Abstract
Purpose
Type 2 diabetes mellitus (T2DM) is a multifactorial disease characterized by insulin resistance. As time progresses, monotherapy often does not provide effective glycemic control, generating the need for an add-on therapy. Hence, multiple oral hypoglycemic agents formulated as a single-dose form called fixed-dose combinations (FDCs) play an essential role in glycemic control. The purpose of this systematic review is to appraise the recently published evidence on the safety, efficacy, and bioavailability of FDCs.
Methods
A comprehensive literature search of PUBMED, Scopus, ScienceDirect.com, ProQuest, SpringerLink, clintrials.gov, Embase, and EBSCO using the key words FDCs, combination therapy, T2DM management, and add-on therapy was conducted. Studies on the safety profile/tolerability, efficacy, and bioavailability of various FDCs of oral hypoglycemic agents were preferred.
Findings
The systematic review of all the publications suggests that FDCs of oral hypoglycemic agents (OHAs) significantly reduce HbA1c and fasting plasma glucose values, thereby efficiently reducing hyperglycemia in patients in whom monotherapy fails. FDCs are the bioequivalent of the concomitant drugs administered as individual components. Improved adherence to FDCs and the absence of serious adverse drug reactions compared with dual therapy play an important role in decreasing the incidence of hyperglycemia in patients with T2DM.
Implications
From this updated review, it was found that metformin was the most widely used component of FDCs with other OHAs. Studies on the safety and efficacy of newly approved OHAs such as sodium glucose cotransporter inhibitors were limited. An increasing number of randomized trials on the safety and efficacy of newly emerging FDCs suggests that they would be better treatment options for T2DM patients.
Key words: bioavailability, fixed-dose combinations, glycemic control, hyperglycemia, monotherapy
Highlights
-
•
Comprehensive analysis of current fixed-dose combinations used in the treatment of type 2 diabetes mellitus
-
•
Most widely used component of fixed-dose combinations is metformin with other oral hypoglycemic agents (eg, like glimepiride, pioglitazone, rosiglitazone, acarbose, and sitagliptin.
-
•
Fixed-dose combinations help to reduce hyperglycemia efficiently; the long-term complication of diabetes could be minimized in these patients, thereby improving the quality of life of patients.
Type 2 diabetes mellitus (T2DM) is a multifactorial disease affecting multiple organ systems.1, 2 It is characterized by the resistance of cells to insulin, thereby causing hyperglycemia.3 It is associated with microvascular and macrovascular complications that in the long run can lead to morbidity and mortality.4, 5
Lifestyle modifications and monotherapy with oral hypoglycemic agents are generally considered first-line intervention for glycemic control.5, 6 As the disease progresses, β cells continue to deteriorate in T2DM patients who require effective glycemic control.7 Most often, the efficacy of monotherapy decreases after a few years of treatment, resulting in ineffective glycemic control, and does not prevent the progression of disease, which requires an additional agent for effective glycemic control.8 For the successful management of both insulin resistance and β-cell dysfunction, there arises a need for combination therapy with agents having complementary mechanisms of action formulated in a single-dose form called fixed-dose combinations (FDCs).9 Sulfonylurea with biguanide and biguanide with thiazolidinedione are the most commonly used fixed-dose combinations.1 A list of approved combination products available in the global market is presented in Table I.3, 5, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 The health care professionals should be aware of the role of these products, including their advantages and disadvantages.
Table I.
Available FDCs of various oral hypoglycemic agents.
| FDCs | Available Doses | Mechanism of Action |
|---|---|---|
| Acarbose + metformin5 | 50 mg/500 mg | Acarbose: intestinal carbohydrate digestion is slowed down |
| Metformin: reduces hepatic gluconeogenesis | ||
| Rosiglitazone + metformin10, 11 | 4 mg/2 g | Rosiglitazone: increases insulin sensitivity |
| 4 mg/2 g | ||
| Sitagliptin + metformin12, 13 | 100 mg/1000 mg 100 mg/2000 mg | Sitagliptin: stimulates postprandial insulin and suppresses glucagon secretion |
| Glimepiride + metformin14, 15 | 1 mg/500 mg | Glimepiride: increases insulin secretion from pancreatic β cells |
| 2 mg/500 mg | ||
| Glibenclamide + metformin14 | 5 mg/500 mg | Glibenclamide: increases insulin secretion from pancreatic β cells |
| Glyburide + metformin16, 17 | 2.5 mg/500 mg | Glyburide: increases insulin secretion from pancreatic β cells |
| 5 mg/500 mg | ||
| Vildagliptin + metformin3, 18 | 50 mg/500 mg | Vildagliptin: stimulates postprandial insulin and suppresses glucagon secretion |
| 50 mg/850 mg | ||
| 50 mg/1000 mg | ||
| Pioglitazone + metformin8, 19 | 30 mg/50 mg | Pioglitazone: increases insulin sensitivity |
| Repaglinide + metformin20, 21 | 1 mg/500 mg | Repaglinide: increases insulin secretion |
| 2 mg/500 mg | ||
| Mitiglinide + metformin22 | 10 mg/500 mg | Mitiglinide: increases insulin secretion |
| Empagliflozin + linagliptin23 | 10 mg/5 mg | Empagliflozin: reduces renal glucose reabsorption |
| 25 mg/5 mg | Linagliptin: stimulates postprandial insulin and suppresses glucagon secretion | |
| Glipizide + metformin24 | 2.5 mg/250 mg | Glipizide: increases insulin secretion from pancreatic β cells |
| 2.5 mg/500 mg | ||
| 5 mg/500 mg | ||
| Rosiglitazone + glimepiride25 | 4 mg/1 mg | Rosiglitazone: increases insulin sensitivity |
| 4 mg/2 mg | Glimepiride: increases insulin secretion from pancreatic β cells | |
| 4 mg/4 mg | ||
| 8 mg/2 mg | ||
| 8 mg/4 mg | ||
| Pioglitazone + glimepiride26 | 30 mg/2 mg | Pioglitazone: increases insulin sensitivity |
| 30 mg/4 mg | Glimepiride: increases insulin secretion from pancreatic β cells | |
| Saxagliptin + metformin27 | 5 mg/500 mg | Saxagliptin: stimulates postprandial insulin and suppresses glucagon secretion |
| 2.5 mg/1000 mg | ||
| 5 mg/1000 mg |
FDCs = fixed-dose combinations.
Advantages of FDCs
-
▪
FDCs help in formulating 2 drugs into a single-dose form, thereby minimizing the medication burden to the patient.
-
▪
The relative adherence rates of T2DM patients can be improved.
-
▪
FDCs improve glycemic control, showing better efficacy.5
-
▪
Medical expenditures due to hospitalization can be reduced.28
-
▪
It decreases the frequency of drug administration in patients with T2DM.29
-
▪
It prevents polypharmacy.18
Disadvantages of FDCs
-
▪
Dose titration will be difficult.
-
▪
A patient who is satisfied taking separate medications may not switch to FDCs.
-
▪
There may be an increase in the number of adverse drug reactions (ADRs).28
-
▪
The combination may affect the bioavailability of agents.22
The objective of this review was to analyze the use of FDCs in glycemic control and their efficacy, safety, and bioavailability in patients with T2DM.
Material and Methods
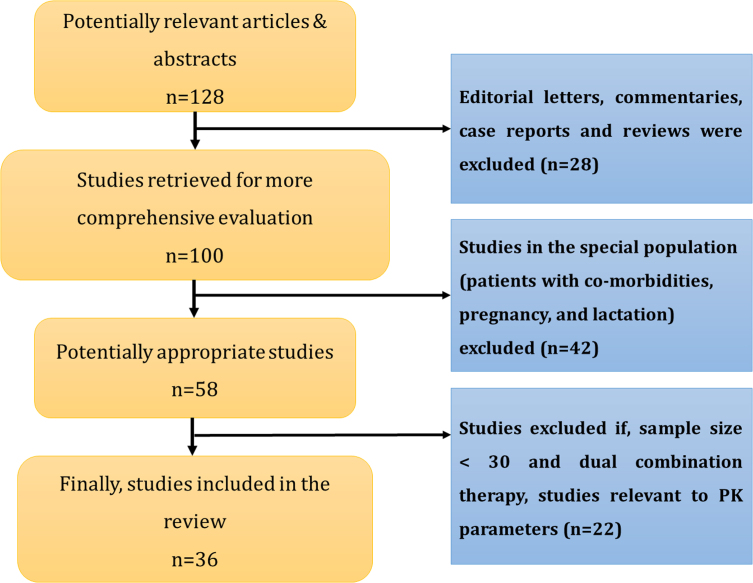
A comprehensive literature search of PUBMED, Scopus, ScienceDirect.com, ProQuest, SpringerLink, clintrials.gov, Embase, and EBSCO using the key words FDCs, combination therapy, T2DM management, and add-on therapy was conducted. The search resulted in the collection of 128 articles. The search was narrowed down to original research articles on FDCs in T2DM. Editorial letters, reviews, case report studies that included <30 patients in the study, and articles related to studies in the special population (patients with comorbidities, pregnancy, and lactation) were excluded. The search was restricted to the articles published in English. The search on FDC therapies was concentrated on their efficacy, safety, tolerability, bioequivalence, adherence, and compliance. Of the 58 appropriate articles collected, 36 were included based on the criteria that the studies were conducted in patients with newly diagnosed T2DM and known cases of T2DM with increased fasting plasma glucose (FPG) levels, increased glycosylated hemoglobin (HbA1c) levels, and increased post-prandial blood sugar levels in the age group of 18 to 80 years. The articles were included irrespective of the sex and race in which the studies were conducted. The various methods used in the studies include open-label, prospective, retrospective, randomized, nonrandomized, double-blind, parallel, placebo-controlled, noninterventional, and crossover studies.
The study characteristics such as author, year of publication, type of study, population size, baseline HbA1c, FPG values, and outcomes such as efficacy and safety of FDCs were noted and checked. The systematic review protocol is represented in Figure 1.
Fig. 1.
Systematic review protocol.
Results and Discussion
The effect of FDCs in the treatment of T2DM was addressed by 9 studies, 2 of which were prospective, 1 was observational, and 7 were randomized, double-blind, parallel studies. The outcomes monitored were HbA1c, FPG, and ADRs. An open-label, prospective, multicenter observational study conducted by Ved et al3 in 2012 on 300 patients with T2DM treated with vildagliptin and metformin FDC showed a highly significant decrease in FBG, postprandial glucose (PPG), and HbA1c values from the baseline at the end of 3 months. The study results showed that FDC of vildagliptin and metformin was effective in reducing the daily dose of insulin in patients with T2DM3 and no data regarding the ADRs was reported.
A large prospective study comprising of 9364 people with T2DM was carried out by Saboo et al30 between the years 2010 and 2012. This open, prospective, multicenter, single-arm, noninterventional study concentrated on the safety and efficacy of acarbose and metformin FDCs. Patients aged older than 18 years of age with T2DM were treated with acarbose (25/50 mg) and metformin (500 mg) FDC for 12 weeks and PPG, HbA1c, fasting blood glucose, and body weight were measured. The study showed that there was a significant decrease in FBG, PPG, HbA1c, and body weight from baseline. The most common ADR was flatulence, and only 5 patients experienced hypoglycemia during the study. The physicians were asked to rate their overall satisfaction as satisfied or not satisfied and tolerability as poor, fair, good, or excellent. Of the patients, 91.8% showed excellent to good tolerance according to the physician’s assessment. In conclusion, this study states that the acarbose and metformin FDC was found to be efficacious, safe, and well tolerated by the patients. However, this study has the limitation of not being placebo controlled or blinded as it was planned for postmarketing surveillance.30
A 24-week observational study performed by Rombopoulos et al18 in 2014 reports about the treatment compliance with the vildagliptin and metformin FDC compared with metformin monotherapy. Of the 659 patients enrolled, medication adherence of 98.9% was found in the FDC group compared with 84.6% in the monotherapy group, but the reduction in HbA1c values was not significant between the groups.18 A randomized, double-blind, parallel study conducted by Wang et al5 in 2013 states that acarbose and metformin significantly reduce HbA1c, FPG, and PPG from baseline (P < 0.0001). Furthermore, this study emphasized the reduction in body weight without a significant risk of hypoglycemia. A 26-week, double-blind, parallel study by Rosenstock et al31 comparing 655 patients with inadequately controlled T2DM treated with the alogliptin and pioglitazone FDC yielded similar reduction in values of HbA1c, FPG, and PPG.
In another randomized, double-blind study carried out by González-Ortiz et al14 comprising of 152 patients divided into 2 treatment arms, in which 1 group was treated with the glimepiride and metformin FDC and other with the glibenclamide and metformin FDC. Greater efficacy in lowering HbA1c and FPG was observed in the glimepiride and metformin group compared with the glibenclamide and metformin group. Compared with the glibenclamide group, the glimepiride group showed a lower incidence of hypoglycemia.
A first randomized, double-blind, phase 3, parallel study was conducted by Lewin et al23 during the years 2011 through 2013 to determine the safety and efficacy of the empagliflozin and linagliptin FDC as initial treatment in patients with T2DM. The study led to a clinically significant reduction in HbA1c in subjects whose baseline values were ≥8.5% compared with the subjects whose baseline values were ≤8.5%. The combination is likely to decrease the weight of the subjects by promoting urinary glucose excretion. None of the subjects included in the study reported confirmed hypoglycemia. The combination was well tolerated, with few patients experiencing a urinary tract infections, genital infections, and hypersensitivity reactions as ADRs of mild to moderate intensity.
A randomized, double-blind, parallel, 16-week multicenter clinical trial was conducted by Chien et al16 in 2007 to evaluate the safety and efficacy of glyburide and metformin. The study reported a significant decrease in HbA1c and FPG values (P<0.0001) in patients treated with FDCs when compared with monotherapy. Of patients in this study, 14.3% reported hypoglycemia. A higher incidence (15.4%) of nervous system side effects such as dizziness and confusion were reported in patients treated with FDCs compared with monotherapy. he study duration was too short to provide information regarding long-term safety. The article states that the combination was well tolerated with improved adherence by simplifying dosage regimen.
In the same year, a 24-week, randomized, double-blind, placebo-controlled study was conducted by Goldstein et al12 to evaluate the effect of the combination therapy of sitagliptin and metformin in patients with T2DM. This study also showed a significant reduction in HbA1c with a lower incidence of hypoglycemia. The patients experienced gastrointestinal ADRs such as abdominal pain, nausea, vomiting, and diarrhea, the incidence of which was similar to the monotherapy group. In conclusion, this combination reduced hyperglycemia significantly with a tolerability profile similar to that of monotherapy with metformin. Another retrospective study conducted by Barner et al6 from 2004 to 2007 states that an FDC of pioglitazone and metformin improved the patient adherence compared with low-dose combination therapy.
A randomized, double-blind study conducted by Derosa et al32 found that patients treated with a rosiglitazone and metformin FDC for 12 months showed a significant reduction in blood pressure and blood sugar levels. Two randomized, open-label studies conducted by Chang et al33 and Migoya et al34 in the years 2012 and 2010 states that FDCs of dapagliflozin and metformin and of sitagliptin and metformin are bioequivalent to the concomitant doses administered as individual components.
A comprehensive systematic review of all the publications suggests that FDCs of oral hypoglycemic agents significantly reduce HbA1c and FPG values, thereby efficiently reducing hyperglycemia in patients who fail to achieve glycemic control with monotherapy. However, there are some limitations for FDCs such as difficulty in dose titration and stability problems between the drugs leading to incompatibilities. Study design, intervention, outcomes, and safety of FDC use in T2DM was shown in Table II,3, 5, 10, 12, 14, 16, 18, 23, 30, 31 and bioavailability of FDCs is shown in Table III.33, 34
Table II.
Studies reporting the use of FDCs in T2DM patients.
| Author | Type of study | Intervention | Outcomes | Safety |
|---|---|---|---|---|
| Ved et al3 (2016) | N = 400, open label, prospective, nonrandomized, multicenter, observational study, 3 months | Vildagliptin (50 mg) + metformin (500, 850, 1000 mg) as FDC | Mean value for FBG, PPG, and HbA1c were significantly reduced after treatment | Not reported in this study |
| Rombopoulos et al18 (2014) | N = 366, multicenter, observational study, 26 weeks | Vildagliptin (50 mg) + metformin (850 mg) as FDC | It resulted in a greater reduction in HbA1c compared with free-dose combination; the patients with FDC were more compliant than with free dose | Not reported in this study |
| Lewin et al23 (2013) | N = 273, phase III, randomized, double- blind, parallel group, 52 weeks | Empagliflozin (25, 10 mg) + linagliptin (5 mg) as FDC | Reduction in HbA1c was significantly greater with FDC compared with individual components | The incidence of ADRs such as UTI, genital infection, were more with empagliflozin 25 mg + linagliptin 10 mg compared with the other compared with the other group but were tolerable with medication |
| Wang et al5 (2012) | N = 233, randomized, double-blind, parallel group, 16 weeks | Acarbose (50 mg) + metformin (500 mg) TDS as FDC | The combination significantly reduced FBS, HbA1c, and PPPG with superior efficacy compared with monotherapy | No hypoglycemia was reported. Mild ADRs such as flatulence and diarrhea were reported in the FDC group |
| Saboo et al30 (2012) | N = 9364, open label, prospective, multicenter, single arm, 12 weeks | Acarbose (25, 50 mg) + metformin (500 mg) as FDC | Significant reductions in body weight, FBG, PPG, HbA1c in the FDC group | Efficacy and tolerability were rated as good and excellent, with no significant risk of hypoglycemia |
| Rosenstock et al31 (2010) | N = 655, double-blind, parallel group, randomized, 26 weeks | Alogliptin (25 mg + pioglitazone (30 mg) QD as FDC | The combination produced greater reductions in HbA1c and FPG than either component monotherapy | The incidence of adverse events was higher compared with monotherapy with alogliptin 25 mg; they were headache, back pain, and UTI. An incidence of mild hypoglycemia was recorded in the FDC group. |
| González-Ortiz et al14 (2008) | N = 152, randomized, double-blind, multicenter, 12 months | Glimepiride (1 g) + metformin (500 mg), 2 tablets QD as FDC | Glimepiride and metformin group showed a greater reduction in FPG, PPBS, and HbA1c compared with glibenclamide and metformin | Mild to moderate hypoglycemia was noted in glimepiride group, which was lower in incidence compared with glibenclamide |
| Goldstein et al12 (2007) | N = 1091, randomized, double-blind, parallel group, 24 weeks | Sitagliptin (50 mg) + metformin (500, 1000 mg) | There was a significant reduction in HbA1c and FPG | The incidence of hypoglycemia and gastrointestinal side effects was higher in the high-dose metformin group. Treatment was generally well tolerated. |
| BID as FDC | ||||
| Chien et al16 (2007) | N = 100, multicenter, randomized, double-blind, parallel group, 16 weeks | Glyburide (2.5, 5 mg) + metformin (500 mg) as FDC | FDC had a greater reduction in FPG, HbA1c compared with monotherapy. The FDC also improved adherence in patients. | The combination was efficacious and well tolerated, and the incidence of gastrointestinal ADRs was lower compared with monotherapy. |
| Bailey et al10 (2005) | N = 568, 24 weeks, multicenter, randomized, double blind, parallel group study | Rosiglitazone 4 and 8 mg; metformin 2 g increased to 3 g at the time of treatment | The FDC showed a significant improvement in HbA1c, FPG values compared with patients treated with a high dose of metformin, ie, 3 g/d | It was well tolerated with a lower incidence of diarrhea, abdominal pain compared with the metformin group. |
ADRs = adverse drug reactions; FBG = fasting blood glucose; FBS = fasting blood sugar; FDC, fixed-dose combination; PPBS = post prandial blood sugar; PPG, postprandial glucose; TDS = three times a day; UTI, urinary tract infection.
Table III.
Bioavailability for FDC Combinations of T2DM.
| Author | Study Design | Intervention | Outcome |
|---|---|---|---|
| Chang et al33 (2015) | N = 72, open-label, randomized, 4-arm crossover study | Dapagliflozin + metformin | The FDC of dapagliflozin and metformin was bioequivalent to individual components both in fed and fasted states. |
| |||
| Migoya et al34 (2010) | N = 48, randomized, open-label, 2-period, crossover study | Sitagliptin + metformin | The FDC combination showed significant reduction in HbA1c and was bioequivalent to individual tablets administered concomitantly in some doses. |
|
FDC = fixed-dose combination; T2DM, type 2 diabetes mellitus.
Summary
The present systematic review of FDCs of various oral hypoglycemic agents suggests that these are beneficial to patients with T2DM in order to achieve their target glycemic levels by effectively controlling hyperglycemia. The review also suggests that the most widely used component of FDCs is metformin with other OHAs such as glimepiride, pioglitazone, rosiglitazone, acarbose, and sitagliptin. Studies on FDCs without metformin as one of the components were found to be fewer in number.
The pharmacokinetic studies on FDCs suggest that these drugs are bioequivalent to the individual components that are coadministered in the same doses, which in turn facilitates the formulation of a single-dose form, thereby reducing the economic burden on patients and increasing patient medication adherence. As FDCs help to reduce hyperglycemia efficiently, the long-term complications of diabetes can be minimized in these patients and thus improve the quality of life of these patients. A search restricted to English-language articles is a limitation of this review.
In conclusion, the favorable effects of FDCs and lack of increased incidence of adverse effects could play an important role in decreasing the increasing global incidence of hyperglycemia due to T2DM compared with dual therapy with individual components.
Conflicts Of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
The study was designed and interpretation was carried out by Dr. Thangavel Mahalingam Vijayakumar. Jayasutha Jayram performed data collection and figure creation. Dr. Thangavel Mahalingam Vijayakumar and Jayasutha Jayram initiated writing followed by Vishnu Meghana Cheekireddy, Dasari Himaja, and Yalamanchili Dharma Teja. Dr. Damodharan Narayanasamy conducted the literature search and framed the manuscript. We thank Dr. K.S. Lakshmi, Dean, SRM College of Pharmacy, SRM University, for providing facilities.
References
- 1.Jung S.H., Chae J.W., Song B.J., Kwon K.I. Bioequivalence comparison of Two Formulations of Fixed-Dose Combination Glimepiride/metformin (2/500 mg) Tablets in Healthy Volunteers. IJPR. 2014;13:365–371. [PMC free article] [PubMed] [Google Scholar]
- 2.Park S.I., Lee H., Oh J., Lim K.S., Jang I.J., Kim J.A., Jung J.H., Yu K.S. A fixed-dose combination tablet of gemigliptin and metformin sustained release has comparable pharmacodynamic, pharmacokinetic, and tolerability profiles to separate tablets in healthy subjects. Drug Des. Dev. Ther. 2015;9:729–736. doi: 10.2147/DDDT.S75980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ved P., Shah S. Evaluation of vildagliptin and fixed dose combination of vildagliptin and metformin on glycemic control and insulin dose over 3 months in patients with type 2 diabetes mellitus. IJEM. 2012;16:S110–S113. doi: 10.4103/2230-8210.94258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valensi P., de Pouvourville G., Bernard N., Chanut-Vogel C., Kempf C., Eymard E., Moisan C., Dallongeville J. Treatment maintenance duration of dual therapy with metformin and Sitagliptin in type 2 diabetes: The ODYSEE observational study. Diabetes Metab J. 2015;41:231–238. doi: 10.1016/j.diabet.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Wang J.S., Huang C.N., Hung Y.J., Kwok C.F., Sun J.H., Pei D., Yang C.Y., Chen C.C., Lin C.L., Sheu W.H. Acarbose plus metformin fixed-dose combination outperforms acarbosemonotherapy for type 2 diabetes. Diabetes Res ClinPract. 2013;102:16–24. doi: 10.1016/j.diabres.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Barner J.C. Adherence to Oral Antidiabetic Agents with Pioglitazone and metformin: Comparison of Fixed-Dose Combination Therapy with Monotherapy and Loose-Dose Combination Therapy. Clin Ther. 2011;33:1281–1288. doi: 10.1016/j.clinthera.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Boulton D.W., Chang M., Griffen S.C., Kitaura C., Lubin S., Pollack A., LaCreta F. Fed and Fasted Single-dose Assessment of Bioequivalence of Dapagliflozin and Metformin Extended-release Fixed-dose Combination Tablets Relative to Single-component Dapagliflozin and Metformin Extended-release Tablets in Healthy Subjects. Clin. Ther. 2016;38:99–109. doi: 10.1016/j.clinthera.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Hiroi S., Matsuno K., Hirayama M., Hayakawa T., Yoshioka N., Kawakami K. Evaluation of the bioequivalence of a fixed dose combination tablet of pioglitazone-metformin versus commercial tablets in healthy Japanese male volunteers. Diabetes Manag. 2012;2:13–20. [Google Scholar]
- 9.Vanderpoel D.R., Hussein M.A., Watson-Heidari T., Perry A. Adherence to a Fixed-Dose Combination of Rosiglitazone Maleate/Metformin Hydrochloride in Subjects with Type 2 Diabetes Mellitus: A Retrospective Database Analysis. Clin.Ther. 2004;26:2066–2075. doi: 10.1016/j.clinthera.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Bailey C.J., Bagdonas A., Rubes J., McMorn S.O., Donaldson J., Biswas N., Stewart M.W. Rosiglitazone/Metformin Fixed-Dose Combination Compared with Uptitrated Metformin Alone in Type 2 Diabetes mellitus: A 24-week, Multicenter, Randomized, Double-Blind, Parallel-Group Study. Clin. Ther. 2005;27:1548–1561. doi: 10.1016/j.clinthera.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Avandamet® (rosiglitazone and metformin hydrochloride). Full prescribing information, GlaxoSmithKline, Research Triangle Park, NC.2008. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Avandamet/pdf/AVANDAMET-PI-MG.PDF. Accessed March 28, 2016.
- 12.Goldstein B.J., Johnson J., Feinglos M.N., Williams-Herman D.E., Lunceford J.K. Effect of Initial Combination Therapy with Sitagliptin, a Dipeptidyl Peptidase-4 Inhibitor, and Metformin on Glycemic Control in Patients with Type 2 Diabetes. Diabetes Care. 2007;30:1979–1987. doi: 10.2337/dc07-0627. [DOI] [PubMed] [Google Scholar]
- 13.JANUMET® (sitagliptin and metformin hydrochloride). Full prescribing information, Merck and Co, Whitehouse Station, NJ.2010. Available at: http://www.merck.com/product/usa/pi_circulars/j/janumet/janumet_pi.pdf. Accessed March 28, 2016.
- 14.González-Ortiz M., Guerrero-Romero J.F., Violante-Ortiz R., Wacher-Rodarte N., Martínez-Abundis E., Aguilar-Salinas C., Islas-Andrade S., Arechavaleta-Granell R., González-Canudas J., RodríGuez-Morán M., Zavala-Suárez E., Ramos-Zavala M.G., Metha R., Revilla-Monsalve C., Beltrán-Jaramillo T.J. Efficacy of glimepiride/metformin combination versus glibenclamide/ metformin in patients with uncontrolled type 2 diabetes mellitus. J. Diabetes Complicat. 2009;23:376–379. doi: 10.1016/j.jdiacomp.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Shin K.H., Kim S.E., Yoon S.H., Cho Jy, Jang I.J., Shin S.G., Yu K.S. Pharmacokinetic Comparison of a New Sustained-Release Formulation of Glimepiride/Metformin 1/500 mg Combination Tablet and a Sustained-release Formulation of Glimepiride/Metformin 2/500 mg Combination Tablet in Healthy Korean Male Volunteers: A Randomised, 2-Sequence, 2-Period, 2-Tretament Crossover Study. Clin. Ther. 2011;33:1809–1818. doi: 10.1016/j.clinthera.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Chien H.H., Chang C.T., Chu N.F., Hsich S.H., Huang Y.Y., Lee I.T., Lee W.J., Tang Y.J., Sheu W.H. Effect of Glyburide-Metformin Combination Tablet in Patients with Type 2 Diabetes. Chin Med Assoc. 2007;70:473–480. doi: 10.1016/S1726-4901(08)70044-3. [DOI] [PubMed] [Google Scholar]
- 17.GLUCOVANCE® (glyburide and metformin hydrochloride). Full prescribing information, Bristol-Myers Squibb, Princeton, Nj.2010. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021178s012lbl.pdf.
- 18.Rombopoulos G., Hatzikou M., Athanasiadis A., Elisaf M. Treatment Compliance with Fixed-Dose Combination of Vildagliptin/Metformin in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Metformin Monotherapy: A 24-Week Observational Study. Int J Endocrinol. 2015;2015:1–15. doi: 10.1155/2015/251485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ACTOPLUS MET® (pioglitazone hydrochloride and metformin hydrochloride) and ACTOPLUS MET XR® (pioglitazone hydrochloride and metformin hydrochloride extended-release). Full prescribing information, Takeda Pharmaceuticals America, Inc., Deerfield, IL, 2010. Available at: http://general.takedapharm.com/content/file.aspx?filetypecode=actoplusmetpi&cacheRandomizer=287cd1f9-1759-4901-a1d6-ac9f8d40db4e; http://general.takedapharm.com/content/file.aspx?filetypecode=ACTOPLUSMETXRPI&cacheRandomizer=8a7806a1-d545-425e-a478-3e3d17f50d6a. Accessed March 28, 2016.
- 20.Hoelscher D., Chu P.L., Lyness W. Fixed Dose Combination Tablet of Repaglinide and Metformin is Bioequivalent to Concomitantly Administered Individual Tablets of Repaglinide and Metformin. Clin Drug Invest. 2008;28:573–582. doi: 10.2165/00044011-200828090-00004. [DOI] [PubMed] [Google Scholar]
- 21.PRANDIMET® (repaglinide and metformin hydrochloride). Full prescribing information, Novo Nordisk, Princeton, NJ.2010. Available at: http://www.novo-pi.com/prandimet.pdf. Accessed March 28, 2016.
- 22.Jung J.A., Kim J.R., Kim S.R., Kim T.E., Lee S.Y., Ko J.W., Huh W. Pharmacokinetics of a Fixed-Dose Combination of Mitiglinide and Metformin versus Concurrent Administration of Individual Formulations in Healthy Subjects. Clin Drug Investig. 2012;32:799–804. doi: 10.1007/s40261-012-0012-6. [DOI] [PubMed] [Google Scholar]
- 23.Lewin A., DeFronzo R.A., Patel S., Liu D., Kaste R., Woerle H.J., Broedi U.C. Initial Combination of Empagliflozin and Linagliptin in Subjects with Type 2 Diabetes. Diabetes Care. 2015;38:394–402. doi: 10.2337/dc14-2365. [DOI] [PubMed] [Google Scholar]
- 24.METAGLIP® (glipizide and metformin hydrochloride). Full prescribing information, Bristol-Myers Squibb, Princeton, NJ.2009. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021460s007lbl.pdf. Accessed March 28, 2016.
- 25.AVANDARYL® (rosiglitazone and glimepiride). Full prescribing information, GlaxoSmithKline, Research Triangle Park, NC.2009. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Avandaryl/pdf/AVANDARYL-PI-MG.PDF. Accessed March 28, 2016.
- 26.DUETACT® (pioglitazone and glimepiride). Full prescribing information, Takeda Pharmaceuticals America, Deerfield, IL.2010. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021925s010s011lbl.pdf. Accessed March 28, 2016.
- 27.KOMBIGLYZE XR® (saxagliptin and extended-release metformin hydrochloride). Full prescribing information, Bristol-Myers Squibb, Princeton, NJ.2010. Available at: http://www.azpicentral.com/kombiglyze-xr/pi_kombiglyze_xr.pdf. Accessed March 28, 2016.
- 28.Balu S., Simko R.J., Quimbo R.M., Cziraky M.J. Impact of fixed-dose and multi-pill combination dyslipidemia therapies on medication adherence and the economic burden of sub-optimal adherence. Curr Med Res Opin. 2009;25:2765–2775. doi: 10.1185/03007990903297741. [DOI] [PubMed] [Google Scholar]
- 29.Gu N., Kim B.H., Rhim H.Y., Chung J.Y., Kim J.R., Shin H.S., Yoon S.H., Cho J.Y., Shin S.G., Jang I.J., Yu K.S. Comparison of the Bioavailability and Tolerability of Fixed-Dose Combination Glimepiride/Metformin 2/500-mg Tablets Versus Separate Tablets: A Single-Dose, Randomized-Sequence, Open-Label, Two-Period Crossover Study in Healthy Korean Volunteers. Clin Ther. 2010;32:1408–1418. doi: 10.1016/j.clinthera.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Saboo Banshi, Chandrasekhara Reddy Gundam, Juneja Subhashchander, Kumar Kedia Ashok, Manjrekar Pravin, Rathod Rahul. Effectiveness and safety of fixed dose combination of acarbose/metformin in Indian Type 2 diabetes patients: Results from observational GLOBE Study. IJEM. 2016;19:129–135. doi: 10.4103/2230-8210.146868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenstock J., Fleck P.R., Inzucchi S.E., Wilson C.A., Seufert J., Mekki Q. Initial Combination Therapy with Alogliptin and Pioglitazone in Drug-Naïve Patients with Type 2 Diabetes. Diabetes Care. 2010;33:2406–2408. doi: 10.2337/dc10-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derosa G., Cicero A.F., Gaddi A.V., Ciccarelli L., Piccinni M.N., Salvadeo S., Pricolo F., Fogari E., Ghelfi M., Ferrari I., Fogari R. Long-term Effects of Glimepiride or Rosiglitazone in Combination with Metformin on Blood Pressure Control in Type 2 Diabetic Patients Affected by the Metabolic Syndrome: A 12-Month, Double-Blind, Randomized Clinical Trial. Clin Ther. 2005;27:1383–1391. doi: 10.1016/j.clinthera.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Chang M., Liu X., Cui D., Liang D., LaCreta F., Griffen S.C., Lubin S., Quamina-Edghill D., Boulton D.W. Bioequivalence, Food Effect and Steady-State Assessment of Dapagliflozin/Metformin Extended-release Fixed-dose Combination Tablets Relative to Single-component Dapagliflozin and Metformin Extended-release Tablets in Healthy Subjects. Clin Ther. 2015;37:1517–1528. doi: 10.1016/j.clinthera.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Migoya E.M., Miller J.L., Gutierrez M., Zheng W., Johnson-Levonas A.O., Liu Q., Matthews C.Z., Wagner J.A., Gottesdiener K.M. Bioequivalence of Sitagliptin/Metformin Fixed-Dose Combination Tablets and Concomitant Administration of Sitagliptin and Metformin in Healthy Adult Subjects. Clin Drug Investig. 2010;30:855–866. doi: 10.1007/BF03256914. [DOI] [PubMed] [Google Scholar]