Abstract
Background
Despite the well-known fact that antibiotics (AB) are not effective against viruses, many patients ask for - and all too often doctors provide – AB for treating URTIs. Over-prescribing of AB is one of the key causes for the development of bacterial resistance, which the U.S. Centers for Disease Control and Prevention (CDC) calls “one of the world's most pressing public health problems”. In addition to the CDC initiated “Get Smart About Antibiotics” campaign, focused on educating doctors the public about the importance of appropriate AB use, other programs tackling this problem include the development of new treatment paradigms. Data published at the Oregon Health & Science University demonstrated that a ‘wait-and-see’ approach, without an AB prescription for the treatment of acute childhood ear infections, was as quick, safe, and effective in resolving the infections as an AB prescription (Spiro DM, Tay KY, Arnold DH, Dziura JD, Baker MD, Shapiro ED. Wait-and-See Prescription for the Treatment of Acute Otitis Media. JAMA 2006; 296:1235-1241).
Objective
To try and reduce inappropriate prescribing practices, a wait and see or delayed approach requires patients to return for a prescription if their symptoms persist or worsen. The aim of this study was to determine whether treatment with Mucinex D (Reckitt Benckiser LLC, Parsippany, New Jersey) lowers the use of antibiotics in the treatment of URTIs when compared with placebo.
Methods
Patients aged 18 to 75 years with symptoms of acute URTIs were randomized to 1200 mg guaifenesin/120 mg pseudoephedrine hydrochloride extended-release, bilayer tablets or matching placebo for 7 consecutive days. Eligible patients met physician’s criteria for antibiotic therapy but were considered suitable for a wait and see approach (withholding antibiotics for ≥48 hours). Patients recorded symptom ratings via an interactive voice response system.
Results
One thousand one hundred eighty-nine patients enrolled; data are presented for the modified intent-to-treat population (n = 1179). At Day 8, significantly fewer patients receiving guaifenesin/pseudoephedrine versus placebo desired antibiotics (4.2% vs 8.0%). No adverse effects were reported due to patients not taking antibiotics. Significant reductions in URTI symptoms were observed for extended-release guaifenesin/pseudoephedrine versus placebo, from Day 1 throughout the study; however, the proportion of patients experiencing overall relief at the Day 4 evening assessment (primary end point) did not reach statistical significance. Treatment-related adverse events were reported in 9.8% and 4.7% of patients receiving guaifenesin/pseudoephedrine and placebo, respectively.
Conclusions
The study found that a wait and see approach was associated with decreased antibiotic use. In addition, the use of a guaifenesin pseudoephedrine combination product provided an effective symptom control compared to a placebo and a well-tolerated first-line strategy for the management of URTIs. This study was not designed to assess the effects of guaifenesin or pseudoephedrine individually. Other limitations include the need for better clinical methods to assess the effectiveness of treatments for acute symptoms of patients with URTIs. ClinicalTrials.gov identifier: NCT01202279.
Key words: antibiotics, extended-release guaifenesin, pseudoephedrine hydrochloride, upper respiratory tract infection
Introduction
Acute upper respiratory tract infections (URTIs) are typically caused by viruses and are, therefore, not treatable with antibiotic agents. However, many patients still request, and are often prescribed, antibiotics for the treatment of URTIs.1, 2 Frequent and inappropriate use of antibiotics is associated with serious consequences such as the increase in cases of Clostridium difficile infection,3 and is driving the widespread and increasing problem of antibiotic resistance.4 Thus it is important to educate patients and health care providers (HCPs) about more prudent use of antibiotics and to encourage alternative treatment strategies and prescribing habits to reduce antibiotic use.4, 5
A wait-and-see approach, also known as a delayed approach to antibiotic prescribing, requires HCPs to instruct patients to return for a prescription if their condition persists or worsens, or to issue a prescription and request that patients refrain from using it unless symptoms persist or worsen. Previously, this approach was shown to be as well tolerated and as effective as antibiotics for the treatment of most childhood ear infections.6
A combined over-the-counter (OTC) expectorant and decongestant containing 1200 mg guaifenesin and 120 mg pseudoephedrine hydrochloride in an extended-release (ER) bilayer tablet formulation (Reckitt Benckiser LLC, Parsippany, New Jersey) has been approved by the US Food and Drug Administration7 for the treatment of chest congestion associated with nasal and sinus congestion. Both of the active ingredients have been shown to reduce URTI symptoms in clinical studies.7, 8, 9, 10, 11, 12, 13, 14 Guaifenesin’s primary indication is the treatment of chest congestion associated with the common cold, but this drug has also been shown to improve symptoms of bothersome mucus and inhibit cough reflex sensitivity,8 reduce cough frequency and intensity10 of nighttime and daytime cough,11 and relieve nasal congestion.
Pseudoephedrine has demonstrated efficacy against nasal congestion, as assessed by both subjective and objective measures14; it also temporarily relieves sinus congestion and pressure.
ER guaifenesin/pseudoephedrine or placebo, in combination with antibiotic therapy, has been investigated in a previous study involving 601 patients with acute URTIs. The ER guaifenesin/pseudoephedrine product was found to improve respiratory symptoms and shorten time to relief compared with placebo.15
However, a major challenge in conducting clinical trials of mucoactive treatments in patients with URTIs is the lack of validated clinical models to assess improvements in symptoms. The likely reason for this is the heterogeneous nature of URTIs, where daily changes in symptoms occur due to natural resolution of the infection.16 This makes any assessments of clinical efficacy for mucoactive drugs challenging17 and subjective patient-reported outcome measures typically lack the precision to differentiate minimally important treatment differences from these natural changes. Attempts to differentiate between active drugs and placebo and define minimally important treatment differences in efficacy studies in patients with URTIs have been unsuccessful.18
Also, studies involving objective measures to assess the treatment effect of mucoactive products have been problematic and resulted in inconsistent outcomes.19, 20 Thus, at present, clinical efficacy of an expectorant in patients with URTIs needs to be based on subjective symptom assessments by the patient.
The aim of the randomized, double-blind, placebo-controlled, parallel-group, multicenter study reported here was to determine whether ER guaifenesin/pseudoephedrine, combined with a wait and see strategy, could offer sufficient symptomatic improvement to give patients with URTIs a treatment alternative and thus reduce the use of, and patient desire for, antibiotics. Further, this study assessed the safety and efficacy of ER guaifenesin/pseudoephedrine compared with placebo in providing first-line symptom relief for acute URTIs.
Patients and Methods
This was a randomized, double-blind, placebo-controlled, parallel-group, multicenter study of ER guaifenesin/pseudoephedrine for the symptomatic treatment of patients with URTIs who sought treatment at a doctor’s office. The first patient was enrolled on October 19, 2009, and the last patient completed on April 2, 2010. Approval for the study was obtained (Chesapeake Research Review, Inc, Columbia, Maryland) and written informed consent was obtained from all patients. This study was conducted according to the Declaration of Helsinki (Recommendations Guiding Physicians in Biomedical Research Involving Human Patients), and complied with the International Conference on Harmonisation Harmonized Tripartite Guidelines for Good Clinical Practice 1996 (Directive 91/507/EEC), and the US Code of Federal Regulations. The study was registered with ClinicalTrials.gov (NCT01202279). The data management of the study was the responsibility of TKL Research, Inc (Rochelle Park, New Jersey). The statistical analysis was conducted by Paragon Biomedical, Inc (Irvine, California). All investigation staff were blinded.
Patient selection
Forty-nine sites in the United States agreed to participate in this study and participants were enrolled at a total of 45 study sites. Adult patients (aged 18–75 years) were eligible to be considered to participate if they presented at a study health care clinic with symptoms indicative of an acute respiratory tract infection (eg, common cold, acute bronchitis, and acute sinusitis). Only patients actively seeking treatment were enrolled; no advertising was carried out to recruit participants. Eligible patients (Table I) with onset of symptoms within the past 5 days were enrolled if they had a total respiratory symptom score ≥ 12 (based on a 0–5 severity rating of 7 respiratory symptoms), with at least 2 out of 3 symptoms; that is, chest congestion, nasal congestion, or thickened mucus, having a score ≥ 3. Participants were required to meet the physician’s normal criteria for identifying patients who should receive antibiotic therapy but who were considered suitable for a wait-and-see approach (withholding antibiotics for ≥48 hours). Exclusion criteria included recurring respiratory symptoms due to chronic allergic rhinitis, sinusitis, or bronchitis; significant comorbidities; receiving treatment with intranasal medications, systemic antihistamine, or bronchodilators; receiving treatment with a monoamine oxidase inhibitor within 2 weeks of enrollment, or sleeping pills, sedatives, tranquilizers, muscle relaxants, or antidepressants within 7 days (except long-term medication that had been administered at a stable dose for ≥3 months), or systemic corticosteroids or antibiotics within 6 weeks; febrile illness >38°C within 7 days of enrollment; onset of symptoms of URTIs within 2 weeks of receiving a seasonal influenza or 2009 H1N1 vaccination; pregnancy or lactating; and participation in another clinical investigation within 4 weeks of enrollment. The use of nonsteroidal anti-inflammatory drugs or other medications to treat URTIs was prohibited during the study, with the exception of low-dose aspirin (81 mg) or acetaminophen (2 × 325 mg) every 4 to 6 hours.
Table I.
Patient characteristics at baseline (modified intent-to-treat population).*
| Guaifenesin/pseudoephedrine (n = 591) | Placebo (n = 588) | |
|---|---|---|
| Age, y | 37.4 (13.7) | 38.8 (13.7) |
| Female sex | 395 (66.8) | 404 (68.7) |
| Race | ||
| White | 494 (83.6) | 485 (82.5) |
| Black/African American | 59 (10.0) | 63 (10.7) |
| Other | 38 (6.4) | 40 (6.8) |
| Diagnosis | ||
| Acute bronchitis | 66 (11.2) | 68 (11.6) |
| Acute sinusitis | 145 (24.5) | 151 (25.7) |
| Rhinitis | 62 (10.5) | 67 (11.4) |
| Nasal congestion | 87 (14.7) | 70 (11.9) |
| Chest congestion | 22 (3.7) | 24 (4.1) |
| Other | 209 (35.4) | 208 (35.4) |
| Baseline total symptom score | 23.4 (4.9) | 23.6 (4.5) |
| Baseline individual symptom scores | ||
| Chest congestion | 3.05 (1.14) | 3.11 (1.11) |
| Thickened mucus | 3.50 (0.90) | 3.49 (0.80) |
| Nasal congestion | 3.55 (0.92) | 3.57 (0.92) |
| Runny nose | 2.93 (1.23) | 3.01 (1.14) |
| Sinus headache | 3.11 (1.35) | 3.11 (1.30) |
| Sinus pressure | 3.39 (1.09) | 3.36 (1.10) |
| Postnasal drip | 3.11 (1.16) | 3.15 (1.07) |
| Baseline WURSS-21 score | ||
| Overall total | 90.4† (22.1) | 91.2 (21.0) |
| Symptom score | 44.0† (10.7) | 44.3 (10.0) |
| Functional score | 36.2† (12.9) | 36.7 (12.6) |
WURSS-21 = Wisconsin Upper Respiratory Symptom Survey.
Values are presented as mean (SD) (age, baseline total symptom score, baseline individual symptom scores, and baseline WURSS-21 score), or n (%) (sex, race, and diagnosis).
n = 590.
Randomization to treatment
The random allocation sequence was generated by the study statistician. At baseline visit each patient was assigned a randomization number and the randomization schedule was blocked in groups of 4 to ensure approximately equal assignment to each treatment group at each site. Each site was given a group of randomized patient packs to assign to patients sequentially by the principal investigator as they were enrolled into the study. Patients received either 1200 mg guaifenesin/120 mg pseudoephedrine ER bilayer tablets or matching placebo tablets. They were instructed to take 1 tablet every morning and evening with a full glass of water for 7 consecutive days, in accordance with product labeling. The first dose of study medication was taken in the clinic and the remainder were taken at home.
At baseline and twice daily, patients recorded symptom ratings via an interactive voice response system (IVRS); the severity of each of 7 symptoms (chest congestion, thickened mucus, nasal congestion, runny nose, sinus headache, sinus pressure, and postnasal drip) as they had experienced them over the previous 12 hours was scored on a scale of 0 to 5 (0 = none, 1 = very mild, 2 = mild or slight, 3 = moderate, 4 = severe, or 5 = as bad as it could be). The IVRS data were retrieved by MERGE eClinical (Chicago, Illinois) (known as etrials Worldwide at the time of the study). MERGE eClinical transferred the data to TKL Research, Inc. (Fair Lawn, NJ). After unblinding, the data were analyzed by Paragon Biomedical, Inc. (Morrisville, NC).
Patients were examined by their HCP on Day 4 and Day 8 (end of study). At Day 8 or end of treatment study visit, patients were asked to respond, on a scale of 0 to 5, to the following question: Was the study medication effective? (0 = not effective at all, 1 = somewhat effective, 2 = moderately effective, 3 = very effective, and 4 = extremely effective). In addition, investigators recorded their end of study assessment of treatment by scoring their answer to the following questions: “Based on the observed treatment outcomes for this patient, would you recommend the study medication for future use before prescribing an antibiotic for the treatment of symptoms associated with an acute upper respiratory infection in this type of patient (Yes or No)?” and, “How satisfied are you with the use of study medication as a first-line treatment before giving antibiotic therapy” (1 = very dissatisfied, 2 = dissatisfied, 3 = neither satisfied nor dissatisfied, 4 = satisfied, and 5 = very satisfied).
Patient quality of life (QoL) during the study was recorded using the Wisconsin Upper Respiratory Symptom Survey questionnaire (WURSS-21), which includes 1 global severity item (How sick do you feel today?), 10 symptom-based items, 9 functional items, and 1 global change item (Compared to yesterday, I feel…).17, 21 This validated QoL tool was completed at baseline (before treatment) and at HCP visits on Day 4 and Day 8 (or end of study).
Adverse events (AEs) were recorded at each visit (either volunteered by the patient, discovered by investigator questioning, or detected through other means) and were defined as any untoward medical occurrence. AEs were coded using MedDRA terminology (MedDRA MSSO, McLean, VA, USA) and rated as mild, moderate, or severe. They were considered treatment emergent if the date of onset was Day 1 (baseline) or later. The investigator classified AEs as not related, remotely, possibly, probably, or definitely related to study medication.
The primary efficacy end point was the proportion of patients experiencing overall relief on the evening of Day 4, based on the IVRS daily diary data, defined as having scored no symptoms worse than 2 = mild or slight.
Secondary efficacy end points included time from baseline to treatment failure, defined as the time when a patient requested and received an antibiotic or was prescribed an antibiotic based on the investigator׳s assessment of symptoms; proportion of patients not requiring an antibiotic; time from baseline to initial overall relief, defined as the first diary time point at which no symptom was scored worse than 2 = mild or slight (ie, no symptoms with a severity rating score of 3 = moderate or more based on a scale range of 0 = none to 5 = as bad as it can be); time from baseline to sustained overall relief, defined as overall relief maintained for at least 24 hours (at least 2 consecutive diary times) and continuing to the final diary time point; total symptom score at each time point; individual symptom scores at each time point; patient׳s overall rating of the efficacy of treatment in relieving symptoms associated with the infection; investigator׳s end of study assessment of treatment; and WURSS-21 total score (QoL).
Sample size calculation
A sample size of 500 patients per group, allowing for a 6% dropout rate, was calculated to provide 90% power to show a statistically significant difference in the primary efficacy end point, using a continuity-corrected χ2 test with an α = 0.05 2-sided significance level. This calculation was based on the results of a previous study, with a different design, of ER guaifenesin/pseudoephedrine compared with placebo in 605 patients.15
Statistical analysis
All efficacy variables were determined using the modified intent-to-treat (mITT) population defined as all randomized patients who received at least 1 dose of study medication with 1 or more postbaseline efficacy measures. The last observation carried forward approach was used for patients who discontinued or those without final visits. The per protocol (PP) population consisted of the mITT population without any major protocol violations. The safety population was defined as all patients who received the study medication (excluding patients who returned all medication unused).
Statistical analyses were performed using SAS statistical software (version 8.2; SAS Institute Inc, Cary, North Carolina) and tested at the 2-sided 5% level. The difference between treatment groups in the primary efficacy end point was analyzed using the Cochran-Mantel-Haenszel test, adjusting for region. In addition, a logistic regression model with treatment, region, baseline total symptom score, antibiotic use (before, or at, Day 4 evening), and treatment by antibiotic use interaction terms was evaluated. Ordinal and binary efficacy secondary end points were compared between treatment groups using a logistic regression model with treatment, region, baseline symptom score, antibiotic use, treatment by antibiotic use, and treatment by time point interaction terms in the model. The changes in individual and total symptom scores from baseline to each postbaseline time point were compared between the 2 treatment groups using a repeated measures ANCOVA model with treatment, region, baseline total symptom score, antibiotic use, treatment by antibiotic use, and treatment by time point interaction terms in the model.
The statistical analyses were compliant with relevant guidance documents such as the International Conference on Harmonisation’s guidelines on statistical principles for clinical trials. However, a noteworthy caveat is that no multiplicity adjustments were applied, so any statistically significant findings should be interpreted with appropriate caution.
Results
Patient population
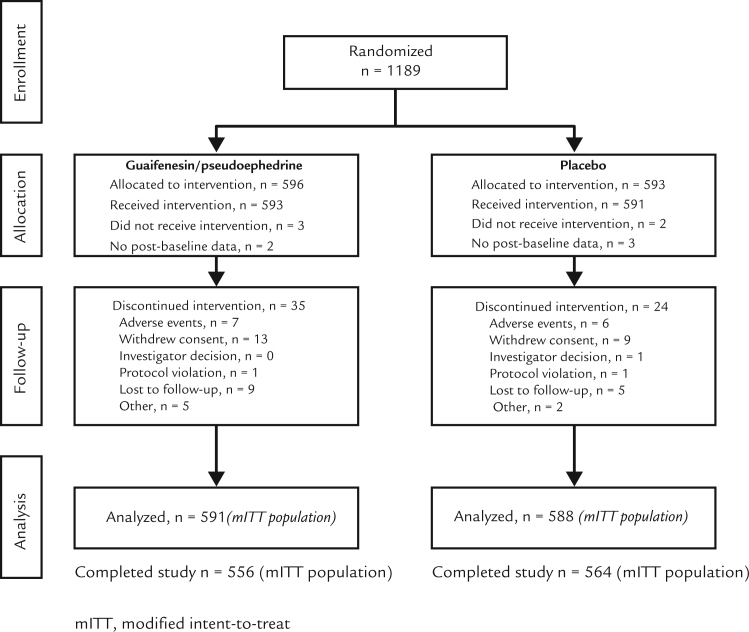
A total of 1189 patients were randomized: 596 to guaifenesin/pseudoephedrine, and 593 to placebo (Figure 1). The mITT and PP population consisted of 591 and 510 patients, respectively, who received guaifenesin/pseudoephedrine, and 588 and 523 patients, respectively, who received placebo. In the mITT population, 556 patients completed the study in the guaifenesin/pseudoephedrine group and 564 in the placebo group. Results are reported for the mITT data set, unless otherwise specified.
Figure 1.
Patient disposition. mITT = modified intent-to-treat.
Treatment groups were balanced with respect to patient baseline characteristics (Table I). Compliance to study medication was good in both treatment groups; the mean number of doses taken was 13.6 and 13.9 in the guaifenesin/pseudoephedrine and placebo groups, respectively (from a total of 14 possible doses).
Reduction of symptoms
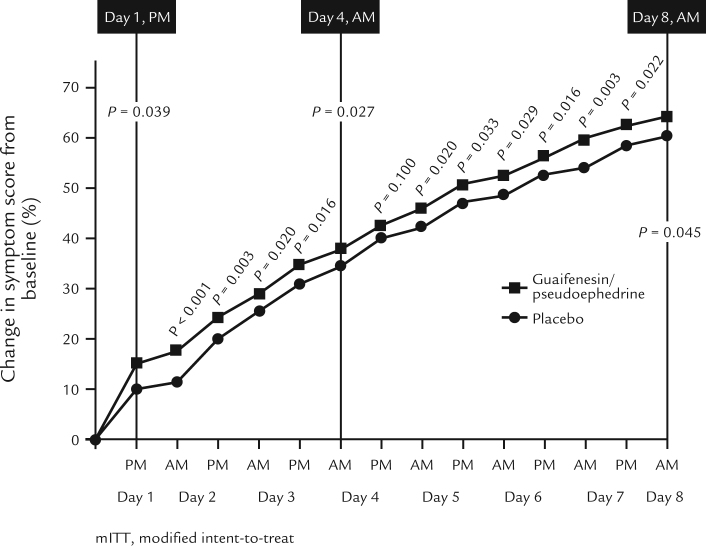
Overall, a statistically significant reduction in total symptom score was seen with guaifenesin/pseudoephedrine over placebo (P = 0.006); this was observed from Day 1 (P = 0.039), with the largest difference seen on the morning of Day 2 (P < 0.001), and remained throughout the course of the study (13 of 14 scoring occasions) until Day 8 (P = 0.045), with the exception of the evening assessment on Day 4 (P = 0.100) (Figure 2). The number of patients experiencing overall relief at the evening of Day 4 (primary end point) was 197 (33.3%) in the guaifenesin/pseudoephedrine group and 187 (31.8%) in the placebo group, which was not statistically significantly different (odds ratio, 0.9; 95% CI, 0.6–1.2; P = 0.441). Statistically significant differences in mean change from baseline in individual symptom scores were observed with guaifenesin/pseudoephedrine over placebo for thickened mucus, nasal congestion, runny nose, sinus headache, sinus pressure, and postnasal drip (all P values < 0.05) but not for chest congestion (Table II). There was a statistically significant improvement in time from baseline to initial overall relief with guaifenesin/pseudoephedrine over placebo (P = 0.005), and time from baseline to sustained overall relief (at least 2 consecutive diary entries) also favored guaifenesin/pseudoephedrine (P = 0.072 [P = 0.029 for the PP population]).
Figure 2.
Time point comparison of change from baseline in total symptom score (modified intent-to-treat population).
Table II.
Change from baseline in individual symptom scores (modified intent-to-treat population).
| Symptom | Time of first significant difference between treatment groups | Mean (SD) change from baseline at time of first significant difference |
P value at time of first significant difference* | Overall P value* | |
|---|---|---|---|---|---|
| Guaifenesin/ pseudoephedrine (n = 591) | Placebo (n = 588) | ||||
| Chest congestion | – | – | – | – | 0.279 |
| Thickened mucus | Day 2 morning | 0.48 (1.02) | 0.28 (0.96) | < 0.001 | 0.014 |
| Nasal congestion | Day 2 morning | 0.59 (1.10) | 0.38 (1.04) | < 0.001 | 0.020 |
| Runny nose | Day 1 evening | 0.43 (1.09) | 0.27 (1.08) | 0.017 | 0.016 |
| Sinus headache | Day 2 morning | 0.75 (1.29) | 0.50 (1.28) | 0.002 | 0.027 |
| Sinus pressure | Day 1 evening | 0.51 (1.05) | 0.26 (1.00) | 0.049 | 0.009 |
| Postnasal drip | Day 2 morning | 0.56 (1.16) | 0.34 (1.08) | 0.004 | 0.041 |
Difference between treatment groups in change from baseline was based on ANCOVA with treatment and region effects, baseline symptom score, time point, antibiotic use, and treatment by antibiotic and treatment by time point terms in the model.
Antibiotic use reduction
The use of a wait-and see-approach in this study resulted in approximately 80% of patients, regardless of treatment group, not receiving antibiotics; that is, 80.9% and 77.0% in the guaifenesin/pseudoephedrine and placebo groups, respectively. This difference did not reach statistical significance in the mITT population (P = 0.116); however, in the PP population, a statistically significant difference was observed (82.5% vs 76.9% for guaifenesin/pseudoephedrine and placebo groups, respectively; P = 0.025). There was no indication that patients experienced any adverse effects due to the withholding of antibiotics using this wait-and-see approach.
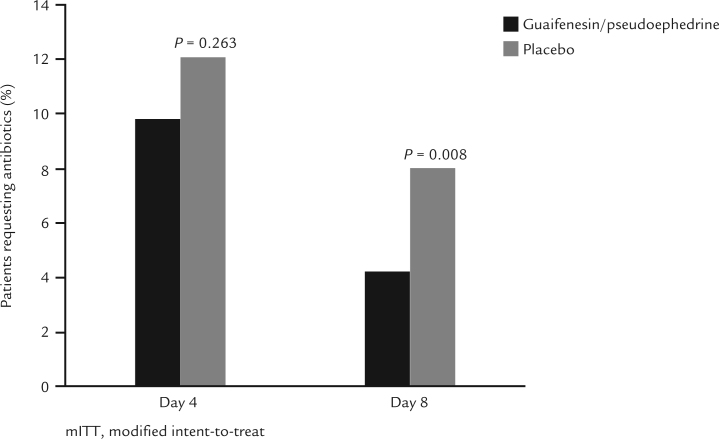
Overall, there was no statistically significant difference between treatment groups in the time to prescription of antibiotics (mean [SD] = 3.9 [2.02] days versus 4.1 [2.15] days for guaifenesin/pseudoephedrine and placebo groups, respectively [P = 0.122]). At both Day 4 and Day 8, fewer patients in the guaifenesin/pseudoephedrine group desired antibiotics compared with those in the placebo group, reaching a significant difference at Day 8 (Day 4: 9.8% vs 12.0%, respectively [P = 0.263]; Day 8: 4.2% vs 8.0%, respectively [P = 0.008]) (Figure 3).
Figure 3.
Percentage of patients requesting antibiotics at Day 4 and Day 8 (modified intent-to-treat population).
Assessment of study treatment
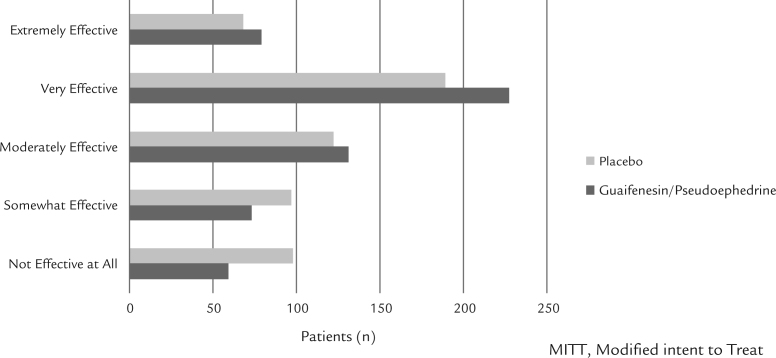
At the Day 4 assessment, significantly more patients receiving guaifenesin/pseudoephedrine, compared with placebo, believed their study medication alleviated their symptoms sufficiently (P = 0.048). In response to the question, Was the study medication effective?, there was a statistically significant difference overall in favor of guaifenesin/pseudoephedrine compared with placebo at Day 8 (P = 0.025) (Figure 4). No difference was observed in the physicians’ assessment of their patients’ treatment.
Figure 4.
Patients’ end of treatment (Day 8) assessment of study medication (modified intent-to-treat population).
Influence of treatment on QoL
Treatment with guaifenesin/pseudoephedrine was associated with an early improvement in QoL compared with placebo, as represented by a statistically significant improvement in the mean change from baseline to Day 4 in WURSS-21 scores for the total overall score (P = 0.024) as well as both subscales; that is the Total Symptom and Total Functional Scores (Table III). Differences in change from baseline in individual WURSS-21 scores significantly favored guaifenesin/pseudoephedrine for 9 symptoms (runny nose, sneezing, scratchy throat, hoarseness, head congestion, think clearly, breathe easily, walk/climb stairs/exercise, and interaction with others; all P values < 0.05).
Table III.
Change from baseline in Wisconsin Upper Respiratory Symptom Survey (WURSS-21) scores (modified intent-to-treat population).
| Guaifenesin/pseudoephedrine (n = 591) | Placebo (n = 588) | P value* | |
|---|---|---|---|
| Overall total WURSS-21 score | |||
| Mean change from baseline to Day 4 | 40.0 | 36.4 | 0.024 |
| Mean change from baseline to Day 8 | 68.0 | 65.4 | 0.085 |
| Total WURSS-21 symptom score | |||
| Mean change from baseline to Day 4 | 19.4 | 17.7 | 0.013 |
| Mean change from baseline to Day 8 | 33.4 | 32.0 | 0.165 |
| Total WURSS-21 functional score | |||
| Mean change from baseline to Day 4 | 16.2 | 14.6 | 0.050 |
| Mean change from baseline to Day 8 | 27.7 | 26.8 | 0.056 |
Difference between treatment groups in change from baseline is based on ANCOVA with treatment, region, baseline total WURSS-21 functional score, antibiotic use, and treatment by antibiotic use terms in the model.
On Day 8, differences between guaifenesin/pseudoephedrine and placebo in mean change from baseline in individual WURSS-21 scores were significantly in favor of guaifenesin/pseudoephedrine for 4 symptoms (runny nose, sneezing, think clearly, and interaction with others; all P values < 0.05). However, no overall statistically significant improvement in QoL was observed between treatments at Day 8.
Safety
During the course of this study there were a total of 70 and 32 treatment-related AEs in the guaifenesin/pseudoephedrine and placebo groups, respectively (safety population: guaifenesin/pseudoephedrine n = 593 and placebo n = 591). In the guaifenesin/pseudoephedrine treatment group, 9.8% of patients experienced a treatment-related AE compared with 4.7% of patients in the placebo group (no serious AEs were reported during the study).
In the guaifenesin/pseudoephedrine group, 8 patients (1.3%) discontinued the study due to an AE compared with 5 patients (0.8%) in the placebo group. The most commonly experienced AEs were insomnia (n = 17) in the guaifenesin/pseudoephedrine group and headache (n = 14) in the placebo group.
There was a statistically significant higher incidence of gastrointestinal distress in patients who received placebo and an antibiotic compared with patients receiving only guaifenesin/pseudoephedrine (8 out of 135 [5.9%] vs 11 out of 479 [2.3%], respectively; P = 0.045 [safety population]).
Discussion
In this study, ER guaifenesin/pseudoephedrine was associated with a statistically significant improvement in URTI symptoms compared with placebo, demonstrating a reduction in total symptom score in 13 of 14 diary assessments (mITT P values ranging between 0.045 and < 0.001 (see Figure 2). This difference from placebo was apparent throughout the study, except for the evening score on Day 4 (P = 0.1). The reason for this discrepancy is unknown, but this isolated outcome was not expected, considering that the symptom score P values are significant for the combination drug versus placebo at both the bracketing time points on Day 4 morning (P = 0.027) and Day 5 morning (P = 0.02). The single, statistically nonsignificant assessment result on Day 4 evening seems to be inconsistent with the other 13 assessments throughout the study and also stands in contrast with the total overall WURSS-21 score, which includes a symptom subscore and showed statistical significance favoring the combination product over placebo at the same Day 4 evening measurement.
Placebo effects in studies of mucoactive drugs in URTI patients have been documented.22, 23 Such a phenomenon could also have influenced the questionable outcome on Day 4 in this study. The main hypothesis to explain this single point deviation in the study results is that there may have been some sort of white coat or Hawthorne effect24 caused by the office visit on Day 4 before patients completed their afternoon/evening assessment. The hypothesis implies that the clinic experience and heightened awareness of being studied on that day may have made patients unsure about what to enter into the IVRS for their self-assessment, contrary to how they had done the scoring at the other time points before the afternoon of Day 4 and for the remainder of the study on the morning of Day 5 and onward. In some way, the examinations and interviews at the clinics seem to have influenced patient perceptions, leading to the elimination of the differentiation between the active treatment and placebo at the afternoon/evening assessment on Day 4 compared with the earlier time points in the study.
The primary end point for this study was not chosen because Day 4 evening is a particularly significant time point for assessing treatment efficacy in a study of this nature. Rather, it was an arbitrary selection based on a prior, differently designed study,15 which also served to determine the sample size. In retrospect, earlier assessment time points might arguably be more relevant, because rapid symptom reduction is a priority for most URTI patients, and the ER drug combination tested in this study achieved a significant reduction in symptom score versus placebo starting on Day 1 and at all time points preceding the Day 4 evening assessment.
The treatment effect reported in this study may raise a question as to what constitutes a clinically meaningful difference between placebo and active treatment. For example, 1 study publication reported that patients with the common cold would accept a small treatment benefit in symptom relief (10% or less),18 and in a review of the effectiveness of nasal decongestants,25 the decrease in subjective symptoms compared with placebo was small but statistically significant at 6%. The difficulty in demonstrating sizeable treatment effects in placebo-controlled URTI studies can, to a large extent, be attributed to the rapidly changing URTI condition and naturally improving symptoms. The other key factor contributing to this difficulty is the absence of relevant objective or validated, subjective clinical methods to capture the gradual changes in symptomatology while the underlying (viral) infection is resolving.16 Therefore, the usually small improvements recorded with active OTC cough and cold treatments compared with placebo in URTI treatment studies may, in contrast, be perceived as being more meaningful by consumers self-treating with such products, because the clinical methods used in the studies do not capture the real-life consumer self-treatment experience with OTC cough and cold products.
The second objective of this study was to examine the effectiveness of a wait-and-see approach in reducing antibiotic prescribing in patients with acute symptoms of a URTI if they are instead offered a treatment for rapid symptom relief. The results of this study encourage the use of such a strategy in that ~80% of patients did not receive, or ask for, an antibiotic, regardless of treatment group. Compared with placebo, fewer patients in the active treatment group (ER guaifenesin/pseudoephedrine) were prescribed an antibiotic than in the mITT and PP populations and statistically significantly fewer patients receiving ER guaifenesin/pseudoephedrine desired antibiotics, compared with the PP population, at Day 8.
It has to be noted that reduction of antibiotic use in the Mucinex D group compared with the placebo was observed at Day 4 and Day 8 in the mITT and PP populations, but statistical significance was only achieved at Day 8 in the PP population (P = 0.033).
These outcomes may potentially be attributed to the redirecting of HCPs on the appropriate prescribing of antibiotics, combined with the observed improvement in symptoms resulting from the ER guaifenesin/pseudoephedrine treatment. Suggestions for HCPs provided by the Centers for Disease Control and Prevention Get Smart About Antibiotics campaign relating to antibiotic use include recommending a specific symptomatic therapy, spending time answering patient questions, and offering a contingency plan if symptoms worsen.5 This study provides evidence that using a wait-and-see approach, combined with the use of ER guaifenesin/pseudoephedrine to improve symptoms, does seem to result in the desired reduction in both requests for, and use of, antibiotics in patients with acute symptoms of URTIs.
Limitations of this study are that the treatment effect was not significant at the arbitrarily chosen primary efficacy end point, and that this outlier result compared with the other 13 symptom scoring occasions cannot be sufficiently explained. Other irregularities, such as the differences in efficacy observed between the active treatment and placebo, that missed statistical significance in the mITT population but were significant in the PP population, highlight that protocol violations (eg, placebo-assigned patients taking additional treatments), may have compromised the strength of this study. This study was also not designed to quantify the individual contribution of guaifenesin or pseudoephedrine to the observed symptom improvements. Although pseudoephedrine is an established decongestant, guaifenesin is a well-recognized expectorant and has been shown to be effective for chest congestion and improvement of cough as well as symptoms of rhinitis and nasal congestion.11, 26, 27 Thus, it is assumed that both agents contributed to the relief of URTI symptoms and the related patient satisfaction.
Lastly, it was logistically and methodologically challenging to implement the wait-and-see strategy in this study because it was difficult to recruit true antibiotic-seeking patients and have a better defined target population. Although the study outcomes point to the potentially beneficial effects of a wait-and-see strategy in reducing antibiotic overuse in URTI patients, a larger sample size and optimized patient recruitment strategies might have resulted in even more convincing results in terms of showing reduced antibiotic requests or desired prescriptions in these patients. Further method development efforts for conducting these kinds of studies are needed.
Conclusions
The results of this wait-and-see study provide additional insights, not only regarding the effectiveness and safety of an ER guaifenesin/pseudoephedrine combination product in reducing acute URTI symptoms, but also in the strategy of providing symptom relief to discourage unwarranted antibiotic use in this patient population. Addressing patient concerns and recommending symptom-relieving products for URTIs early on can meet patient expectations for rapid relief and diminish their desire for antibiotic prescriptions.
Further research in the assessment of symptomatic treatments for the management of acute URTI symptoms is needed. Data from this trial may inform the design and execution of future studies of URTI patient populations to identify better clinical methods and encourage more prudent use of antibiotics for viral URTIs.
Conflicts of Interest
This study was supported by Reckitt Benckiser LLC, Parsippany, New Jersey.
Helmut Albrecht is a consultant to Reckitt Benckiser LLC, Parsippany, New Jersey. Eric Guenin and Tim Shea are current employees and Gail Solomon is a former employee of Reckitt Benckiser LLC, Parsippany, New Jersey. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
The authors thank Erin Steward (formerly an employee of Reckitt Benckiser LLC, Parsippany, New Jersey) and Robert Smith (formerly a consultant of Reckitt Benckiser LLC, Parsippany, New Jersey) for providing statistical analysis, and Elements Communications Ltd (Westerham, Kent, United Kingdom), for providing medical writing assistance (supported by Reckitt Benckiser LLC, Parsippany, New Jersey).
Edward Septimus: study design, literature review, data interpretation, and writing.
Helmut H. Albrecht: was involved in the planning (incl. lit. search), protocol generation, investigator meeting, data interpretation & reporting and preparation of publication manuscript.
Gail Solomon: Data interpretation.
Tim shea: study design, data collection.
Eric Guenin: data interpretation, literature search, writing.
References
- 1.Gonzales R., Bartlett J.G., Besser R.E., Hickner J.M., Hoffman J.R., Sande M.A. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: background. Ann Intern Med. 2001;134:490–494. doi: 10.7326/0003-4819-134-6-200103200-00015. [DOI] [PubMed] [Google Scholar]
- 2.Roumie C.L., Halasa N.B., Grijalva C.G., Edwards K.M., Zhu Y., Dittus R.S. Trends in antibiotic prescribing for adults in the United States – 1995 to 2002. J Gen Intern Med. 2005;20:697–702. doi: 10.1111/j.1525-1497.2005.0148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly C.P., LaMont J.T. Clostridium difficile — more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 4.Fleming-Dutra K.E., Hersh A.L., Shapiro D.J., Bartoces M., Enns E.A., File T.M., Jr Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA. 2016;315:1864–1873. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 5.Get Smart: Know When Antibiotics Work. http://www.cdc.gov/getsmart/. Accessed January 2013.
- 6.Spiro D.M., Tay K.Y., Arnold D.H., Dziura J.D., Baker M.D., Shapiro E.D. Wait-and-see prescription for the treatment of acute otitis media: a randomized controlled trial. JAMA. 2006;296:1235–1241. doi: 10.1001/jama.296.10.1235. [DOI] [PubMed] [Google Scholar]
- 7.Mucinex D NDA Dossier at Drugs@FDA Website. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021585 Accessed September 2016.
- 8.Dicpinigaitis P.V., Gayle Y.E. Effect of guaifenesin on cough reflex sensitivity. Chest. 2003;124:2178–2181. doi: 10.1378/chest.124.6.2178. [DOI] [PubMed] [Google Scholar]
- 9.Yuta A., Baraniuk J.N. Therapeutic approaches to mucus hypersecretion. Curr Allergy Asthma Rep. 2005;5:243–251. doi: 10.1007/s11882-005-0044-6. [DOI] [PubMed] [Google Scholar]
- 10.Robinson R.E., Cummings W.B., Deffenbaugh E.R. Effectiveness of guaifenesin as an expectorant: a cooperative double-blind study. Curr Ther Res Clin Exp. 1977;22:284–296. [Google Scholar]
- 11.Storms W., Farrar J.R. Guaifenesin in rhinitis. Curr Allergy Asthma Rep. 2009;9:101–106. doi: 10.1007/s11882-009-0015-4. [DOI] [PubMed] [Google Scholar]
- 12.Scarupa M.D., Kaliner M.A. Adjuvant therapies in the treatment of acute and chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:251–262. [PubMed] [Google Scholar]
- 13.Becker D.G. Medical treatment of sinusitis. J Long Term Eff Med Implants. 2003;13:195–205. doi: 10.1615/jlongtermeffmedimplants.v13.i3.60. [DOI] [PubMed] [Google Scholar]
- 14.Eccles R., Jawad M.S., Jawad S.S., Angello J.T., Druce H.M. Efficacy and safety of single and multiple doses of pseudoephedrine in the treatment of nasal congestion associated with common cold. Am J Rhinol. 2005;19:25–31. [PubMed] [Google Scholar]
- 15.LaForce C., Gentile D.A., Skoner D.P. A randomized, double-blind, parallel-group, multicenter, placebo-controlled study of the safety and efficacy of extended-release guaifenesin/pseudoephedrine hydrochloride for symptom relief as an adjunctive therapy to antibiotic treatment of acute respiratory infections. Postgrad Med. 2008;120:53–59. doi: 10.3810/pgm.2008.07.1791. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht H. Can Big Data Analyses Help Speed Up the Clinical Development of Mucoactive Drugs for Symptomatic RTIs? Lung. 2016;194:31–34. doi: 10.1007/s00408-016-9846-7. [DOI] [PubMed] [Google Scholar]
- 17.Barrett B., Brown R., Mundt M. Comparison of anchor-based and distributional approaches in estimating important difference in common cold. Qual Life Res. 2008;17:75–85. doi: 10.1007/s11136-007-9277-2. [DOI] [PubMed] [Google Scholar]
- 18.Barrett B., Harahan B., Brown D., Zhang Z., Brown R. Sufficiently Important Difference for Common Cold: Severity Reduction. Ann Fam Med. 2007;5:216–223. doi: 10.1370/afm.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin B.K. An in vitro comparison of the mucoactive properties of guaifenesin, iodinated glycerol, surfactant, and albuterol. Chest. 1999;116:195–200. doi: 10.1378/chest.116.1.195. [DOI] [PubMed] [Google Scholar]
- 20.Smith S.M., Schroeder K., Fahey T. Over-the-counter (OTC) medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD001831.pub4. CD001831. [DOI] [PubMed] [Google Scholar]
- 21.Barrett B., Brown R., Mundt M., Safdar N., Dye L., Maberry R. The Wisconsin upper respiratory symptom survey is responsive, reliable, and valid. J Clin Epidemiol. 2005;58:609–617. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle W.J., Cohen S. Common cold. In: Eccles R., Weber O., editors. Etiology of the common cold: Modulating factors. Birkenhäuser Verlag; Basel: 2009. pp. 149–186. [Google Scholar]
- 23.Eccles R. The powerful placebo in cough studies? Pulm Pharmacol Ther. 2002;15:303–308. doi: 10.1006/pupt.2002.0364. [DOI] [PubMed] [Google Scholar]
- 24.McCambridge J., Witton J., Elbourne D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taverner D., Latte G.J. Nasal decongestants for the common cold. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD001953.pub3. CD001953. [DOI] [PubMed] [Google Scholar]
- 26.Wawrose S.F., Tami T.A., Amoils C.P. The role of guaifenesin in the treatment of sinonasal disease in patients infected with the human immunodeficiency virus (HIV) Laryngoscope. 1992;102:1225–1228. doi: 10.1288/00005537-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Rosen E.J., Calhoun K.H. Alterations of nasal mucociliary clearance in association with HIV infection and the effect of guaifenesin therapy. Laryngoscope. 2005;115:27–30. doi: 10.1097/01.mlg.0000150678.83602.d4. [DOI] [PubMed] [Google Scholar]