Abstract
Background
Sitagliptin, a dipeptidyl peptidase-4 inhibitor, is widely used in patients with type 2 diabetes. However, the pleiotropic effects of sitagliptin is not well understood.
Objective
To assess the clinical efficacy and safety of sitagliptin on atherosclerosis, β-cell function, and glycemic control in Japanese patients with type 2 diabetes.
Methods
A prospective observational study of 270 patients with type 2 diabetes mellitus was carried out. Patients (aged 64.3 [12.4] years, body mas index 25.2 [4.3]) with glycated hemoglobin >6.9% (52 mmol/mol) or fasting plasma glucose >130 mg/dL were treated with sitagliptin for 12 months. The primary end point was glycated hemoglobin level changes from baseline to 3 months. The secondary end points included changes in several biomarkers related to inflammation and β-cell function from baseline to 3 months, as well as changes in glycated hemoglobin level from baseline to 12 months.
Results
Glycated hemoglobin levels were significantly lower in patients treated with sitagliptin for 3 months than at baseline (8.1% [1.4%]–7.3% [1.2%]) (65 [16.9]–56 [13.1] mmol/mol]) (P < 0.0001), which continued after 12 months (7.4% [1.3%]) (56 [15.2] mmol/mol) (P < 0.0001). In addition, a marker of vascular-specific inflammation, pentraxin-3, and a marker of β-cell function (proinsulin/insulin ratio), respectively, were lower after treatment with sitagliptin for 3 months than at baseline (1.88 [0.78]–1.65 [0.63] ng/mL [P = 0.0038] and 0.20 [0.14]–0.17 [0.11] [P = 0.01], respectively). On the other hand, a biomarker reflecting whole body inflammation; that is, high-sensitivity C-reactive protein level, was unchanged. Adverse events occurred in 14 patients (5.18%).
Conclusions
Sitagliptin may have beneficial effects on vascular inflammation and β-cell function in Japanese patients with type 2 diabetes. Pentraxin-3 may be an early predictive marker for detecting the antiatherosclerotic effects of dipeptidyl peptidase-4.
Key words: pentraxin-3, dipeptidyl peptidase-4 inhibitor, sitagliptin, proinsulin/insulin ratio
Introduction
The incidence of type 2 diabetes is increasing worldwide and can lead to cardiovascular disease (CVD), which is a major cause of mortality in these patients.1 Several clinical studies have shown that inadequate glycemic control is a risk factor for CVD.2 However, there is little evidence that glycemic control and treatment with any antidiabetes agent actually reduces the incidence of CVD.
Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that was approved in Japan as an antidiabetes agent in 2009. DPP-4 inhibitors improve glucose metabolism by inducing incretin hormones that stimulate insulin secretion and inhibit glucagon secretion in a glucose-dependent manner, thereby improving glycemic control with a lower risk of hypoglycemia or weight gain.3, 4, 5, 6 Therefore, sitagliptin, a relatively new agent, is becoming widely used in newly diagnosed and poorly controlled patients with type 2 diabetes in Japan.7
Recently, the potential antiatherosclerotic effects of DPP-4 inhibitors have been attracting attention. Glucagon-like peptide-1 (GLP-1) receptors are expressed in various cardiovascular tissues, including endothelial cells.8 In a rodent model of atherosclerosis, GLP-1 receptor agonists,9 and DPP-4 inhibitors,10, 11, 12 including sitagliptin, were shown to inhibit atherosclerosis. However, the underlying mechanisms by which these incretin-related drugs exert antiatherosclerotic effects are not fully understood. The pathogenesis of atherosclerosis is characterized by various mechanisms such as inflammation, lipid metabolism, cell proliferation, and oxidative stress. Therefore, measurements of biomarkers related to those proatherosclerotic factors are valuable for clarifying the mechanisms by which DPP-4 inhibitors exert antiatherosclerotic effects. To clarify whether sitagliptin has protective effects on vascular-specific or nonspecific inflammation, we measured the level of pentraxin-3 (PTX3) as well as high-sensitivity C-reactive protein (hs-CRP) in the present study.
Several reports have shown that DPP-4 inhibitors have protective effects on β-cell function.13, 14, 15, 16 β-Cell dysfunction is important in the pathogenesis of type 2 diabetes. In particular, Asian patients with type 2 diabetes are characterized by a relatively lower body mass index than other ethnic populations.17 In addition, insulin secretory defects are more prominent in Asian patients than in white patients.18, 19 Therefore, preservation of β-cell function is an important strategy in the treatment of Asian patients with diabetes. In several studies, DPP-4 inhibitors preserved β-cell function in a model of mice with diabetes.13, 14 However, histologic analyses are rarely performed in humans; therefore, it remains unknown whether DPP-4 inhibitors also have a protective effect on β-cell function in humans. Thus, assessment of β-cell function using biomarkers such as homoeostasis model assessment of β-cell function and proinsulin/insulin (PI/I) ratio is necessary to determine the protective effects of DPP-4 inhibitors in human β cells.
In the present prospective and observational study, the efficacy of sitagliptin for glycemic control was assessed for 3 months in newly diagnosed or poorly controlled patients with type 2 diabetes. In addition, we investigated the pleiotropic effects of sitagliptin on atherogenic factors and β-cell function.
Subjects, Materials, and Methods
Study design and participants
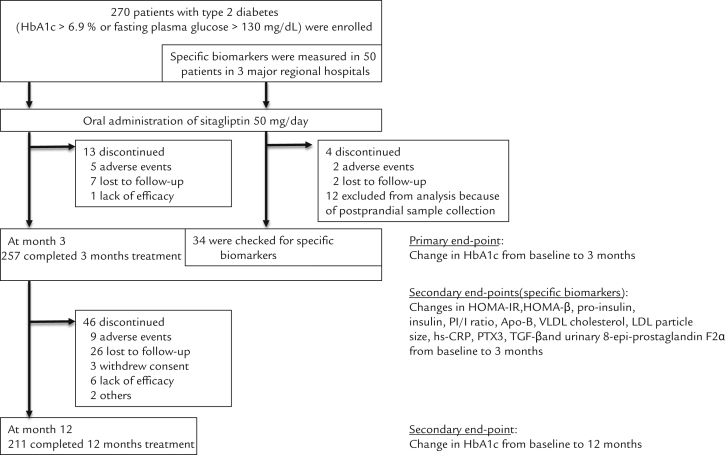
This open-label, central registration, multicenter, prospective, and pragmatic observational study (Sitagliptin Investigation on Glycemic Effects in Yokohama: SINGLE-Y) was carried out from March 2011 to April 2013 at 42 sites (39 general practitioners and 3 major regional hospitals) in Yokohama, Japan. Eligible study participants included adults (aged ≥20 years) with poorly controlled type 2 diabetes (glycated hemoglobin [HbA1c] >6.9% [52 mmol/mol] or fasting plasma glucose >130 mg/dL) receiving diet and exercise therapy over the course of 1 month or receiving oral glucose-lowering agents (eg, sulfonylureas, biguanides, and thiazolidinediones as monotherapies or in combination). The primary exclusion criteria included any patient with diabetes who had experienced diabetes-related ketoacidosis or diabetes-related coma within 6 months; patients with a severe infection, severe trauma, or planning any surgery; pregnant or breastfeeding women; and patients receiving treatment with insulin, glinides, or α-glucosidase inhibitors. All patients provided written informed consent. The medical expenses were paid by the participants and their insurance in accordance with normal clinical practice. The expenses for exploratory clinical tests were paid by the research funding. The present study was approved by the ethics committee at Yokohama Rosai Hospital, Saiseikai Yokohama-shi Tobu Hospital, and Showa University Fujigaoka Hospital.
Eligible patients were administered sitagliptin (50 mg/d) for 3 months. The doses of sulfonylureas were adjusted according to the recommendations of Japan Diabetes Society (glimepiride <2 mg/d, glibenclamide <1.25 mg/d, and gliclazide <40 mg/d). Doses of sitagliptin and other glucose-lowering agents were unchanged within the first 3 months; thereafter, changes in the dose of sitagliptin (25–100 mg/d) or other glucose-lowering agents were permitted. Changes in background medication such as antihypertension, antiplatelet, or lipid-lowering drugs were allowed in case of medical necessity.
Study end points
A flow chart of study participants throughout the study and end points are shown in Figure 1. The primary end point included changes in HbA1c from baseline to 3 months. The secondary end points were as follows: changes in HbA1c from baseline to 12 months; changes in apolipoprotein B-48, VLDL cholesterol, particle size of LDL cholesterol, hs-CRP, PTX3, transforming growth factor-β, and urinary 8-epi-prostaglandin F2α, homeostasis model assessment of insulin resistance, homeostasis model assessment of β-cell function, plasma insulin concentrations, plasma proinsulin concentrations, and plasma PI/I ratios from baseline to 3 months. Secondary end point measurements were only performed at Yokohama Rosai Hospital, Saiseikai Yokohama-shi Tobu Hospital, and Showa University Fujigaoka Hospital. Postprandial samples were excluded from the analysis. In addition, lipid profiles, liver enzymes, kidney function, and uric acid levels were measured. The collected data included the patients׳ demographic characteristics, medical histories, anthropometric data, blood analysis for glycemic control parameters, all adverse events, and hypoglycemic events. These data were collected to the study center for data management from the patients׳ medical records by the investigators using a case report form that was anonymized. Laboratory analyses were done at clinical test companies; specifically, BML Corporation (Tokyo, Japan) or SRL Corporation (Tokyo, Japan) trusted by each hospital or clinic. Measurements of specific biomarkers were centrally carried out by SRL Corporation. At each visit, body weight, blood pressure, and pulse rate were measured using weighting scales and automated sphygmomanometers at each hospital or clinic. Safety profile and tolerability were assessed by each investigator throughout the study. Adverse events were defined as any untoward medical occurrence that did not necessarily have a causal relationship with the treatment, including abnormalities on clinical laboratory measurements. If participants experienced adverse events, all details (eg, date, intensity, duration, outcome, and relationship to the drug) were documented and reported to the principal investigator by each investigator. Investigators assessed medication adherence by asking patients directly.
Figure 1.
Flow chart of study participants and study end points throughout the study. Data include the number of study participants. HbA1c = glycated hemoglobin; HOMA-IR = homoeostasis model assessment of insulin resistance; HOMA-β = homoeostasis model assessment of β-cell function, PI/I = pro-insulin/insulin ratio; Apo-B = apolipoprotein B; hs-CRP = high sensitivity C-reactive protein; PTX3 = pentraxin-3; TGF-β = transforming growth factor-β; 8-epi-PGF2α = 8-epi-prostaglandin F2α.
Statistical analysis
Data are given as means (SD). Before the analysis, the data distribution was assessed by the Shapiro-Wilk test. Paired t tests or Wilcoxon signed-rank tests were used to compare the parameters before and after the observation period. One-way repeated measures ANOVA was used for comparison of values at baseline, after 3 months, and at 12 months. Pairwise associations were examined by Pearson’s correlation coefficient test. In all statistical analyses, P < 0.05 was considered statistically significant. JMP 12 software (SAS Institute Inc, Cary, NC) was used for all analyses.
Results
Baseline characteristics of the study participants
A total of 270 Japanese patients with type 2 diabetes (165 men and 105 women) met the criteria and participated in the present study. Characteristics at baseline were as follows: age 64.3 (12.4) years; body mass index 25.2 (4.3); plasma glucose 200.8 (70.7) mg/dL; HbA1c 8.1% (1.4%) (65 [16.9] mmol/mol); systolic and diastolic blood pressure 130 [16] mm Hg and 77 [11] mm Hg, respectively; total cholesterol 200 [37] mg/dL; HDL cholesterol 54.4 [14.0] mg/dL; LDL cholesterol 112 [39] mg/dL; triglycerides 166 [96] mg/dL; and estimated glomerular filtration rate 70.1 [21.5] mL/min/1.73 m2 (Table I).
Table I.
Baseline characteristics of the study participants.*
| Characteristic | Study values |
|---|---|
| Sex, n | |
| Male | 165 |
| Female | 105 |
| Age, y | 64.3 (12.4) |
| Body mass index | 25.2 (4.3) |
| Plasma glucose level, mg/dL | 200.8 (70.7) |
| Glycated hemoglobin | |
| % | 8.1 (1.4) |
| mmol/mol | 65 (16.9) |
| Fasting insulin, μU/mL | 16.2 (24.5) |
| Systolic blood pressure, mm Hg | 130 (16) |
| Diastolic blood pressure, mm Hg | 77 (11) |
| Total cholesterol level, mg/dL | 200 (37) |
| HDL cholesterol level, mg/dL | 54.4 (14.0) |
| LDL cholesterol level, mg/dL | 112 (39) |
| Triglyceride level, mg/dL | 166 (96) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 70.1 (21.5) |
| Smoking, % | 29.2 |
| Drinking, % | 42.2 |
| Hypertension, % | 52.6 |
| Dyslipidemia, % | 57.4 |
Data are presented as number, mean (SD), or percentage.
Primary end point
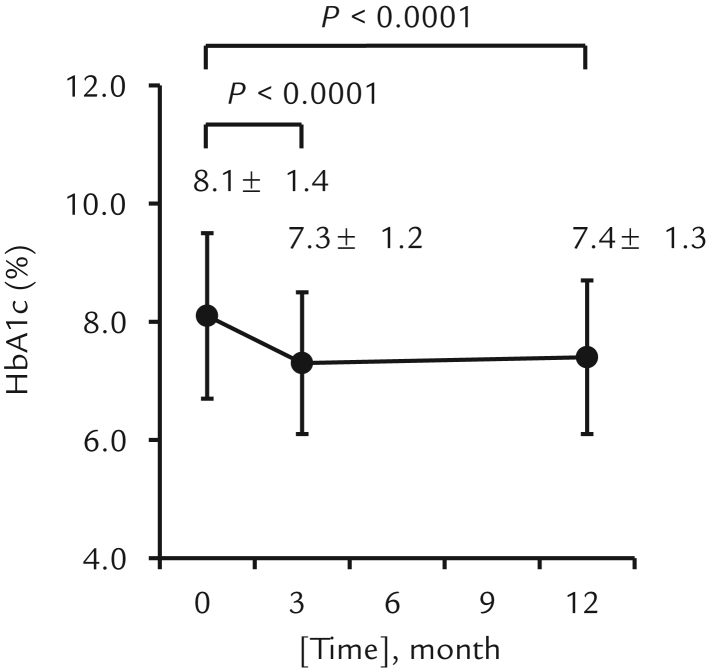
Patients receiving sitagliptin as a monotherapy or combination therapy had significantly lower HbA1c values 3 months after treatment than at baseline (8.1% [1.4%]–7.3% [1.2%]) (65 [16.9]–56 [13.1] mmol/mol) (P < 0.0001) (Figure 2). This glucose-lowering effect of sitagliptin was seen both in men (7.9% [1.3%]–7.2% [1.1%]) (55 [13.0]–63 [13.9] mmol/mol) (P < 0.0001) and women (8.4% [1.5%]–7.4% [1.2%]) (68 [16.9]–58 [13.3] mmol/mol) (P < 0.0001).
Figure 2.
Changes in glycated hemoglobin (HbA1c) from baseline to 3 and 12 months. Error bars represent SD.
Secondary end points
Patients receiving sitagliptin as a monotherapy or combination therapy had significantly lower HbA1c values 12 months after treatment than at baseline (8.1% [1.4%]–7.4% [1.3%]) (65 [16.9]–57 [15.1] mmol/mol; P < 0.0001) (Figure 2).
Changes in other secondary end points are summarized in Table II. Thirty-four patients were checked for specific biomarkers. After 3 months, patients treated with sitagliptin had significantly lower PTX3 levels than at baseline (1.88 [0.78]–1.63 [0.63] ng/mL; P = 0.0038). There was no significant correlation between the changes in PTX3 levels and the changes in HbA1c values (r = –0.07; P = 0.69). In addition, the changes in PTX3 levels were not correlated to HbA1c values at baseline (r = –0.19; P = 0.29). Therefore, it is assumed that sitagliptin treatment decreased PTX3 levels independent from the glycemic control. In contrast, the hs-CRP levels were unchanged with sitagliptin treatment. Changes in biomarkers related to lipid profiling such as VLDL cholesterol level, apolipoprotein-B 48 concentration, and LDL cholesterol particle size were not significant. Similarly, there were no significant differences in transforming growth factor-β, levels, a marker of cell proliferation, or urinary 8-epi-prostaglandin F2α levels, a marker of oxidative stress, with sitagliptin treatment.
Table II.
Changes in biomarkers related to atherogenic factors and β-cell function (N = 34).*
| Biomarker | Baseline | At 3 mo | P value (vs baseline) |
|---|---|---|---|
| Glycated hemoglobin | 0.0067 | ||
| % | 7.68 (1.08) | 7.17 (1.17) | |
| mmol/mol | 53 (26.4) | 44 (30.2) | |
| Apolipoprotein B-48, mg/mL | 5.81 (7.29) | 6.38 (7.88) | 0.5 |
| VLDL cholesterol level, mg/dL | 27.4 (8.8) | 25.6 (9.3) | 0.4 |
| LDL particle size, Å | 270 (4.7) | 268 (6.0) | 0.1 |
| High-sensitivity C-reactive protein, mg/dL | 0.118 (0.78) | 0.095 (0.103) | 0.2 |
| Pentraxin-3 level, ng/mL | 1.88 (0.78) | 1.63 (0.63) | 0.0038 |
| Transforming growth factor-β, ng/mL | 2.39 (2.76) | 1.85 (1.32) | 0.3 |
| urinary 8-epi-prostaglandin F2α, pg/mg creatinine | 331 (141) | 339 (171) | 0.7 |
| Homoeostasis model assessment of insulin resistance | 4.47 (4.66) | 7.64 (7.24) | 0.17 |
| Homoeostasis model assessment of β-cell function | 38.9 (26.1) | 82.9 (62.9) | 0.07 |
| Insulin level, pmol/L | 60.1 (49.9) | 59.3 (35.3) | 0.91 |
| Proinsulin level, pmol/L | 9.89 (5.79) | 8.37 (5.12) | 0.02 |
| Proinsulin/insulin ratio | 0.20 (0.14) | 0.17 (0.11) | 0.01 |
Data are presented as mean (SD).
In this study, we also investigated biomarkers related to β-cell function. Patients had significantly lower plasma proinsulin concentrations and PI/I ratios after sitagliptin treatment than at baseline (9.89 [5.79]–8.37 [5.12] pmol/L [P = 0.02] and 0.20 [0.14]– 0.17 [0.11] [P = 0.01], respectively). In contrast, changes in plasma insulin concentrations, homoeostasis model assessment of β-cell function, and homoeostasis model assessment of insulin resistance, a marker of insulin resistance, were unchanged. Changes in other observations are shown in Table III. No significant change in body weight was observed. Statistically significant changes were observed in diastolic blood pressure, total cholesterol level, HDL cholesterol level, serum creatinine level, and uric acid level after sitagliptin treatment.
Table III.
Changes in other observations.*
| Observation | Baseline | At 3 mo | At 12 mo |
|---|---|---|---|
| Body weight, kg | 65.5 (13.7) | 64.9 (13.3) | 65.3 (13.7) |
| Systolic blood pressure, mm Hg | 129.5 (16.2) | 127.1 (13.4) | 127.4 (13.4) |
| Diastolic blood pressure, mm Hg† | 76.6 (11.2) | 74.2 (9.7) | 74.2 (10.2) |
| Pulse rate, bpm | 74.9 (13.6) | 73.6 (12.8) | 73.1 (11.1) |
| Total cholesterol level, mg/dL† | 200.1 (37.4) | 196.2 (41.1) | 192.4 (36.0) |
| LDL cholesterol level, mg/dL | 111.8 (30.2) | 107.8 (26.4) | 111.2 (27.5) |
| HDL cholesterol level, mg/dL† | 54.4 (13.9) | 53.9 (13.8) | 53.5 (14.1) |
| Triglyceride level, mg/dL | 166.0 (92.4) | 157.3 (90.6) | 152.4 (87.2) |
| Aspartate aminotransferase level, U/L | 26.1 (13.1) | 25.2 (11.8) | 25.2 (10.8) |
| Alanine aminotransferase level, U/L | 30.2 (21.6) | 28.0 (18.2) | 27.3 (17.2) |
| γ-glutamyltranspeptidase level, U/L | 53.4 (67.8) | 50.6 (64.8) | 49.2 (72.6) |
| Total bilirubin level, mg/dL | 0.7 (0.4) | 0.6 (0.2) | 0.7 (0.4) |
| Blood urea nitrogen level, mg/dL | 15.9 (5.1) | 16.0 (4.9) | 16.4 (5.8) |
| Creatinine level, mg/dL† | 0.77 (0.23) | 0.78 (0.23) | 0.80 (0.25) |
| Uric acid level, mg/dL† | 5.2 (1.3) | 5.6 (1.3) | 5.5 (1.3) |
Data are presented as mean (SD).
P < 0.05 compared with baseline, after 3 months, and at 12 months as determined by 1-way repeated measures ANOVA.
Safety
Adverse events are summarized in Table IV. Adverse events were reported by 14 (5.18%) of 270 participants. Deaths occurred in 4 participants (attributable to cerebral infarction in 2 participants, sepsis in 1 participant, and gallbladder cancer in 1 participant). Patient withdrawals from the study were attributed to skin eruptions, flatulence, constipation, nausea, joint pain, arrhythmias, and renal dysfunction. There were no episodes of hypoglycemia observed in the present study.
Table IV.
Observed adverse events
| Type of adverse event | Result* |
|---|---|
| Any adverse event | 14 (5.18) |
| Cerebral infarction | 2 (0.74) |
| Sepsis | 1 (0.37) |
| Gallbladder cancer | 1 (0.37) |
| Skin eruption | 3 (1.11) |
| Flatulence | 2 (0.74) |
| Constipation | 1 (0.37) |
| Joint pain | 1 (0.37) |
| Nausea | 1 (0.37) |
| Arrhythmia | 1 (0.37) |
| Renal dysfunction | 1 (0.37) |
Data are presented as n (%).
Discussion
The present study clearly shows that sitagliptin significantly improves glycemic control in Japanese patients with newly diagnosed or poorly controlled type 2 diabetes. In addition, PTX3 and PI/I ratios were significantly lower after receiving sitagliptin treatment for 3 months than at baseline.
In the present study, sitagliptin had glucose-lowering effects as determined by HbA1c values, which were approximately 0.7% lower at 12 months than at baseline. This finding is in agreement with that of recent clinical studies of sitagliptin20, 21 and other DPP-4 inhibitors.22, 23 Importantly, the reduction was achieved without weight gain or an increased risk of hypoglycemia. Sitagliptin exerts glucose-lowering effects with glucose dependency; thus, the risk of hypoglycemia is low unless used with high doses of sulfonylureas. In the present study, the doses of sulfonylureas were adjusted according to the recommendation of Japan Diabetes Society. These adjustments may have contributed to the absence of hypoglycemic events.
In the present study, PTX3 levels were significantly lower after treatment with sitagliptin for 3 months than at baseline. PTX3 is an acute inflammatory biomarker produced by peripheral tissues and reflects impaired vascular endothelial function.24 In contrast, CRP is produced mainly in the liver in response to stimulation of various cytokines, reflecting whole-body inflammation. The expression of PTX3 has been found to increase in patients with CVD.25, 26 In a model of acute myocardial infarction, PTX3-deficient mice showed exacerbated heart damage with higher circulating levels of interleukin-6 than wild-type mice. In addition, this phenotype was reserved by exogenous PTX3.27 Therefore, it is assumed that increased PTX3 may have protective effects against cardiac tissue damage. In diabetes patients, plasma PTX3 levels are positively correlated with the development of retinopathy, whereas hs-CRP levels are not.28 Another report29 showed that plasma PTX3 levels are associated with carotid intima-media thickness in type 1 diabetes patients, independent from glycemic control. Thus, PTX3 may be an accurate predictor of vascular inflammation in patients with diabetes. To our knowledge, the present study is the first to show a decrease in PTX3 levels attributable to treatment with DPP-4 inhibitors. No previous study was identified through a search of Medline or Embase to December 2016 using the key words dipeptidyl peptidase-4 inhibitors and pentraxin-3. PTX3 seems to be an early predictive marker for the antiatherosclerotic effects of DPP-4 inhibitors.
Three recent randomized clinical studies showed that DPP-4 inhibitors neither reduced nor increased the risk of cardiovascular events in patients with type 2 diabetes with a history of CVD or at high risk for CVD.20, 22, 23 These results cannot exclude possible benefits of DPP-4 inhibitors for CVD because there were careful regulations of risk factors with statins, antiplatelet drugs, and/or antihypertensive drugs in a large proportion of those patients. In rodent studies, treatment with DPP-4 inhibitors reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice11 and LDL receptor-deficient mice.12 In human studies, intima-media thickness progression was inhibited to a greater extent in patients receiving sitagliptin for 12 months than that of diet controlled patients with coronary artery disease and impaired glucose tolerance.30 Therefore, early and effective glycemic control with DPP-4 inhibitors may reduce the incidence of CVD in the long term.
The present study showed a decrease in PI/I ratios with sitagliptin treatment, suggesting a protective effect on β-cell function. An elevated PI/I ratio reflects β-cell dysfunction.31, 32 Previous basic studies showed protective effect of DPP-4 inhibitors on β cells. Mu et al33 reported that β-cell mass and β-cell to α-cell mass were higher in high-fat diet and streptozotocin-induced mice with diabetes receiving sitagliptin treatment than that of placebo, whereas glipizide had no effect. GLP-1 has also been shown to increase β-cell mass in rodents by promoting β-cell replication and differentiation of β-cell precursors and by inhibiting apoptosis.34, 35Although molecular mechanisms by which these incretin hormones promote β-cell proliferation or inhibit apoptosis are still not fully understood, it is likely that the protective effects of DPP-4 inhibitors are attributable to increased circulating incretin hormones. Use of DPP-4 inhibitors may be an effective strategy for protecting β-cell function in patients with type 2 diabetes, especially with impaired insulin secretory defects commonly seen in Asians.
The present study has some limitations. First, we did not include a placebo group for comparison purposes. Second, the number of participants enrolled was small and the treatment period of 3 months may have been too short. In particular, measurements of biomarkers related to β-cell function and atherogenic factors, including PI/I ratio and PTX3, were performed at only 3 major regional hospitals, which further limited the number of patients included in the statistical analysis. Larger, placebo-controlled studies with longer follow-up periods are required. Third, we cannot all exclude the potential influence of dropouts on results, because we used paired t tests or Wilcoxon signed-rank tests without the missing data of dropouts in the analysis in this pragmatic observational study.
Conclusions
The present study suggests that sitagliptin treatment was associated with improved glycemic control in this small, select group of Japanese patients with newly diagnosed or poorly controlled type 2 diabetes. In addition, sitagliptin may have pleiotropic effects on vascular inflammation and β-cell function. Treatment with sitagliptin may be a useful strategy for Japanese patients with type 2 diabetes for reducing the risk of CVD and preserving β-cell function.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
This study was supported by a grant from the Waksman Foundation of Japan. The authors thank Shogo Shishikura, MPH, of Teikyo University Graduate School of Public Health, for his advice from an academic viewpoint, including references for revising the manuscript. The authors also thank the Sitagliptin Investigation on Glycemic Effects in Yokohama investigators for participating in this study and the clinical staff who participated in this study.
List of investigators: Matsuo Taniyama, Chiho Sugisawa, Rie Tadokoro, Toru Iizaka, Yasuyoshi Takahashi, Go Koizumi, Fumiko Otsuka, Shotaro Sato, Mariko Higa, Hiromi Ouchi, Takamasa Ichijo, Eiko Yoshida, Futoshi Ebara, Yoko Murai, Akira Honda, Masahiko Namiki, Kosuke Azuma, Sumio Azuma, Jun Ishibashi, Tetsuhiko Tsumuraya, Hikaru Yagi, Yasushi Ogihara, Ryohei Mikura, Sadao Kawase, Takashi Iizuka, Sigeki Akabane, Satoshi Hashimono, Toshihiro Yamazaki, Keiko Arai, Makoto Hasegawa, Toshiko Chino, Nobuyoshi Ishii, Manabu Wakakura, Masashi Uesato, Makoto Takase, Shinichiro Asaki, Hiroshi Yamada, Kosuke Minamisawa, Yoshiro Suzuki, Eiichi Arita, Hideki Tatsuta, Hiromi Himeno, Yoko Matsuzawa, Jun Saito, Masao Omura, Yuya Tsurutani, and Tetsuo Nishikawa.
M.O. and T.N. designed and conducted the study. Y.T. analyzed the data, interpreted the results and wrote the manuscript. Y.M., J.S., M.H and M.T. contributed to data acquisition and discussion.
References
- 1.Haffner S.M., Lehto S., Rönnemaa T., Pyörälä K., Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Stratton I.M., Adler A.I., Neil H.A., Matthews D.R., Manley S.E., Cull C.A. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschner P., Kipnes M.S., Lunceford J.K., Sanchez M., Mickel C., Williams-Herman D.E. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637. doi: 10.2337/dc06-0703. 29/12/2632 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Arnolds S., Dellweg S., Clair J., Dain M.P., Nauck M.A., Rave K. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509–1515. doi: 10.2337/dc09-2191. dc09-2191 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamoto Y., Taniguchi T., Nonaka K., Okamoto T., Okuyama K., Arjona Ferreira J.C. Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J. 2010;57:383–394. doi: 10.1507/endocrj.k09e-272. JST.JSTAGE/endocrj/K09E-272 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Takahara M., Shiraiwa T., Kaneto H., Katakami N., Matsuoka T.A., Shimomura I. Efficacy of sitagliptin on blood glucose fluctuation in Japanese type 2 diabetic patients with basal-supported oral therapy. Endocr J. 2012;59:1131–1136. doi: 10.1507/endocrj.ej12-0220. DN/JST.JSTAGE/endocrj/EJ12-0220 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Kohro T., Yamazaki T., Sato H., Harada K., Ohe K., Komuro I. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J. 2013;54:93–97. doi: 10.1536/ihj.54.93. [DOI] [PubMed] [Google Scholar]
- 8.Wei Y., Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. 0014-5793(94)01430-9 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Arakawa M., Mita T., Azuma K., Ebato C., Goto H., Nomiyama T. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. db09-1694 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vittone F., Liberman A., Vasic D., Ostertag R., Esser M., Walcher D. Sitagliptin reduces plaque macrophage content and stabilises arteriosclerotic lesions in Apoe (-/-) mice. Diabetologia. 2012;55:2267–2275. doi: 10.1007/s00125-012-2582-5. [DOI] [PubMed] [Google Scholar]
- 11.Matsubara J., Sugiyama S., Sugamura K., Nakamura T., Fujiwara Y., Akiyama E. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012;59:265–276. doi: 10.1016/j.jacc.2011.07.053. S0735-1097(11)04630-4 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Shah Z., Kampfrath T., Deiuliis J.A., Zhong J., Pineda C., Ying Z. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. CIRCULATIONAHA.111.041418 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu J., Woods J., Zhou Y.P., Roy R.S., Li Z., Zycband E. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes. 2006;55:1695–1704. doi: 10.2337/db05-1602. 55/6/1695 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Takeda Y., Fujita Y., Honjo J., Yanagimachi T., Sakagami H., Takiyama Y. Reduction of both beta cell death and alpha cell proliferation by dipeptidyl peptidase-4 inhibition in a streptozotocin-induced model of diabetes in mice. Diabetologia. 2012;55:404–412. doi: 10.1007/s00125-011-2365-4. [DOI] [PubMed] [Google Scholar]
- 15.Pospisilik J.A., Martin J., Doty T., Ehses J.A., Pamir N., Lynn F.C. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes. 2003;52:741–750. doi: 10.2337/diabetes.52.3.741. [DOI] [PubMed] [Google Scholar]
- 16.Ahren B., Winzell M.S., Wierup N., Sundler F., Burkey B., Hughes T.E. DPP-4 inhibition improves glucose tolerance and increases insulin and GLP-1 responses to gastric glucose in association with normalized islet topography in mice with beta-cell-specific overexpression of human islet amyloid polypeptide. Regul Pept. 2007;143:97–103. doi: 10.1016/j.regpep.2007.03.008. S0167-0115(07)00087-0 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Yoon K.H., Lee J.H., Kim J.W., Cho J.H., Choi Y.H., Ko S.H. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. S0140-6736(06)69703-1 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Fukushima M., Suzuki H., Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pr. 2004;66(Suppl 1):S37–S43. doi: 10.1016/j.diabres.2003.11.024. S0168-8227(04)00160-3 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Tripathy D., Carlsson M., Almgren P., Isomaa B., Taskinen M.R., Tuomi T. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes. 2000;49:975–980. doi: 10.2337/diabetes.49.6.975. [DOI] [PubMed] [Google Scholar]
- 20.Green J.B., Bethel M.A., Armstrong P.W., Buse J.B., Engel S.S., Garg J. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015 doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 21.Nonaka K., Kakikawa T., Sato A., Okuyama K., Fujimoto G., Kato N. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79:291–298. doi: 10.1016/j.diabres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 22.White W.B., Cannon C.P., Heller S.R., Nissen S.E., Bergenstal R.M., Bakris G.L. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 23.Scirica B.M.M.M., Bhatt D.L.L.L., Braunwald E., Steg P.G.G.G., Davidson J., Hirshberg B. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 24.Inoue K., Kodama T., Daida H. Pentraxin 3: a novel biomarker for inflammatory cardiovascular disease. Int J Vasc Med. 2012;2012:657025. doi: 10.1155/2012/657025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peri G., Introna M., Corradi D., Iacuitti G., Signorini S., Avanzini F. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K., Sugiyama A., Reid P.C., Ito Y., Miyauchi K., Mukai S. Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arter Thromb Vasc Biol. 2007;27:161–167. doi: 10.1161/01.ATV.0000252126.48375.d5. 01.ATV.0000252126.48375.d5 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Salio M., Chimenti S., De Angelis N., Molla F., Maina V., Nebuloni M. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. CIRCULATIONAHA.107.749234 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Yang H.S., Woo J.E., Lee S.J., Park S.H., Woo J.M. Elevated plasma pentraxin 3 levels are associated with development and progression of diabetic retinopathy in korean patients with type 2 diabetes mellitus. Invest Ophthalmol Vis Sci. 2014;55:5989–5997. doi: 10.1167/iovs.14-14864. [DOI] [PubMed] [Google Scholar]
- 29.Katakami N., Kaneto H., Sakamoto F., Takahara M., Irie Y., Fujisawa K. Plasma pentraxin 3 levels are associated with carotid IMT in type 1 diabetic patients. Diabetes Res Clin Pract. 2013;99:185–191. doi: 10.1016/j.diabres.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa S., Shimano M., Watarai M., Koyasu M., Uchikawa T., Ishii H. Impact of sitagliptin on carotid intima-media thickness in patients with coronary artery disease and impaired glucose tolerance or mild diabetes mellitus. Am J Cardiol. 2014;114:384–388. doi: 10.1016/j.amjcard.2014.04.050. S0002-9149(14)01117-5 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Roder M.E., Porte D., Jr., Schwartz R.S., Kahn S.E. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:604–608. doi: 10.1210/jcem.83.2.4544. [DOI] [PubMed] [Google Scholar]
- 32.Roder M.E., Dinesen B., Hartling S.G., Houssa P., Vestergaard H., Sodoyez-Goffaux F. Intact proinsulin and beta-cell function in lean and obese subjects with and without type 2 diabetes. Diabetes Care. 1999;22:609–614. doi: 10.2337/diacare.22.4.609. [DOI] [PubMed] [Google Scholar]
- 33.Mu J., Petrov A., Eiermann G.J., Woods J., Zhou Y.P., Li Z. Inhibition of DPP-4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur J Pharmacol. 2009;623:148–154. doi: 10.1016/j.ejphar.2009.09.027. S0014-2999(09)00793-6 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Drucker D.J. Glucagon-like peptide-1 and the islet beta-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–5148. doi: 10.1210/en.2003-1147. [pii] [DOI] [PubMed] [Google Scholar]
- 35.Perfetti R., Hui H. The role of GLP-1 in the life and death of pancreatic beta cells. Horm Metab Res. 2004;36:804–810. doi: 10.1055/s-2004-826167. [DOI] [PubMed] [Google Scholar]