable output of this assay. The analysis script is only slightly impacted by mature parasite stages at growth arrest as the script does not distinguish individual merozoites contained within a schizont as multiple separate parasites prior to and including 35 h of age. It does, however, detect approximately 15–20% more parasites than a ring stage parasite population, based solely on more of the parasite population imaged fulfilling the requirements of size and parasite intensity within the total population. Thus, Emax values obtained for drugs such as lumefantrine and pyrimethamine can be suboptimal in comparison to puromycin. Post 35 h of age, a few schizonts may have ruptured and higher levels of parasite numbers may be detected in this instance. Hence the reason why time points greater than 35 h for a parasite with a cell cycle duration of 40 h is the last time point where compound additions are made. The assay is routinely performed for high throughput compound screening purposes (Williamson et al., 2016, Hameed et al., 2014, Avery et al., 2014, Le Manach et al., 2014, Lotharius et al., 2014) with similar conditions described within this experiment (2–3% rings, 0.3%H), apart from the use of highly synchronous parasites and a reduced total assay time of 65 h in this instance compared to the standard 72 h. This high content imaging assay and stage evaluation approach has previously been utlilized in the determination of the stage specificity of the clinical candidate SJ733 (Jiménez-Díaz et al., 2014), where this compound was determined to be ring stage active, with equivalent parasite sensitivity at all ages post RBC invasion.
2.3.1. Compound preparation
All compounds were sourced from Sigma Aldrich Australia. Artemisone, artemether, artesunate, artemisinin and DHA tested in the independent historical experiment were obtained from the Medicines for Malaria Venture validation set. Artemisinin, DHA, chloroquine, puromycin, pyrimethamine and lumefantrine DMSO stock solutions were diluted in dose in DMSO to generate dose response curves (DRC) within a 384-well polypropylene plate, in triplicate point for each dose. (Artemisinin was represented twice within the compound DRC plate and therefore data presented within this study is representative of 6 data points for each condition tested in comparison with 3 data points for the other drugs tested). The DRC stock compounds were diluted 1 μL in 25 μL of water for addition to parasite containing assay plates. A new compound dilution plate was prepared for each time point addition to parasite containing plates.
2.3.2. IC50 shift with parasite age post RBC invasion
A schematic representation of the experimental set-up for the evaluation of parasite tolerance to the anti-malarial drugs is presented in Fig. 2.
Fig. 2.
Schematic diagram for IC50shift experiment for determining parasite tolerance to anti-malarial drugs. Parasite cultures between 0 and 2 h of age were isolated for each parasite culture tested (Fig. 1). Each respective culture was dispensed into 15 × 384-well Perkin Elmer cell carrier imaging plates. All plates were placed within their relevant incubation conditions and at each compound addition time point (identified by a bold red arrow) a single plate for each of the parasite culture conditions was removed from incubation and 5 μl of diluted compound added. The order in which the 5 μl of diluted compounds were added to the parasite culture containing plates was maintained at each time point. The parasite assay plates containing the compounds were placed back into their respective incubation conditions. This was performed for each of the 15 designated time points throughout the parasite asexual intra-erythrocytic life cycle, as indicated by each red arrow up to and including 35 h post RBC invasion. All the plates were then stained with DAPI at the 65 h post RBC invasion time point and imaged on the Opera HTS confocal imaging system. Parasite numbers were analysed as described previously (Duffy and Avery, 2012). The number of parasites were normalized to in-plate control images (5 μM puromycin or 0.4% DMSO) and expressed as a % inhibition of parasite proliferation. The compound concentration was then plotted against % inhibition of parasite proliferation. The data was analysed using nonlinear regression, sigmoidal dose response (variable slope) with no top or bottom constraints analysis. The IC50 for each compound at each compound addition time point throughout the intra-erythrocytic cycle was calculated. The fold change in IC50 values for each compound was calculated by dividing the IC50 obtained at each subsequent time point by that obtained at the first time point of 3 h post RBC invasion. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In addition to preparing the test plates for the parasite tolerance studies, two mL of each 3D7 culture were also simultaneously dispensed into 12-well plates and 5 μM of each compound added to each well containing culture. A concentration of 5 μM was used for each compound to provide a maximal saturable dose of compound suitable for determining, in general, when the compound has its onset of action. It is noted that this concentration is not relevant to that present in vivo and does not take into account in vivo half-lives of compounds such as DHA. Geimsa stained smears of the 12-well plates, containing culture with 5 μM compound added at 3 h post RBC invasion, were made at 37 h post RBC invasion and compared to those from cultures with no compound to enable morphological determination of parasite stage of arrest. Specifically, to determine if parasite growth was arrested at ring stage or whether the parasite continued to develop through to mature trophozoite demonstrating a delayed effect.
2.4. Independent historical data: 3D7-ALB – reduced oxygen conditions
The evaluation utilizing 3D7-ALB parasite under reduced oxygen (5%) at comparable compound addition times was undertaken independently. In this instance, the parasite was cultured for greater than 1 year using the protocol described previously (Duffy and Avery, 2012). Two weeks before compound sensitivity testing was performed, the parasite culture was sorbitol synchronized and parasites isolated as described in section 2.1(Age defined parasite synchronization and isolation). Evaluation of cell cycle duration was not performed in this instance, as cell cycle duration and the influence of culturing conditions was not a concern at this point in time. The data generated from this experiment was analysed as described previously. This data will be referred to as 3D7-ALB-H from here on.
3. Results and discussion
3.1. Evaluation of parasite culture specific asexual lifecycle duration
The 3D7 parasites demonstrated up to 5 h variation in lifecycle duration under the different conditions tested in this comparison: 3D7 S/A: 40 h; 3D7-ALB-CO2: 44 h, and the 3D7-ALB (3D7-ALB-H) culture 45 h (evaluation was made to the closest hour). Briolant et al. (2007) demonstrated the extension of the cell cycle duration under hyperoxic conditions by increasing the duration of schizogeny. The increase in schizogeny was also demonstrated to be parasite strain specific with W2 having a reduced increase in schizogeny extension than 3D7.
3.2. Effect of culture condition on 3 h post RBC invasion IC50 values
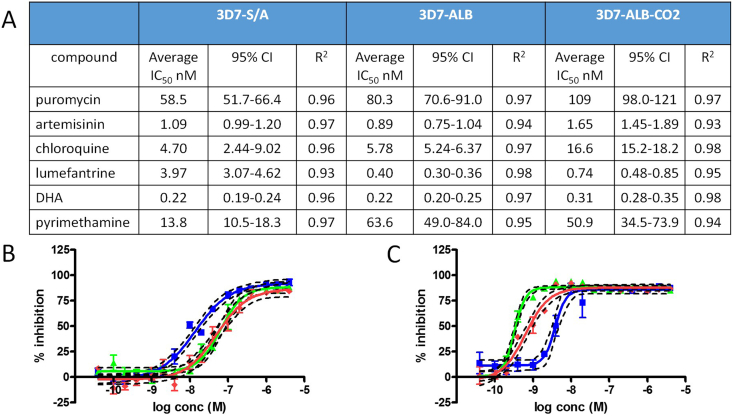
Fig. 3 contains the IC50 values obtained for the compounds tested against 3 h post RBC invasion parasites. Also included are the IC50 graphs for pyrimethamine and lumefantrine.
Fig. 3.
IC50values for the 5 drugs plus puromycin, 16 dose DRC for all three parasite cultures 3 h post RBC invasion. Data is from a single controlled biological replicate performed in triplicate wells for all compounds apart from artemisinin, which was performed twice in triplicate within the same experiment. A. Table of compound average IC50 nM values, 95% confidence Intervals and R2 for all three parasite/culture conditions. B. pyrimethamine IC50 DRC (average IC50 ±SEM with 95%CI), C. lumefantrine IC50 DRC (average IC50 ±SEM with 95%CI). Blue: 3D7-S/A, green: 3D7-ALB, and red: 3D7-ALB-CO2 parasite cultures.
At the first compound addition time point of 3 h post RBC invasion, the various parasite cultures demonstrated differential susceptibilities for pyrimethamine, lumefantrine and to a lesser extent chloroquine. 3D7-ALB and 3D7-ALB-CO2 demonstrated a 3.5–4.5 fold increased tolerance to pyrimethamine in comparison to the 3D7-S/A culture. The 3D7-ALB and 3D7-ALB-CO2 cultures demonstrated a 10 and 5.4 fold increase in sensitivity to lumefantrine in comparison to 3D7-S/A, respectively. These data clearly demonstrated differential effects of parasite culturing and assay conditions on parasite sensitivities to pyrimethamine and lumefantrine. It is possible that lumefantrine bound to serum lipid components which are elevated in Albumax II®, therefore reducing available compound for action, resulting in the difference in activity observed between parasites cultured in S/A and those in ALB medium. However, as the subsequent time points indicate variations between the different 3D7 parasite cultures this effect does not appear to be related to increased tolerance to lumefantrine over time. 3D7-ALB-CO2 demonstrated a very mild increase in tolerance to chloroquine (approximately 3 fold) in comparison to 3D7-S/A but no difference with 3D7-ALB. Briolant et al. (2007) also determined that 3D7 parasite cultured in hyperoxic conditions did not affect the IC50 for chloroquine significantly, although the W2 strain did show some increased activity at 5% O2 in comparison to those at 10 and 21% O2. The data for 5% O2 in the Briolant et al. (2007) study for W2, however, has a large activity range from the three separate experiments (15–168 nM). It is possible that hyperoxia associated effects may be different from strain to strain. All three parasite cultures were equally susceptible to artemisinin, DHA and puromycin in the studies we performed. This data highlights the potential influence of media components and incubation conditions on the outcomes from anti-malarial compound screening when utilizing highly synchronous parasite preparations.
3.3. Effect of media components and incubation conditions on parasite tolerance to artemisinin, DHA, puromycin, pyrimethamine and lumefantrine throughout the asexual intra-erythrocytic lifecycle
Based on the elongation of the cell cycle duration of 3D7 parasites cultured in ALB media or incubated under hyperoxic conditions in comparison to S/A in this study, we hypothesised that the loss of activity for artemisinin previously witnessed from ring stage parasites to trophozoites, would be delayed by 4–5 h if the length of asexual lifecycle duration alone was singularly impacting on the parasites’ tolerance to the drugs. However, the complete opposite was witnessed. Parasites cultured in ALB only or ALB in hyperoxic conditions demonstrated tolerance to artemisinin much earlier after RBC invasion than that for the same parasite grown in S/A and normoxic conditions.
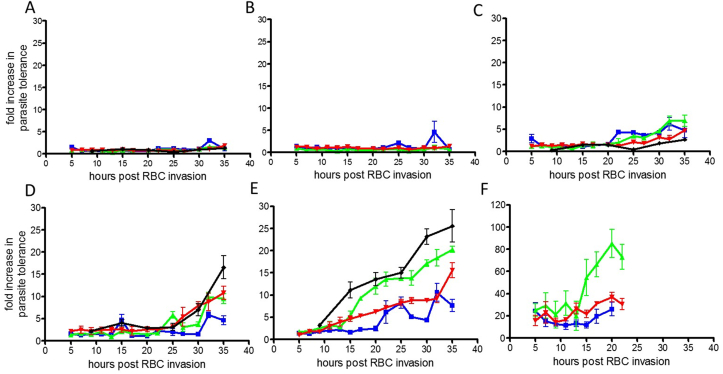
The influence of culturing conditions on the five drugs tested plus puromycin are discussed below. Fig. 4 presents the activity profiles of the five drugs plus puromycin, and comparison with data obtained for 3D7-ALB-H.
Fig. 4.
Compound age of effect. Time post RBC invasion against fold increase in parasite tolerance (fold increase in IC50 ± standard deviation for at least 3 well replicates per dose for chloroquine, pyrimethamine, puromycin, DHA and lumefantrine and 6 for artemisinin (tested twice in triplicate)), per time post RBC invasion. A. puromycin, B. pyrimethamine, C. chloroquine, D. DHA, E. artemisinin, and F. lumefantrine. The green line is data for 3D7-ALB, Blue 3D7-S/A and the red line 3D7-ALB-CO2 (hyperoxic condition). The Black line is data from 3D7-ALB-H cultured parasite for puromycin, chloroquine, DHA and artemisinin in duplicate. The scale for the fold increase in parasite tolerance is 0–30 for all compounds apart from E, lumefantrine which is 0–120 due to substantial parasite tolerance to lumefantrine in comparison to the other 5 compounds. The data for later parasite ages for lumefantrine was not obtainable due to complete loss of compound activity post late trophozoite stage relating to the age of parasite arrest. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Puromycin was demonstrated to arrest parasite growth at young ring stage by Giemsa stained thin blood smear. The three 3D7 parasite cultures demonstrate no alteration in tolerance to puromycin (RNA translation inhibitor) at any age post RBC invasion. This was also confirmed by the independent historical data for 3D7-ALB-H. The compound is fast acting and equally active at all stages of parasite development.
Parasites at 35 h of age, when treated with pyrimethamine, were still equally sensitive at levels comparable to that of parasites treated at 3 h post RBC invasion. However, in contrast to puromycin, when ring stage parasites were incubated with pyrimethamine, to determine the parasite stage of arrest, development of the parasite was seen to progress to mature trophozoite size, but with no daughter merozoites present. The parasite stage of arrest and the consistent IC50 values for 3 h and 35 h indicate a delayed action which does not have an effect until late trophozoite stage. This data aligns with the inhibition of DNA and RNA synthesis by inhibiting dihydrofolate reductase (DHFR) the recognized drug target for pyrimethamine.
Parasite stage of arrest for chloroquine was determined at late ring to young trophozoite (approximately 15–20 h) where hemozoin deposits were just becoming visible on Giemsa stained smears. All three parasite cultures treated with chloroquine, a 4-aminoquinoline which has its effect via haem detoxification, demonstrate a slow increase in tolerance after 20–22 h parasite ages culminating in a 4–6 fold increase in tolerance at 35 h. There was no discernible difference in the action of chloroquine for any of the parasite culturing conditions (inclusive of the independent historical 3D7-ALB-H data) with only slight loss of activity against the more mature trophozoites and schizont forms (4–6 fold post 30 h). This is comparable to another study by Le Manach et al. (2013) where a two-fold difference in activity of 18–20% reduction in growth at 1.6 × IC50 for ring stage parasites was reported in comparison to approximately 40% reduction in growth against shizonts at the same concentration, correlating with the target for chloroquine involving haem detoxification.
Parasite stage of arrest for DHA was observed to be ring stage parasites. None of the three parasite cultures demonstrated any noticeable loss of sensitivity to DHA up to 22 h of age. Post 30 h of age some mild loss of activity (5–15 fold at 35 h) was demonstrated under all of the parasite culture conditions tested. The data for the 3D7-ALB culture from this study and from an independent historical study, 3D7-ALB-H, demonstrate comparable activity up to 27 h with some minor differences post 27–30 h RBC invasion. In general, the parasite tolerance to all three test culture conditions (plus 3D7-ALB-H) was maintained with only minor variations post 30–32 h.
Parasite stage of arrest for artemisinin was also observed to be ring stage. However, the activity profiles obtained for artemisinin were different to those for DHA. All three 3D7 parasite cultures (plus 3D7-ALB-H) were equally susceptible to artemisinin from 3 to 13 h post RBC invasion. After 13 h, susceptibility to artemisinin for all four 3D7 cultures began to diverge. 3D7-ALB and 3D7-ALB-H plus 3D7-ALB-CO2 all demonstrated increased tolerance to artemisinin post 13 h whilst 3D7-S/A did not demonstrate any loss of sensitivity to artemisinin until post 20 h. By 20 h post RBC invasion 3D7-ALB and 3D7-ALB-H demonstrated a 2 fold greater tolerance over 3D7-ALB-CO2 and 4 fold that of 3D7-S/A. Although 3D7-S/A demonstrated a gain in tolerance between 20 and 27 h (maximum 8 fold gain), after 25 h a small resurgence of susceptibility occurred, though not returning to early ring stage levels of susceptibility, which plateaued until post 35 h where an increase in parasite tolerance was once more observed. This pattern for 3D7-S/A is similar to that obtained by Klonis et al., in media consisting of 4% serum and 5 mg/ml Albumax II®, however the onset of parasite tolerance in the study presented here was delayed 12 h in comparison to that observed by Klonis et al. Also in contrast to the data obtained for DHA in this study, Klonis et al. (2013) also observed a similar profile for DHA as for artemisinin, although to a lesser extent. The differences reported between these studies could be due to altered dynamics relating to compound wash out versus continued exposure, in addition to the 3D7 strain used, which apparently has a longer cell cycle duration as experimental time points of 44–48 h were used for their study. The independent historical data for compound sensitivity, and that obtained within this study for the effect of artemisinin on 3D7-ALB cultured parasites clearly demonstrate comparable profiles with gain of parasite tolerance occurring between 10 and 13 h post RBC invasion, reaching a plateau from 20 to 27 h, then gaining further tolerance to artemisinin in the later part of the parasite asexual development.
Dunnett's multiple comparison analysis was performed to determine if parasite culture conditions significantly altered the parasite response to artemisinin at each time post RBC invasion. In comparison to the 3D7-S/A parasite, statistically significant differences (p value 0.0001) were obtained for both 3D7-ALB and 3D7-ALB-CO2 at 17, 20, 27, 30 and 35 h post RBC invasion. This statistical analysis indicates that parasite culture conditions significantly altered sensitivity and tolerance of the same parasite stock to artemisinin drug challenge at several time points throughout the intra-erythrocytic lifecycle.
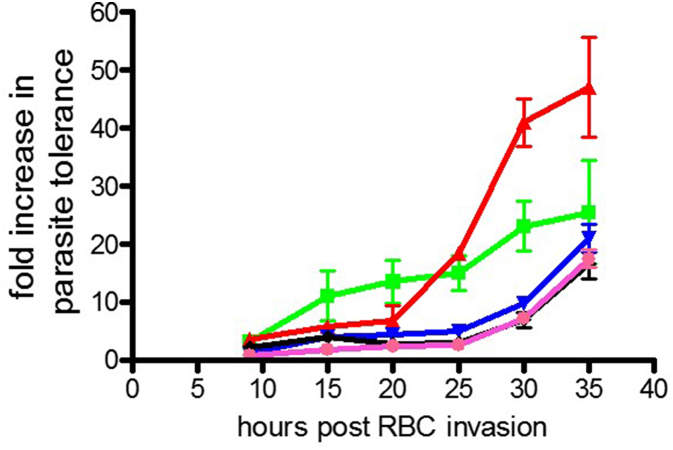
In the historical data, artemether, artesunate, and artemisone were also tested and the fold increase in parasite tolerance presented in Fig. 5.
Fig. 5.
Comparison of parasite tolerance to five artemisinin derivatives. Historical data for 3D7-ALB parasite and 5% O2 incubation conditions. Average fold increase in parasite tolerance is shown (Increase in IC50 value ± standard deviation (duplicate point/well testing for 16 doses)) in relation to parasite age in hrs post RBC invasion. Black, DHA; Pink, artesunate; Blue, artemisone; green, artemisinin; Red, artemether. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
DHA, artesunate and artemisone all displayed the same profile of comparable parasite susceptibility until approximately 25–30 h post RBC invasion, at which point a gradual increase in parasite tolerance comparable to that observed for artemisinin at 35 h was witnessed. This last data point however is the only parasite age where artemisinin has corresponding activity with DHA, artesunate and artemisone as it demonstrates a distinctly different profile at earlier time points. Preliminary data suggested that artemether also exhibits a unique profile to all other artemisinin derivatives tested including artemisinin. Artemether activity was comparable to DHA, artesunate and artemisone until 20 h post RBC invasion. Subsequent to this a fast acquisition of parasite tolerance with a final fold increase in tolerance of 47 at 35 h post RBC invasion was demonstrated. Differences in the stage of effect profile for individual artemisinin derivatives has also been demonstrated by Skinner et al. (1996). Artemether is known to be bound to serum proteins at high levels but it is unlikely in this instance to be the sole reason for the marked loss of activity demonstrated against the parasites at ages post RBC invasion times greater than 20 h.
Lumefantrine parasite stage of arrest was demonstrated to be mature trophozoite. The parasite responses to lumefantrine treatment (potential B-hematin inhibitor, hemazoin) were very extreme in relation to parasite age. With as little as a 2 h difference in parasite age, i.e. from 3 to 5 h post RBC invasion, the parasite tolerance of all three parasite culture conditions had already increased by 10–20 fold. Parasite tolerance continued to increase for all cultures with compound addition to mature rings and trophozoites. The gain in parasite tolerance was demonstrated to be dependent on parasite culture conditions throughout long term continuous culture, with 3D7-ALB and 3D7-ALB-CO2 both demonstrating greater fold increases in parasite tolerance to that of 3D7-S/A. By 20 h post RBC invasion the increase in 3D7-ALB tolerance to lumefantrine was almost 80 fold. At time points post 20–23 h activity was minimal not permitting IC50 values to be obtained. This data demonstrates the requirement for compound contact with the parasite almost immediately after RBC invasion for optimum parasite sensitivity, but the parasite does not stop developing until trophozoite stage. There is therefore only a small window of opportunity for lumefantrine to have its maximal effect within a single asexual cycle of proliferation.
Also of importance is the observation that both 3D7-ALB and 3D7-ALB-CO2, differing only in the level of oxygen available, demonstrate differential tolerance profiles for both artemisinin and lumefantrine in this specific comparison. Increased parasite tolerance to both drugs within normoxic (5%O2) compared to hyperoxic (21% O2) conditions was witnessed. This suggests that both media components and oxygen levels, during the four months of continuous culture may result in alterations of parasite sensitivity and tolerance to anti-malarial compounds, perhaps even counteracting or influencing the effect of one of the other parameters.
These data clearly show differential compound sensitivities and tolerances, for not only the parasites cultured in the various environments, but also for compounds often considered to be interchangeable for applications within experimental design, for example DHA and artemisinin.
Five recent investigations into transcriptional variation within the malaria parasite provide a potential rationale for why long term in vitro continuous culturing conditions may be fundamental to the outcome of experimental investigations; and why they are as relevant as any other parameter within experimental design. Transcriptional variation within the malaria parasite results in functional variation affecting genes involved in a number of processes including lipid metabolism, protein folding, red blood cell remodelling and transcriptional regulation. Rovira-Graells et al. (2012) demonstrated the epigenetic plasticity of the malaria parasite when comparing transcriptional variation in genetically identical 3D7 parasites sourced from two separate laboratories. Heat shock exposure to one of the 3D7 parental lines and two sub clones demonstrated a sub clone specific ability to withstand the heat shock stress over the parent. The 3D7 parental parasite was then demonstrated to be able to adapt to overcome its sensitivity to heat shock. Sub clone specific transcriptional variations resulting in phenotypic differences has also been demonstrated by Kafsack et al. (2014) using the same two 3D7 parental parasites. Gametocyte formation was demonstrated in one 3D7 culture but not for the other, with some sub clones of the gametocyte producing 3D7 parasite able to produce higher numbers of gametocytes than others. This investigation ultimately resulted in the identification of the high correlation of the DNA-binding protein PfAP2-G and gametocytogenesis initiation.
Two other studies by Singh et al. (2007) and Tilly et al. (2014), have specifically investigated the influence of some culture conditions, Albumax II® versus 10% serum, on both transcription (trophozoite and ring stage respectively) and phenotypic alterations including: growth and compound sensitivity (Singh et al., 2007), and cyto-adhesion, knob structure and protein localization (Tilly et al., 2014). Lastly Torrentino-Madamet et al. (2011) also performed transcription analysis of the influence of hyperoxia throughout the asexual intra-erythrocytic life cycle, observing stage specific transcriptional variations dependant on oxygen levels used for in vitro culture. These studies all demonstrate that in vitro environmental conditions can cause altered phenotypes within Plasmodium falciparum.
Within this study our aim was to determine if employing different in vitro culturing conditions for the same parasite stock could manifest in altered parasite tolerance and sensitivity to a range of anti-malarial compounds. We believe that the data presented indicate that long term continuous in vitro culturing conditions do significantly alter parasite tolerance to certain anti-malarial drugs at different stages of their intra-erythrocytic life cycle.
It is tempting to try and extrapolate the relevance of this data in relation to potential mechanisms involved in this alteration of parasite tolerance to selected anti-malarial drugs. Although 3D7 was originally cloned from NF54, prior to starting this culturing programme, single cell cloning of the culture was not performed. However, the comparison used parasites from the same Pf 3D7 nitrogen stock, clearly showing that parasites with an altered sensitivity and tolerance to compounds can be generated from parasites from the same parental stock, when exposed to different long term in vitro culture conditions. In hindsight, clonal selection of the 3D7 strain used at the initiation of the culturing programme would have been beneficial for elucidating if the underlying mechanism for the altered drug sensitivities was by clonal selection or some form of adaptation of the parasite.
The next step is to interrogate the impact of the three culture conditions for 3D7 (3D7-S/A, 3D7-ALB and 3D7-ALB-CO2) on proteomic, genomic, translational, lipidomic and metabolomic profiles, at several specific ages post RBC invasion, in order to determine what changes have occurred for these parasites when cultured under these conditions. Ultimately, we aim to elucidate what the consequences are to the parasite under the variable culturing conditions and the relationship with artemisinin and lumefantrine tolerance variations.
Acknowledgements
The authors wish to thank and acknowledge the Australian Red Cross Blood Bank for the provision of fresh red blood cells, without which this research could not have been performed. Many thanks to Shane Maher for performing and providing technical assistance for the stage of arrest and cell cycle duration experiments. This work was supported by the Australian Research Council (LP120200557 awarded to VMA).
References
- Ariey F., Witkowski B., Amaratunga C., Beghain J., Langlois A.C., Khim N., Kim S., Duru V., Bouchier C., Ma L., Lim P. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery V.M., Bashyam S., Burrows J.N., Duffy S., Papadatos G., Puthukkuti S., Sambandan Y., Singh S., Spangenberg T., Waterson D., Willis P. Screening and hit evaluation of a chemical library against blood-stage Plasmodium falciparum. Malar. J. 2014;13(1):190. doi: 10.1186/1475-2875-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briolant S., Parola P., Fusaï T., Madamet-Torrentino M., Baret E., Mosnier J., Delmont J.P., Parzy D., Minodier P., Rogier C., Pradines B. Influence of oxygen on asexual blood cycle and susceptibility of Plasmodium falciparum to chloroquine: requirement of a standardized in vitro assay. Malar. J. 2007;6(1):44. doi: 10.1186/1475-2875-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranmer S.L., Magowan C., Liang J., Coppel R.L., Cooke B.M. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 1997;91(3):363–365. doi: 10.1016/s0035-9203(97)90110-3. [DOI] [PubMed] [Google Scholar]
- Duffy S., Avery V.M. Development and optimization of a novel 384-well anti-malarial imaging assay validated for high-throughput screening. Am. J. Trop. Med. Hyg. 2012;86(1):84–92. doi: 10.4269/ajtmh.2012.11-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S., Loganathan S., Holleran J.P., Avery V.M. Large-scale production of Plasmodium falciparum gametocytes for malaria drug discovery. Nat. Protoc. 2016;11(5):976–992. doi: 10.1038/nprot.2016.056. [DOI] [PubMed] [Google Scholar]
- Guiguemde W.A., Anang A., Shelat A.A., Bouck D., Duffy S., Crowther G.J., Davis P.H., Smithson D., Connelly M., Clark J., Zhu F., Jiménez-Díaz M.B., Martinez M.S., Wilson E., Tripathi A.K., Gut J., Sharlow E.R., Bathurst I., El Mazouni F., Fowble J.W., Forquer I., McGinley P.L., Castro S., Angulo-Barturen I., Ferrer S., Rosenthal P.L., DeRisi J.L., Sullivan D.J., Jr., Lazo J.S., Roos D.S., Riscoe M.K., Phillips M.A., Rathod P.K., Van Voorhis W.C., Avery V.M., Guy R.K. Chemical genetics of Plasmodium falciparum. Nature. 2010;465(7296):311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed P.S., Chinnapattu M., Shanbag G., Manjrekar P., Koushik K., Raichurkar A., Patil V., Jatheendranath S., Rudrapatna S.S., Barde S.P., Rautela N., Awasthy D., Morayya S., Narayan C., Kavanagh S., Saralaya R., Bharath S., Viswanath P., Mukherjee K., Bandodkar B., Srivastava A., Panduga V., Reddy J., Prabhakar K.R., Sinha A., Jimenez-Diaz M.B., Martinez M.S., Angulo-Barturen I., Ferrer S., Sanz L.M., Gamo F.J., Duffy S., Avery V.M., Magistrado P.A., Lukens A.K., Wirth D.F., Waterson D., Balasubramanian V., Iyer P.S., Narayanan S., Hosagrahara V., Sambandamurthy V.K., Ramachandran S. Aminoazabenzimidazoles, a novel class of orally active antimalarial agents. J. Med. Chem. 2014;57(13):5702–5713. doi: 10.1021/jm500535j. [DOI] [PubMed] [Google Scholar]
- Jiménez-Díaz M.B., Ebert D., Salinas Y., Pradhan A., Lehane A.M., Myrand-Lapierre M.E., O'Loughlin K.G., Shackleford D.M., De Almeida M.J., Carrillo A.K., Clark J.A., Dennis A.S., Diep J., Deng X., Duffy S., Endsley A.N., Fedewa G., Guiguemde W.A., Gomez M.G., Holbrook G., Horst J., Kim C.C., Liu J., Lee M.C., Matheny A., Santos Martinez M., Miller G., Rodriguez-Alejandre A., Sanz L., Sigal M., Spillman N.J., Stein P.D., Wang Z., Zhu F., Waterson D., Knapp S., Shelat A., Avery V.M., Fidock D.A., Gamo F.J., Charman S.A., Mirsalis J.C., Ma H., Ferrer S., Kirk K., Angulo-Barturen I., Kyle D.E., DeRisi J.L., Floyd D.M., Guy R.K. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl. Acad. Sci. 2014;111(50):E5455–E5462. doi: 10.1073/pnas.1414221111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack B.F., Rovira-Graells N., Clark T.G., Bancells C., Crowley V.M., Campino S.G., Williams A.E., Drought L.G., Kwiatkowski D.P., Baker D.A., Cortés A., Llinas M. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507(7491):248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonis N., Xie S.C., McCaw J.M., Crespo-Ortiz M.P., Zaloumis S.G., Simpson J.A., Tilley L. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc. Natl. Acad. Sci. 2013;110(13):5157–5162. doi: 10.1073/pnas.1217452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979:418–420. [PubMed] [Google Scholar]
- Le Manach C., Scheurer C., Sax S., Schleiferböck S., Cabrera D.G., Younis Y., Paquet T., Street L., Smith P., Ding X.C., Waterson D. Fast in vitro methods to determine the speed of action and the stage-specificity of anti-malarials in Plasmodium falciparum. Malar. J. 2013;12(1):424. doi: 10.1186/1475-2875-12-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Manach C., Gonzàlez Cabrera D., Douelle F., Nchinda A.T., Younis Y., Taylor D., Wiesner L., White K.L., Ryan E., March C., Duffy S., Avery V.M., Waterson D., Witty M.J., Wittlin S., Charman S.A., Street L.J., Chibale K. Medicinal chemistry optimization of antiplasmodial imidazopyridazine hits from high throughput screening of a SoftFocus kinase library: part 1. J. Med. Chem. 2014;57(6):2789–2798. doi: 10.1021/jm500098s. [DOI] [PubMed] [Google Scholar]
- Lotharius J., Gamo-Benito F.J., Angulo-Barturen I., Clark J., Connelly M., Ferrer-Bazaga S., Parkinson T., Viswanath P., Bandodkar B., Rautela N., Bharath S., Duffy S., Avery V.M., Möhrle J.J., Guy R.K., Wells T. Repositioning: the fast track to new anti-malarial medicines? Malar. J. 2014;13(1):143. doi: 10.1186/1475-2875-13-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader J., Botha M., Theron A., Lauterbach S.B., Rossouw C., Engelbrecht D., Wepener M., Smit A., Leroy D., Mancama D., Coetzer T.L. Nowhere to hide: interrogating different metabolic parameters of Plasmodium falciparum gametocytes in a transmission blocking drug discovery pipeline towards malariaelimination. Malar. J. 2015;14(1):213. doi: 10.1186/s12936-015-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira-Graells N., Gupta A.P., Planet E., Crowley V.M., Mok S., de Pouplana L.R., Preiser P.R., Bozdech Z., Cortés A. Transcriptional variation in the malaria parasite Plasmodium falciparum. Genome Res. 2012;22(5):925–938. doi: 10.1101/gr.129692.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba K.J., Kirk K. pH regulation in the intracellular malaria parasite, Plasmodium falciparum H+ extrusion via a V-type H+-ATPase. J. Biol. Chem. 1999;274(47):33213–33219. doi: 10.1074/jbc.274.47.33213. [DOI] [PubMed] [Google Scholar]
- Singh K., Agarwal A., Khan S.I., Walker L.A., Tekwani B.L. Growth, drug susceptibility, and gene expression profiling of Plasmodium falciparum cultured in medium supplemented with human serum. J. Biomol. Screen. 2007;12(8):1109–1114. doi: 10.1177/1087057107310638. [DOI] [PubMed] [Google Scholar]
- Skinner T.S., Manning L.S., Johnston W.A., Davis T.M. In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int. J. Parasitol. 1996;26(5):519–525. doi: 10.1016/0020-7519(96)89380-5. [DOI] [PubMed] [Google Scholar]
- Srivastava K., Singh S., Singh P., Puri S.K. In vitro cultivation of Plasmodium falciparum: studies with modified medium supplemented with ALBUMAX II and various animal sera. Exp. Parasitol. 2007;116(2):171–174. doi: 10.1016/j.exppara.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Tilly A.K., Thiede J., Metwally N., Lubiana P., Bachmann A., Roeder T., Rockliffe N., Lorenzen S., Tannich E., Gutsmann T., Bruchhaus I. Type of in vitro cultivation influences cytoadhesion, knob structure, protein localization and transcriptome profile of Plasmodium falciparum. Sci. Rep. 2014;5:16766. doi: 10.1038/srep16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrentino-Madamet M., Alméras L., Desplans J., Le Priol Y., Belghazi M., Pophillat M., Fourquet P., Jammes Y., Parzy D. Global response of Plasmodium falciparum to hyperoxia: a combined transcriptomic and proteomic approach. Malar. J. 2011;10(1):4. doi: 10.1186/1475-2875-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J.B. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Van Huyssen W., Rieckmann K.H. Disposable environmental chamber for assessing the drug susceptibility of malaria parasites. Trop. Med. Parasitol. 1993;44(4):329–330. [PubMed] [Google Scholar]
- Williamson A.E., Ylioja P.M., Robertson M.N., Antonova-Koch Y., Avery V., Baell J.B., Batchu H., Batra S., Burrows J.N., Bhattacharyya S., Calderon F., Charman S.A., Clark J., Crespo B., Dean M., Debbert S.L., Delves M., Dennis A.S., Deroose F., Duffy S., Fletcher S., Giaever G., Hallyburton I., Gamo F.J., Gebbia M., Guy R.K., Hungerford Z., Kirk K., Lafuente-Monasterio M.J., Lee A., Meister S., Nislow C., Overington J.P., Papadatos G., Patiny L., Pham J., Ralph S.A., Ruecker A., Ryan E., Southan C., Srivastava K., Swain C., Tarnowski M.J., Thomson P., Turner P., Wallace I.M., Wells T.N., White K., White L., Willis P., Winzeler E.A., Wittlin S., Todd M.H. Open source drug discovery: highly potent antimalarial compounds derived from the tres cantos arylpyrroles. ACS central Sci. 2016;2(10):687–701. doi: 10.1021/acscentsci.6b00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B., Lelièvre J., Barragán M.J.L., Laurent V., Su X.Z., Berry A., Benoit-Vical F. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob. agents Chemother. 2010;54(5):1872–1877. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E., DeRisi J.L. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 2011;9(8):e1001138. doi: 10.1371/journal.pbio.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]