Abstract
Background
Although antithrombotic agents are widely used for cardiac and cerebrovascular disease prevention, they increase the risk of gastrointestinal (GI) bleeding.
Objective
To examine GI bleeding risk in association with an esophagogastroduodenoscopy (EGD) biopsy performed in patients without cessation of antithrombotic therapy.
Methods
This study was prospectively conducted at 14 centers. EGD biopsies were performed in patients receiving antithrombotic agents without cessation, as well as age- and sex-matched controls not receiving antithrombotic therapy. Patients treated with warfarin before the biopsy had a prothrombin time-international normalized ratio level <3.0. The proportion of GI bleeding events was compared between the groups.
Results
The patient group (n = 277) underwent a total of 560 biopsies while continuing antithrombotic therapy, of whom 24 were receiving multiple antiplatelet drugs, and 9 were receiving both antiplatelet and anticoagulant agents. The control patients (n = 263) underwent 557 biopsies. The upper-GI bleeding rate within 30 days after the EGD biopsy did not increase in patients without cessation of antithrombotic treatment, regardless of receiving single or multiple antithrombotic agents.
Conclusions
We found no significant increase in upper-GI bleeding risk following an EGD biopsy in patients taking antithrombotic agents, suggesting its safety without the need for antithrombotic treatment interruption.
Key words: antiplatelet drug, anticoagulant drug, esophagogastroduodenoscopy, biopsy, gastrointestinal bleeding
Introduction
Antithrombotic agents have important roles for medical treatment and prevention of various diseases. Of those, antiplatelet drugs such as low-dose aspirin and clopidogrel are used for prevention of cerebrovascular disease and coronary arterial disease.1, 2, 3, 4 On the other hand, anticoagulant drugs, including warfarin and direct oral anticoagulants (DOACs) are given for a variety of clinical conditions, such as prevention of recurrent venous thromboembolism and stroke in patients with atrial fibrillation.5, 6, 7, 8 Thus, antithrombotic agents have been commonly used in recent years as both primary and secondary drugs to prevent cerebrovascular and coronary diseases in the expanding elderly population.
Although use of antithrombotic agents contributes to prevention of various vascular diseases, patients treated with those are at greater risk of gastrointestinal (GI) bleeding.9, 10, 11, 12, 13, 14, 15 Therefore, diagnostic and therapeutic endoscopic procedures should be performed under consideration of bleeding risk. Indeed, cessation of antithrombotic drugs before an endoscopic examination is thought to reduce the risk of GI bleeding.12, 13, 16, 17, 18, 19 However, thrombosis caused by cessation of those drugs is closely associated with more serious complications including increased mortality.
Several retrospective studies have shown that an endoscopic biopsy is not related to increased GI bleeding in patients who continue to use antithrombotic drugs.20, 21, 22, 23 In addition, endoscopic biopsy procedures have been safely performed in patients taking warfarin by appropriately controlling the prothrombin time-international normalized ratio (PT-INR).24, 25 As a result, recent guidelines for gastrointestinal endoscopy do not recommend cessation of antithrombotic drugs before an endoscopic biopsy.26, 27 However, evidence thoroughly showing the safety of an endoscopic biopsy in patients taking antithrombotic agents remains insufficient. To clearly elucidate this issue, we performed the present multicenter prospective observational study to determine bleeding risk in patients undergoing antithrombotic treatment according to guidelines presented by the Japan Gastroenterological Endoscopy Society (JGES).27
Materials and Methods
This study was prospectively conducted from January 2013 to August 2014 at 14 centers (2 university hospitals, 12 general hospitals) in Japan. The study protocol was approved by the Shimane University Institutional Committee on Ethics as well as by the ethics committee of each institution (UMIN000013520). Informed consent was obtained from all patients. All endoscopic biopsy procedures performed in patients receiving antithrombotic agents were carried out according to the JGES guidelines.27
Patients being treated at the participating hospitals who received antiplatelet (single or multiple) and/or anticoagulant agents, and who underwent a diagnostic esophagogastroduodenoscopy (EGD) biopsy during the study period were enrolled. The EGD examinations and biopsy procedures were performed by well-trained endoscopy specialists at each hospital who carefully observed the upper-GI tract of their patients and sometimes used magnifying endoscopy for diagnosis when necessary to avoid an unnecessary biopsy. All biopsies were carefully performed using forceps for various reasons, including the presence of symptoms (heartburn, dysphagia, epigastralgia, and discomfort), screening or surveillance of cancer, and closer examination of cancer before endoscopic or surgical therapy. The actual number of samples obtained was used as the number of biopsy procedures in each case. PT-INR level was measured in each patient receiving warfarin within 7 days before EGD and a diagnostic biopsy procedure was permitted when that value was <3.0. Patients administered DOACs received those drugs on the day of the EGD examination and biopsy procedure. Emergency EGD cases as well as patients with cessation of antithrombotic drugs before the EGD biopsy were excluded from the study. Patients not receiving antithrombotic therapy and who underwent a diagnostic EGD biopsy procedure were enrolled as control patients. We attempted to match the control group by age (±5 years) and gender with the patients receiving antithrombotic agents at a 1 to 1 ratio. After confirming hemostasis at the biopsy site, the endoscope was removed. The primary outcome of this study was incidence of upper-GI bleeding following an EGD biopsy. Patients who came to the emergency room or outpatient clinic for symptoms such as tarry stool or hematemesis within 30 days after the EGD biopsy underwent a blood test, and those with upper-GI bleeding were confirmed by the presence of anemia.
Statistical analyses were conducted using a χ2 test, with a P value < 0.05 considered to be significant. All calculations were performed using SPSS version 19.0 for Windows (IBM-SPSS Inc, Armonk, New York).
Results
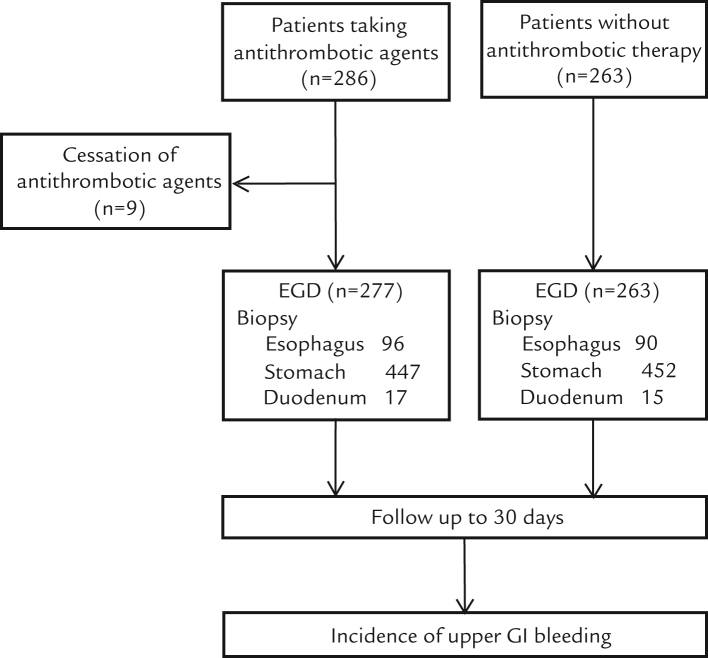
A flow chart of the study protocol is presented in Figure 1. We initially enrolled 286 patients who were taking antithrombotic agents, of whom 9 were excluded from analysis due to cessation of antithrombotic agents before EGD. In addition, 263 patients not receiving antithrombotic therapy were enrolled as control patients. The number in the control group was less than that of patients taking antithrombotic agents, because appropriate age- and sex-matched patients could not be found at several of the participating institutions during the study period.
Figure 1.
Flow chart of the study protocol.
Patient characteristics are shown in Table I. There were no significant differences in regard to gender or average age between the groups. Details of the antithrombotic drugs used are shown in Table I. As for anticoagulant agents, warfarin (n = 45) and DOACs (dabigatran, n = 12 and rivaroxaban, n = 7) were given, whereas low-dose aspirin (100 mg, n = 122 and 81 mg, n = 7), clopidogrel (n = 37), ethyl icosapentate (n = 21), limaprost alfadex (n = 20), and cilostazol n = 17) were frequently given as antiplatelet agents. Twenty-four patients were receiving multiple antiplatelet drugs (clopidogrel and aspirin together, n = 5), and 9 were administered both antiplatelet and anticoagulant agents. The rates of proton pump inhibitor (PPI) and histamine H2-receptor antagonist (H2-blocker) use were significantly higher in patients receiving antithrombotic agents compared with the control group.
Table I.
Patient characteristics.*
| Taking antithrombotic agents without cessation | No current antithrombotic therapy | P value | |
|---|---|---|---|
| No. | 277 | 263 | – |
| Gender, male/female | 192/85 | 187/76 | 0.719 |
| Age, y | 73.9 (8.6) | 72.6 (8.1) | 0.074 |
| Antithrombotic agent | |||
| Anticoagulant agent (n = 64) | |||
| Warfarin | 45 | – | – |
| Dabigatran | 12 | – | – |
| Rivaroxaban | 7 | – | – |
| Antiplatelet agent | |||
| Low-dose aspirin | – | – | |
| 100 mg | 122 | ||
| 81 mg | 7 | ||
| Clopidogrel | 37 | – | – |
| Ethyl icosapentate | 21 | – | – |
| Limaprost alfadex | 20 | – | – |
| Cilostazol | 17 | – | – |
| Dipyridamole | 4 | – | – |
| Ticlopidine hydrochloride | 3 | – | – |
| Sarpogrelate hydrochloride | 3 | – | – |
| Ifenprodil tartrate | 3 | – | – |
| Others | 9 | – | – |
| Multiantiplatelet users† | 24 | – | – |
| Antiplatelet and anticoagulant agent users | 9 | – | – |
| Antiacid agent | |||
| Proton pump inhibitor | 150 | 75 | <0.001 |
| Histamine H2-receptor antagonist | 41 | 17 | 0.003 |
| No. of biopsies | |||
| Esophagus | 96 | 90 | 0.950 |
| Stomach | 447 | 452 | 0.359 |
| Duodenum | 17 | 15 | 0.863 |
Values for age are given as mean (SD).
Clopidogrel and aspirin together (n = 5).
The total number of biopsy procedures performed in the group of patients who were receiving antithrombotic agents was 560 (esophagus, n = 96; stomach, n = 447; and duodenum, n = 17) and in the control group that number was 557 (esophagus, n = 90; stomach, n = 452; and duodenum, n = 15). During the follow-up period (within 30 days after the EGD biopsy), we did not find upper-GI bleeding in either group (Table II), although thrombosis was observed in an elderly female patient taking warfarin for treatment of atrial fibrillation and diabetes mellitus. She was hospitalized for a cardiogenic cerebral embolism at 25 days after the biopsy, then recovered with appropriate treatment and was discharged from the hospital on day 6 after admission.
Table II.
Major bleeding and thrombosis within 30 days after esophagogastroduodenoscopy biopsy.
| Patients taking antithrombotic agents without cessation | Patients without antithrombotic therapy | |
|---|---|---|
| Major bleeding | 0 | 0 |
| Thrombosis | 1* | 0 |
Female patient taking warfarin for treatment of atrial fibrillation and diabetes mellitus.
Discussion
In the present multicenter, prospective, observational study, we investigated the risk of upper-GI bleeding following an EGD biopsy procedure in patients taking antithrombotic agents as well as control group patients without antithrombotic therapy. Our results indicated that use of single or multiple antiplatelet and/or anticoagulant drugs does not increase upper-GI bleeding within 30 days after an EGD biopsy.
When an endoscopic biopsy is performed in patients who have stopped antithrombotic therapy, thromboembolism is a more serious complication and draws more attention than hemorrhage.12, 13, 16, 17, 18, 19 Thus, it is considered important to elucidate the safety of an endoscopic biopsy in patients without cessation of that therapy. Previous studies regarding management of antithrombotic agents in patients undergoing an invasive endoscopic procedure, such as biopsy, polypectomy, endoscopic mucosal resection, or endoscopic submucosal dissection, have revealed a low prevalence of GI bleeding events regardless of antithrombotic therapy.20, 21 More recently, a study that focused on the EGD biopsy procedure showed that upper-GI bleeding risk was not increased in patients without cessation of antithrombotic therapy.22 However, those results were obtained from retrospective studies or investigations conducted by a single center or without a control group.
Although several reports have indicated that aspirin alone in standard doses does not increase the rate of bleeding after an endoscopic biopsy,28, 29, 30 few prospective studies have appropriately evaluated the safety of antithrombotic therapy in patients undergoing an EGD biopsy. Ono et al28 investigated hemostasis as well as late-phase bleeding risk in patients taking antithrombotic agents up to 2 weeks after an EGD biopsy, and found a low level of risk for either event in patients who continued antithrombotic therapy. However, that was a single-arm study conducted at a single center. Another prospective study also demonstrated that continuation of antithrombotic agents did not increase bleeding risk after an EGD biopsy.29 Nevertheless, although that was a multicenter investigation, several factors, including age and gender, were different between the comparison groups.
To thoroughly verify the safety of continuing antithrombotic therapy in association with an EGD biopsy, we designed a prospective, multicenter study and enrolled patients who continued antithrombotic therapy during the procedure (n = 277, 560 biopsy procedures), as well as an age- and sex-matched control group who did not receive antithrombotic agents (n = 263, 557 procedures). Our findings clearly showed that hemostasis and late-phase bleeding for up to 30 days after the EGD biopsy did not increase in the patients without cessation of antithrombotic agents regardless of biopsy site, indicating the safety of this procedure while continuing to receive antithrombotic therapy.
Warfarin, an anticoagulant drug, is widely used for prevention of thrombosis and thromboembolism. Previous studies have revealed that bleeding risk in association with an EGD biopsy did not increase in patients with a PT-INR level within the therapeutic range (2.0–3.0).24, 25 In this regard, Western guidelines as well as those of the JGES currently recommend performance of an EGD biopsy procedure without cessation of warfarin within that therapeutic range.26, 27 In the present study, we investigated patients taking warfarin (PT-INR < 3.0) with or without antiplatelet agents, and found no increase in upper-GI bleeding following an EGD biopsy, which confirmed similar findings reported in recent studies. Thus, we consider that an EGD biopsy can be safely performed in patients taking warfarin alone or that combined with antiplatelet agents who have a controlled INR level. In addition to warfarin, DOACs have been developed as a new anticoagulant type.5, 6, 7, 8 In the present as well as other recent studies, the risk of upper-GI bleeding in association with an EGD biopsy has not been found to increase in patients who continued DOAC treatment. However, evidence in this regard remains insufficient.
There are several limitations to our study. First, the sample size was relatively small. Previous studies have indicated that upper-GI bleeding related to a biopsy procedure is rare regardless of current administration of antithrombotic agents27, 28, 29, 30; thus, studies with a much larger scale are necessary to thoroughly investigate the proportion of patients taking antithrombotic agents who experience that complication and its risk compared with a control group. Unfortunately, we were not able to conduct such an investigation with an appropriate sample size to fully evaluate the study purpose. Second, in the present study, we defined bleeding cases by confirming the presence of anemia in patients who came to the emergency room or outpatient clinic for symptoms such as tarry stool or hematemesis within 30 days after undergoing an EGD biopsy. Although possible symptoms in relation to upper-GI bleeding were carefully explained to the patients when they underwent the biopsy, we might not have been completely aware of various adverse events that occurred following that procedure. Furthermore, the proportions of patients who used PPIs and H2 blockers were higher among those receiving antithrombotic agents compared with the control patients in our study, although we did not find a higher incidence of peptic ulcers, upper-GI bleeding, or gastroesophageal reflux diseases in our patients taking those antacid agents. Because PPIs and H2 blockers are often used as antithrombotic agents for prevention of upper-GI bleeding in chronic cases, they may have had an influence on the incidence of upper-GI bleeding after the EGD biopsy in our cohort. A large-scale prospective study is needed to elucidate the preventive effect of antacids on GI bleeding in patients who have undergone an EGD biopsy.
Conclusions
Results of our prospective multicenter study showed no significant increase in the proportion of upper-GI bleeding complications following an EGD biopsy in patients receiving antithrombotic medication, suggesting the safety of such a low-risk procedure without the need to interrupt antithrombotic treatment.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
The authors thank the staff and patients who participated in this study. No funding source had involvement in the design, analysis, or writing of the manuscript. All authors designed the study protocol and carried out the research. Y. Takafumi, S. Ishihara and Y. Kinoshita performed the statistical analysis and wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Leon M.B., Baim D.S., Popma J.J., Gordon P.C., Cutlip D.E., Ho K.K., Giambartolomei A., Diver D.J., Lasorda D.M., Williams D.O., Pocock S.J., Kuntz R.E. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339:1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 2.Lansberg M.G., O׳Donnell M.J., Khatri P., Lang E.S., Nguyen-Huynh M.N., Schwartz N.E., Sonnenberg F.A., Schulman S., Vandvik P.O., Spencer F.A., Alonso-Coello P., Guyatt G.H., Akl E.A., American College of Chest Physicians Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e601S–e6636. doi: 10.1378/chest.11-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brighton T.A., Eikelboom J.W., Mann K., Mister R., Gallus A., Ockelford P., Gibbs H., Hague W., Xavier D., Diaz R., Kirby A., Simes J., ASPIRE Investigators Low-dose aspirin for preventing recurrent venous thromboembolism. N. Engl. J. Med. 2012;367:1979–1987. doi: 10.1056/NEJMoa1210384. [DOI] [PubMed] [Google Scholar]
- 4.Okada S., Morimoto T., Ogawa H., Sakuma M., Soejima H., Nakayama M., Sugiyama S., Jinnouchi H., Waki M., Doi N., Horii M., Kawata H., Somekawa S., Soeda T., Uemura S., Saito Y. Effect of low-dose aspirin on primary prevention of cardiovascular events in Japanese diabetic patients at high risk. Circ J. 2013;77:3023–3028. doi: 10.1253/circj.cj-13-0307. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Q., Lau Y.C., Senoo K., Lane D.A., Hong K., Lip G.Y. Non-vitamin K antagonist oral anticoagulants (NOACs) in patients with concomitant atrial fibrillation and heart failure: a systemic review and meta-analysis of randomized trials. Eur J Heart Fail. 2015;17:1192–1200. doi: 10.1002/ejhf.343. [DOI] [PubMed] [Google Scholar]
- 6.Rubboli A., Agewall S., Huber K., Lip G.Y. New-onset atrial fibrillation after recent coronary stenting: Warfarin or non-vitamin K-antagonist oral anticoagulants to be added to aspirin and clopidogrel? A viewpoint. Int J Cardiol. 2015;196:133–138. doi: 10.1016/j.ijcard.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Senoo K., Lau Y.C., Dzeshka M., Lane D., Okumura K., Lip G.Y. Efficacy and safety of non-vitamin K antagonist oral anticoagulants vs. warfarin in Japanese patients with atrial fibrillation – meta-analysis. Circ J. 2015;79:339–345. doi: 10.1253/circj.CJ-14-1042. [DOI] [PubMed] [Google Scholar]
- 8.Gómez-Outes A., Terleira-Fernández A.I., Calvo-Rojas G., Suárez-Gea M.L., Vargas-Castrillón E. Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups. Thrombosis. 2013;2013:640723. doi: 10.1155/2013/640723. Epub 2013 Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensen H.T., Mellemkjaer L., Blot W.J., Nielsen G.L., Steffensen F.H., McLaughlin J.K., Olsen J.H. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol. 2000;95:2218–2224. doi: 10.1111/j.1572-0241.2000.02248.x. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S., Zhao F., Mehta S.R., Chrolavicius S., Tognoni G., Fox K.K. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. 6. [DOI] [PubMed] [Google Scholar]
- 11.Hallas J., Dall M., Andries A., Andersen B.S., Aalykke C., Hansen J.M., Andersen M., Lassen A.T. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study. BMJ. 2006;333:726. doi: 10.1136/bmj.38947.697558.AE. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt D.L., Scheiman J., Abraham N.S., Antman E.M., Chan F.K., Furberg C.D., Johnson D.A., Mahaffey K.W., Quigley E.M., Harrington R.A., Bates E.R., Bridges C.R., Eisenberg M.J., Ferrari V.A., Hlatky M.A., Kaul S., Lindner J.R., Moliterno D.J., Mukherjee D., Schofield R.S., Rosenson R.S., Stein J.H., Weitz H.H., Wesley D.J., American College of Cardiology Foundation. American College of Gastroenterology. American Heart Association . Vol. 118. 2008. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents; pp. 1894–1909. (Circulation). [DOI] [PubMed] [Google Scholar]
- 13.Becker R.C., Scheiman J., Dauerman H.L., Spencer F., Rao S., Sabatine M., Johnson D.A., Chan F., Abraham N.S., Quigley E.M., American College of Cardiology and the American College of Gastroenterology Management of platelet-directed pharmacotherapy in patients with atherosclerotic coronary artery disease undergoing elective endoscopic gastrointestinal procedures. J Am Coll Cardiol. 2009;54:2261–2276. doi: 10.1016/j.jacc.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Antithrombotic Trialists׳ (ATT) Collaboration. Baigent C., Blackwell L., Collins R., Emberson J., Godwin J., Peto R., Buring J., Hennekens C., Kearney P., Meade T., Patrono C., Roncaglioni M.C., Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metz A.J., Bourke M.J., Moss A., Williams S.J., Swan M.P., Byth K. Factors that predict bleeding following endoscopic mucosal resection of large colonic lesions. Endoscopy. 2011;43:506–511. doi: 10.1055/s-0030-1256346. [DOI] [PubMed] [Google Scholar]
- 16.Palareti G., Legnani C., Guazzaloca G., Frascaro M., Grauso F., De Rosa F., Fortunato G., Coccheri S. Activation of blood coagulation after abrupt or stepwise withdrawal of oral anticoagulants–a prospective study. Thromb Haemost. 1994;72:222–226. [PubMed] [Google Scholar]
- 17.Wahl M.J. Dental surgery in anticoagulated patients. Arch. Intern. Med. 1998;158:1610–1616. doi: 10.1001/archinte.158.15.1610. [DOI] [PubMed] [Google Scholar]
- 18.Blacker D.J., Wijdicks E.F., McClelland R.L. Stroke risk in anticoagulated patients with atrial fibrillation undergoing endoscopy. Neurology. 2003;61:964–968. doi: 10.1212/01.wnl.0000086817.54076.eb. [DOI] [PubMed] [Google Scholar]
- 19.Wijeysundera D.N., Wijeysundera H.C., Yun L., Wąsowicz M., Beattie W.S., Velianou J.L., Ko D.T. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation. 2012;126:1355–1362. doi: 10.1161/CIRCULATIONAHA.112.102715. [DOI] [PubMed] [Google Scholar]
- 20.Ono S., Fujishiro M., Hirano K., Niimi K., Goto O., Kodashima S., Yamamichi N., Koike K. Retrospective analysis on the management of anticoagulants and antiplatelet agents for scheduled endoscopy. J Gastroenterol. 2009;44:1185–1189. doi: 10.1007/s00535-009-0127-6. [DOI] [PubMed] [Google Scholar]
- 21.Iwatsuka K., Gotoda T., Kusano C., Fukuzawa M., Sugimoto K., Itoi T., Kawai T., Moriyasu F. Clinical management of esophagogastroduodenoscopy by clinicians under the former guidelines of the Japan Gastroenterological Endoscopy Society for patients taking anticoagulant and antiplatelet medications. Gastric Cancer. 2014;17:680–685. doi: 10.1007/s10120-013-0333-z. [DOI] [PubMed] [Google Scholar]
- 22.Fujita M., Shiotani A., Murao T., Ishii M., Yamanaka Y., Nakato R., Matsumoto H., Tarumi K., Manabe N., Kamada T., Hata J., Haruma K. Safety of gastrointestinal endoscopic biopsy in patients taking antithrombotics. Dig Endosc. 2015;27:25–29. doi: 10.1111/den.12303. [DOI] [PubMed] [Google Scholar]
- 23.Shiffman ML, Farrel MT, Yee YS. Risk of bleeding after endoscopic biopsy or polypectomy in patientsaking aspirin or other NSAIDS. Gastrointest Endosc. 994;40:458-62. [DOI] [PubMed]
- 24.Gerson L.B., Triadafilopoulos G., Gage B.F. The management of anticoagulants in the periendoscopic period for patients with atrial fibrillation: a decision analysis. Am J Gastroenterol. 2000;95:1717–1724. doi: 10.1016/j.amjmed.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 25.Gerson L.B., Michaels L., Ullah N., Gage B., Williams L. Adverse events associated with anticoagulation therapy in the eridndoscopic period. Gastrointest Endosc. 2010;71:1211–1217. doi: 10.1016/j.gie.2009.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ASGE Standards of Practice Committee. Anderson M.A., Ben-Menachem T., Gan S.I., Appalaneni V., Banerjee S., Cash B.D., Fisher L., Harrison M.E., Fanelli R.D., Fukami N., Ikenberry S.O., Jain R., Khan K., Krinsky M.L., Lichtenstein D.R., Maple J.T., Shen B., Strohmeyer L., Baron T., Dominitz J.A. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060–1070. doi: 10.1016/j.gie.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto K., Fujishiro M., Kato M., Higuchi K., Iwakiri R., Sakamoto C., Uchiyama S., Kashiwagi A., Ogawa H., Murakami K., Mine T., Yoshino J., Kinoshita Y., Ichinose M., Matsui T., Japan Gastroenterological Endoscopy Society Guidelines for endoscopic management of non-variceal upper gastrointestinal bleeding. Dig Endosc. 2016;28:363–378. doi: 10.1111/den.12639. [DOI] [PubMed] [Google Scholar]
- 28.Ono S., Fujishiro M., Kodashima S., Takahashi Y., Minatsuki C., Mikami-Matsuda R., Asada-Hirayama I., Konno-Shimizu M., Tsuji Y., Mochizuki S., Niimi K., Yamamichi N., Kaneko M., Yatomi Y., Koike K. Evaluation of safety of endoscopic biopsy without cessation of antithrombotic agents in Japan. J Gastroenterol. 2012;47:770–774. doi: 10.1007/s00535-012-0538-7. [DOI] [PubMed] [Google Scholar]
- 29.Ara N., Iijima K., Maejima R., Kondo Y., Kusaka G., Hatta W., Uno K., Asano N., Koike T., Imatani A., Shimosegawa T. Prospective analysis of risk for bleeding after endoscopic biopsy without cessation of antithrombotics in Japan. Dig Endosc. 2015;27:458–464. doi: 10.1111/den.12407. [DOI] [PubMed] [Google Scholar]
- 30.Whitson M.J., Dikman A.E., von Althann C., Sanyal S., Desai J.C., Bamji N.D., Kornacki S., Harpaz N., Bodian C.A., Cohen L.B., Miller K.M., Aisenberg J. Is gastroduodenal biopsy safe in patients receiving aspirin and clopidogrel a prospective, randomized study involving 630 biopsies. J Clin Gastroenterol. 2011;45:228–233. doi: 10.1097/MCG.0b013e3181eb5efd. [DOI] [PubMed] [Google Scholar]