Abstract
Background and Purpose
Anorexia nervosa (AN) is a serious psychiatric condition characterized by excessive body weight loss and disturbed perceptions of body shape and size, often associated with excessive physical activity. There is currently no effective drug‐related therapy of this disease and this leads to high relapse rate. Clinical data suggest that a promising therapy to treat and reduce reoccurrence of AN may be based on the use of drugs that target the endocannabinoid (EC) system, which appears dysregulated in AN patients.
Experimental Approach
The activity‐based anorexia (ABA) rodent model mimics the severe body weight loss and increased physical activity, as well as the neuroendocrine disturbances (i.e. hypoleptinaemia and hypercortisolaemia) in AN. This study investigated whether cannabinoid agonists can effectively modify anorexic‐like behaviours and neuroendocrine changes in rats subjected to a repeated ABA regime that mimics the human condition in which patients repeatedly undergo a recovery and illness cycle.
Key Results
Our data show that subchronic treatment with both the natural CB1/CB2 receptor agonist Δ9‐tetrahydrocannabinol and the synthetic CB1/CB2 receptor agonist CP‐55,940 significantly reduced body weight loss and running wheel activity in ABA rats. These behavioural effects were accompanied by an increase in leptin signalling and a decrease in plasma levels of corticosterone.
Conclusion and Implications
Taken together, our results further demonstrate the involvement of the EC system in AN pathophysiology and that strategies which modulate EC signalling are useful to treat this disorder, specifically in patients where physical hyperactivity plays a central role in its progression and maintenance.
Abbreviations
- AN
anorexia nervosa
- CB1 receptor
cannabinoid type‐1 receptor
- CP
CP‐55,940
- EC
endocannabinoid system
- THC
Δ9‐tetrahydrocannabinol
Introduction
Anorexia nervosa (AN) is a serious psychiatric condition characterized by a lower normal body weight as a consequence of rigid dietary restriction associated with an intense fear of weight gain due to disturbed perceptions of body shape and size (APA, 2013). In 80% of AN patients, physical hyperactivity also occurs in response to the need to control and maintain a reduced body weight, thereby contributing to the progression of the disorder (Davis et al., 1997; Gümmer et al., 2015; Achamrah et al., 2016). AN onset is typically in adolescence, affects more women than men and often is accompanied by significant psychiatric comorbidities, such as depression, anxiety and/or obsessive–compulsive disorder (Hudson et al., 2007; Mattar et al., 2012; Smink et al., 2012). Since there is currently no effective drug‐related therapy, AN has a low rate of recovery as a large percentage of patients relapse within 1 year of terminating a specialized treatment programme (Carter et al., 2004; Bergh et al., 2013). Consequently, AN tends to be chronic and disabling, with considerable tolls on physical and mental health (Marzola and Kaye, 2015; Mehler and Brown, 2015). Furthermore, AN has the highest mortality rate (~5.6%) of any mental illness (Arcelus et al., 2011; Keshaviah et al., 2014). Thus, discovery of molecular targets for the development of specific medications to treat and reduce its reoccurrence is of critical importance.
Clinical data suggest that a promising pharmacotherapy may be based on the use of drugs that target the endocannabinoid (EC) system, which appears dysregulated in AN patients. For example, elevated plasma levels of the EC anandamide, as well as increased cannabinoid CB1 receptor availability, were found in women with AN compared with healthy controls (Monteleone et al., 2005; Gérard et al., 2011). Moreover, heightened levels of CB1 receptor mRNA in the blood of AN patients further support the notion of impaired EC signalling in this disorder (Frieling et al., 2009). These effects are likely to represent a compensatory up‐regulation in order to compensate for a hypoactivity of the EC system in anorexic states (van der Stelt and Di Marzo, 2003; Di Marzo, 2008). The EC system has numerous physiological functions, including a crucial role for leptin in the regulation of appetite and energy balance by controlling both homeostatic and hedonic aspects of food intake mainly in the CNS (Di Marzo and Matias, 2005). Specifically, by activating the CB1 receptors expressed in brain regions involved in energy modulation (i.e. hypothalamus and mesocorticolimbic system), both endogenous and exogenous cannabinoids are capable of promoting food intake, modifying the release of orexigenic and anorexic mediators, as well as reinforcing hedonic valuation of food (Di Marzo and Matias, 2005; Jager and Witkamp, 2014).In contrast, specific antagonists at the CB1 receptor exert an opposite effect: they suppress food intake and reduce body weight in laboratory animals (Carai et al., 2006). Given the EC system's role in controlling feeding behaviour, it is not surprising that its dysregulation could be connected to the pathophysiology of AN, and that symptoms and progression can be attenuated by normalizing EC signalling through pharmacological treatments based on specific cannabinoids (Di Marzo, 2009).
Animal models are very useful for investigating neurobiological substrates and pharmacological determinants of human disorders, like AN (Casper et al., 2008). The activity‐based anorexia (ABA) rodent model is among the most commonly used for AN studies; animals undergo restricted feeding schedules (1–2 h·day−1) with free access to a running wheel (Routtenberg and Kuznesof, 1967; Routtenberg, 1968). These two factors, when applied simultaneously, model key aspects of human AN, specifically hyperactivity and reduced body weight as well as neuroendocrine disturbances such as dysregulation of appetite‐regulating hormones (i.e. decreased basal plasma levels of leptin) and activation of the hypothalamic–pituitary–adrenal axis (HPA; i.e. increased basal plasma levels of corticosterone) (Burden et al., 1993; Davis et al., 1997; Adan et al., 2011). However, animal models present some limitations since they can reproduce only some traits of the pathology. For example, psychological components such as obsessing over body weight and shape cannot be easily assessed in animals (Casper et al., 2008). Using this model, exposure to the natural CB1/CB2 receptor partial agonist Δ9‐tetrahydrocannabinol (THC) has been previously shown to attenuate the weight loss associated with the development of ABA (Verty et al., 2011). Moreover, ABA development has been associated with an in vivo increase in CB1 receptor availability in different brain areas (Casteels et al., 2014).
As mentioned before, AN tends to be chronic and patients repeatedly undergo a recovery and illness cycle, and the ABA model can also be used to investigate this occurrence by subjecting the animals to the effect of repeated ABA regime (Chowdhury et al., 2015; Aoki et al., 2017). The present study was performed to extend data published by Verty et al. (2011), investigating the effects of THC on weight loss and hyperactivity in rats exposed to a repeated ABA regime, to better assess the effectiveness of pharmacological treatments (Chowdhury et al., 2015; Aoki et al., 2017). For the first time, we evaluated the effects of the synthetic CB1/CB2 receptor agonist CP‐55,940 (CP) to compare the effects of a full cannabinoid receptor agonist in comparison with the partial agonist THC (Castaneto et al., 2014). In clinical reports neuroendocrine disturbances, such as hypoleptinaemia and hypercortisolaemia, have been proposed as diagnostic markers for AN (Misra and Klibanski, 2014), therefore, we decided to evaluate the effect of CB1/CB2 receptor agonists on plasma levels of both leptin and corticosterone.
Methods
Animals
Female Sprague Dawley rats (Envigo, Italy) weighing 125–150 g at the start of the study were used. Animals were housed in a climate‐controlled animal room (21 ± 2°C; 60% humidity) under a reversed 12 h light/12 h dark cycle (lights on at 12:00 h) and fed standard rat chow and water ad libitum. Young female rats were chosen because >90% of human anorexic patients are adolescent young women (Hudson et al., 2007). All procedures and experiments were carried out in an animal facility according to Italian (D.L. 26/2014) and European Council directives (63/2010) and in compliance with the approved animal policies by the Ethical Committee for Animal Experiments at the University of Cagliari (Sardinia, Italy) and the Italian Department of Health (286/2016). All possible efforts were made to minimize animal pain and discomfort, as well as reduce the number of experimental subjects. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Apparatus
Standard polycarbonate cages [48(h) × 32 (w) × 47(d) cm] or polycarbonate cages equipped with running wheels (35 cm in diameter) served has experimental chambers. Each activity wheel cage had a magnetic switch and LCD revolution counter; the switch continuously counted whole revolutions of the activity wheel (Ugo Basile, Varese, Italy).
Experimental design
After 1 week of acclimatization, rats were divided into four groups matched for initial body weight and then housed individually in standard home cages (sedentary rats) or home cages equipped with a running wheel (running rats): (1) ‘Control’ rats received 24 h food access but no access to the activity wheel; (2) ‘Restricted’ rats were allowed food access for 1.5 h·day−1 but no access to the activity wheel; (3) ‘Exercise’ rats received 24 h food and activity wheel access; (4) ‘ABA’ rats were allowed food for 1.5 h·day−1 and had 24 h activity wheel access.
Animals were adapted to their housing conditions for 7 days with ad libitum food and running wheel access (adaptation period). Body weight, food intake and running wheel activity (RWA) (where applicable) were recorded daily within 30 min of the start of the 12 h dark cycle in order to obtain a stable baseline (BL). After adaptation, ABA rats were exposed to two bouts of the ABA regime separated by 10 days of recovery (Figure 1) (Chowdhury et al., 2014; Aoki et al., 2017).
Figure 1.
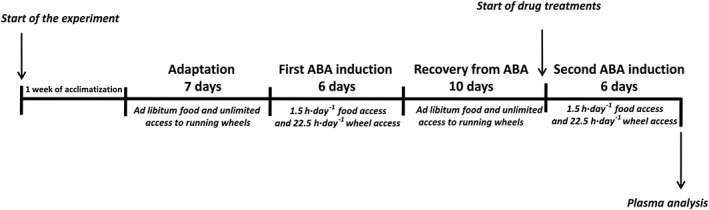
Schematic representation of the experimental design.
First ABA induction
On the last day of the adaptation period, at the onset of the 12 h dark cycle, food was removed from the cages of ABA rats and restricted access to food commenced. For ethical reasons, rats were not allowed to lose more than 22–25% of their initial body weight (Gutierrez, 2013). Therefore, in order to avoid a drop larger than 25%, rats were maintained on this restricted scheduled feeding for a maximum of 6 days (Verty et al., 2011). During each day of the restricted feeding schedule, rats were given unlimited access to food for 1.5 h at the onset of the 12 h dark phase. Access to running wheels was blocked during the 1.5 h feeding period to prevent RWA from competing with eating. Food consumption was measured by weighing the food before and after the 1.5 h access period. The same restricted‐feeding schedule was imposed on the Restricted group of rats, while the Control and Exercise groups of rats continued to receive ad libitum access to food for 24 h. In all four experimental groups, body weight and RWA (where applicable) were recorded daily within 30 min of the start of the 12 h dark cycle. At the same time, food intake was measured for Control and Exercise rats by weighing it every 24 h.
Recovery from ABA
On day 6 of the restricted feeding schedule and after the 1.5 h access to food, ABA and Restricted rats were allowed food ad libitum for 24 h for 10 days for weight restoration, while Control and Exercise rats continued to receive 24 h food access. During this recovery phase, Exercise and ABA rats had unlimited access to running wheels (Dixon et al., 2003; Ratnovsky and Neuman, 2011). In all experimental groups, body weight, food intake and RWA (where applicable) were recorded daily within 30 min of the start of the 12 h dark cycle.
Second ABA induction
At the end of the recovery phase (day 10), before the start of the 12 h dark cycle, animals from each experimental group (n = 42 rats per group) were randomly divided into subgroups according to their pharmacological treatment: (i) THC‐treated rats received either 0.0 (vehicle), 0.5 or 0.75 mg·kg−1 THC (n = 7 rats per dose); (ii) CP‐treated rats received either 0.0 (vehicle) 0.03 or 0.06 mg·kg−1 CP (n = 7 rats per dose). Drug doses were chosen based on preliminary experiments (we discarded doses that resulted in a decrease in spontaneous locomotor activity; data not shown) and according to the literature (Järbe and DiPatrizio, 2005; Dodd et al., 2009). Day 1 of drug treatment represented the start of the second ABA induction in which ABA rats were exposed to the ABA paradigm described above. During the second ABA induction, all rats from all experimental groups were injected daily for 6 days with either vehicle or drug 30 min before the onset of the 12 h dark phase. At the onset of the 12 h dark phase, ABA and Restricted rats were given unlimited access to food for 1.5 h, and food consumption was measured by weighing the food before and after the access period. Control and Exercise rats continued to receive 24 h food access. Running wheel access was blocked (where appropriate) during the feeding period as described above; otherwise, animals had unlimited access. Body weights and RWA were recorded daily just prior to drug injections. At the same time, food intake was measured for ad libitum‐fed rats.
Plasma analysis
All rats were killed at the end of the 12 h light phase on day 6 of the second ABA induction. Plasma leptin and corticosterone levels were measured using a commercially available ELISA kit according to the manufacturer's protocols (EZRL‐83K/Rat Leptin ELISA, EMD Millipore, St. Charles, Missouri, USA; Corticosterone Elisa Kit ADI‐900‐097, Enzo Life Sciences, Lausen, Switzerland). Trunk blood was collected into K3EDTA tubes, centrifuged at 3000× g for 15 min at 4 ± 2°C and then plasma was stored at −20°C for hormone analysis.
Statistical analysis
Body weight, food intake and RWA are presented as mean ± SEM and were analysed by two‐way ANOVA for repeated measures with two factors being groups as a between‐subjects factor and time (days) as within‐subjects factor and a repeated factor. Within each experimental group, body weight, food intake and RWA from pharmacologically‐treated rats are presented as the mean ± SEM and were analysed either by repeated measures two‐way ANOVA with treatment as a between‐subjects factor and time (days) as a within‐subjects and a repeated factor, or one‐way ANOVA with groups as a between‐subject factor. Plasma levels are presented as the mean ± SEM and were analysed by one‐way ANOVA with groups as a between‐subject factor. Post hoc comparisons were made with Newman–Keuls multiple comparison or Bonferroni tests, where appropriate. Analysis of results was carried out using Graph Pad Prism® 5 for Windows (Graph Pad software, USA). In all cases, differences with a P < 0.05 were considered significant. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Drugs
The natural CB1/CB2 receptor partial agonist THC (THC Pharm, Frankfurt am Main, Germany, 1 g·5 mL−1 in ethanol solution) and the synthetic CB1/CB2 receptor agonist CP (Tocris Bioscience, Bristol, UK) were dissolved in 2% Tween‐80, 2% ethanol and 96% saline. All drugs were injected i.p. in a volume of 1 mL·kg−1.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/2016 (Alexander et al., 2015).
Results
First ABA induction
With the restricted feeding schedule, body weight significantly decreased in both ABA and Restricted rats compared with Control and Exercise groups (Figure 2A). Two‐way ANOVA revealed a significant main effect of group × time interaction [F(18,984) = 269.91, P < 0.05]. Moreover, weight loss was more pronounced in ABA compared with Restricted rats starting at day 2 and progressively decreased to a total of ~21% loss. Restricted rats exhibited a significant but stable weight loss of 10%. Both Control and Exercise rats presented an increase in body weight with no significant difference between them. Due to the restricted‐feeding schedule, daily food intake was lower in the ABA and Restricted rats compared with ad libitum‐fed rats, and two‐way ANOVA revealed a significant main effect of group × time interaction [F(18,984) = 32.45, P < 0.05; Figure 2B]. Moreover, Exercise rats consumed significantly more food starting from day 4 than Control rats. The weight loss in ABA rats was accompanied by a concomitant and progressive increase in RWA which became significant from day 2 of the ABA protocol when compared with that of Exercise rats (Figure 2C). Two‐way ANOVA revealed a significant main effect of group × time interaction [F(6,492) = 23.56, P < 0.05]. On day 6, ABA rats displayed a 161% increase in RWA relative to the BL. In contrast, Exercise rats exposed to the running wheel without any food restriction showed only a modest but stable increase in RWA (+71% from BL).
Figure 2.
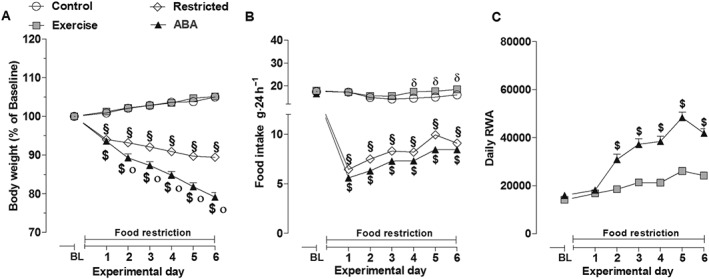
Body weight (A), food intake (B) and RWA (C) in Control, Exercise, Restricted and ABA groups during the 6 days of the first ABA induction. Results are presented as the mean ± SEM (n = 42 rats per group). Statistical analysis was performed by two‐way ANOVA followed by Bonferroni post hoc test (body weight: °P < 0.05 vs Restricted rats, $P < 0.05 and §P < 0.05 vs Exercise and Control rats; Food intake: $P < 0.05 and §P < 0.05 vs Exercise and Control rats; RWA: $ P < 0.05 vs Exercise rats).
Recovery from ABA
ABA rats recovered to baseline body weight within the first 4 days of the recovery phase (Figure 3A). Nonetheless, they weighed significantly less than the other main experimental groups at the end of this period, and two‐way ANOVA revealed a significant main effect of group × time interaction [F(30,1640) = 23.29, P < 0.05]. However, Restricted rats recovered their 10% weight loss within the first day of recovery, and by day 4, their body weight was not statistically different from that of ad libitum‐fed rats. There were no significant differences in body weight between Control and Exercise rats. With regard to daily food intake, two‐way ANOVA also revealed a significant main effect of group × time interaction [F(27,1476) = 7.09, P < 0.05; Figure 3B]. Food intake was significantly higher in ABA rats than the other groups. In contrast, Restricted rats returned to baseline levels of food intake by day 8 of recovery. Moreover, Exercise rats continued to consume significantly more food compared with Control rats. During the first 3 days of recovery, the RWA of ABA rats was significantly lower compared with Exercise rats, and two‐way ANOVA revealed a significant main effect of group × time interaction [F(9,738) = 14.45, P < 0.05; Figure 3C].
Figure 3.
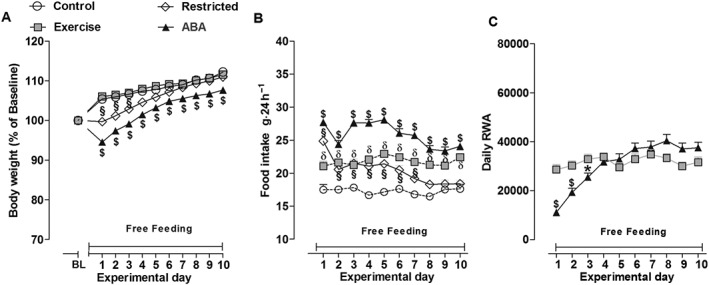
Body weight (A), food intake (B) and RWA (C) in Control, Exercise, Restricted and ABA groups during the 10 days of recovery from ABA. Results are presented as the mean ± SEM (n = 42 rats per group). Statistical analysis was performed by two‐way ANOVA followed by Bonferroni post hoc test (Body weight: $P < 0.05 vs Restricted, Exercise and Control rats, §P < 0.05 vs Exercise and Control rats; food intake: $P < 0.05 vs Restricted, Exercise and Control rats, §P < 0.05 and δP < 0.05 vs Control rats; RWA: $P < 0.05 vs Exercise rats).
Second ABA induction
Effect of THC administration
As shown in Figure 4, no significant main effect of treatment × time interaction was observed for the body weight of the Control, Exercise and Restricted groups [two‐way ANOVA Control: F(12,108) = 0.90, P > 0.05; Exercise: F(12,108) = 0.35, P > 0.05; Restricted: F(12,108) = 0.95, P > 0.05; Figure 4A–C]. In contrast, the body weight of ABA rats was modified by THC treatment (0.5 and 0.75 mg·kg−1). Two‐way ANOVA revealed a significant main effect of treatment × time interaction [F(12,108) = 1.94, P < 0.05]; compared with vehicle‐treated ABA rats, post hoc analysis showed that 0.75 mg·kg−1of THC significantly reduced body weight loss on days 6 and 7 (−20 vs −15.2% and −21.8 vs −16.72% respectively). THC administration did not change food intake in ad libitum‐fed rats [two‐way ANOVA, main effect of treatment × time interaction: Exercise, F(10,90) = 1.76, P > 0.05; Control, F(10,90) = 0.89, P > 0.05; Figure 4E, F]. However, a significant increase in feeding was observed in both Restricted and ABA rats only on day 1 of treatment [two‐way ANOVA: main effect of treatment × time interaction Restricted F(10,90) = 2.80, P <0.05; ABA F(10,90) = 3.89, P <0.05] (Figure 4G, H). Compared with vehicle‐treated rats, post hoc analysis revealed that only the dose of 0.5 mg·kg−1 was effective in the restricted rats (+105%) and that both doses of THC effectively increased food intake in ABA rats (0.5 mg·kg−1: +125%; 0.75 mg·kg−1: +134%). THC treatment did not affect the RWA of Exercise rats according to two‐way ANOVA of the treatment × time interaction [F(12,108) = 0.71, P > 0.05] and treatment alone [F(2,108) = 0.19, P >0.05; Figure 5A]. However, with regard to THC impact on RWA of ABA rats, two‐way ANOVA revealed a significant effect of treatment [F(2,108) = 6.20, P < 0.05] and time [F(6,108) = 4.87, P < 0.05], but the interaction between treatment and time did not have a significant effect [F(12,108) = 1.41, P > 0.05]. Subsequent individual one‐way ANOVAs showed that both doses of THC effectively reduced RWA on days 2 [F(2,18) = 8.40, P < 0.05] and 3 [F(2,18) = 7.470, P < 0.05] compared with vehicle‐treated rats, and 0.75 mg·kg−1 of THC was also effective on days 4 [F(2,18) = 4.695, P < 0.05] and 5 [F(2,18) = 3.529, P < 0.05; Figure 5B].
Figure 4.
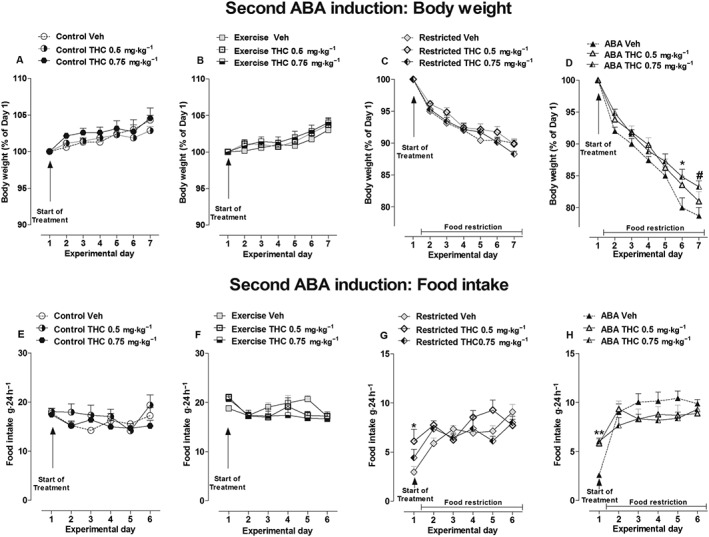
Effect of THC administration (0.5 and 0.75 mg·kg−1) on body weight (A–C) and food intake (E–H) in Control, Exercise, Restricted and ABA groups during the 6 days of the second ABA induction. Results are presented as the mean ± SEM (n = 7 rats per dose). Statistical analysis was performed by two‐way ANOVA followed by Bonferroni post hoc test (body weight: *P < 0.05 vs vehicle; food intake: *P < 0.05 vs vehicle).
Figure 5.
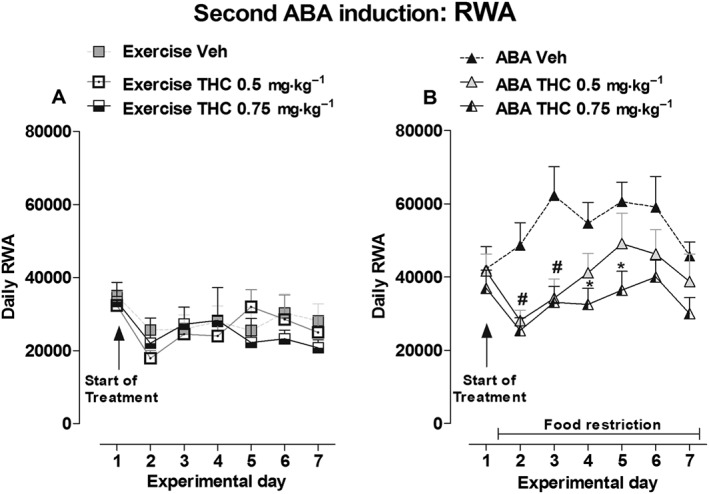
Effect of THC administration (0.5 and 0.75 mg·kg−1) on RWA in Exercise (A) and ABA (B) rats during the 6 days of the second ABA induction. Results are presented as the mean ± SEM (n = 7 rats per dose). Statistical analysis was performed by one‐way ANOVA followed by Newman–Keuls post hoc test (*P < 0.05 vs vehicle).
Effect of CP administration
Over the CP (0.03 and 0.06 mg·kg−1) treatment period, there were no significant differences in body weight between vehicle‐ and CP‐treated rats in the Control, Exercise and Restricted groups [two‐way ANOVA, main effect of treatment × time interaction: Control: F(12,108) = 0.78, P > 0.05; Exercise: F(12,108) = 1.63, P > 0.05; Restricted F(12,108) = 1.01, P > 0.05; Figure 6A–C]. In contrast, the body weight of ABA rats was modified. Two‐way ANOVA showed that interaction between treatment and time had a significant effect [F(12,108) = 2.15, P < 0.05]; post hoc analysis showed that while only 0.06 mg·kg−1of CP prevented weight loss relative to the vehicle on day 6 (−13.74 vs −17.46% respectively), both doses prevented weight loss on day 7 (vehicle: −21.11%, CP 0.03 mg·kg−1: −17.17%,CP 0.06 mg·kg−1: −14.68%; Figure 6D). Similar to THC, CP‐treatment did not modify food intake in ad libitum‐fed rats since two‐way ANOVA did not detect any main effect of treatment × time interaction: Exercise: F(10,90) = 1.28, P > 0.05; Control: F(10,90) = 1.72, P > 0.05; (Figure 6E, F). On day 1 of treatment, both doses of CP affected food intake in Restricted rats by 103 and 107%, respectively, versus vehicle‐treated rats [two‐way ANOVA of treatment × day interaction: F(10,90) = 1.96, P < 0.05; Figure 6G]. At the same time point (day 1), both doses of CP also significantly increased food intake of ABA rats [two‐way ANOVA treatment × time interaction: F(10,90) = 2.82, P < 0.05]. As shown in Figure 6H, post hoc analysis showed that CP increased food intake in ABA rats at both doses tested compared with vehicle (0.03 mg·kg−1: +82%, P < 0.05; 0.06 mg·kg−1: +88%, P < 0.05). Finally, no consequence of CP‐treatment was found on the RWA of Exercise rats [two‐way ANOVA of treatment × day interaction: F(12,108) = 0.76, P > 0.05; treatment: F(2,108) = 0.31, P > 0.05; Figure 7A]. However, CP treatment significantly affected RWA in ABA rats [two‐way ANOVA, main effect of treatment × day interaction: F(12,108) = 2.45, P < 0.05; Figure 7B]. Post hoc analysis showed that the higher dose of CP effectively reduced RWA compared with vehicle‐treated rats starting from the day 2 of treatment.
Figure 6.
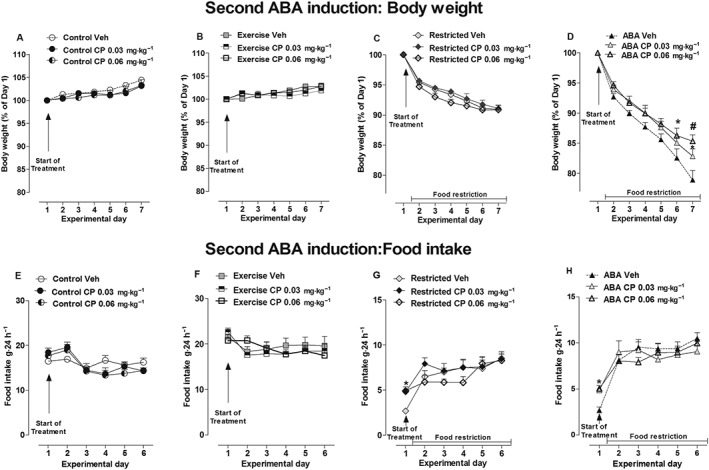
Effect of CP administration (0.03 and 0.06 mg·kg−1) on body weight (A–C) and food intake (E–H) in Control, Exercise, Restricted and ABA groups during the 6 days of the second ABA induction. Results are presented as the mean ± SEM (n = 7 rats per dose). Statistical analysis was performed by two‐way ANOVA followed by Bonferroni post hoc test (body weight: *P < 0.05 vs vehicle; food intake: *P < 0.05 vs vehicle).
Figure 7.
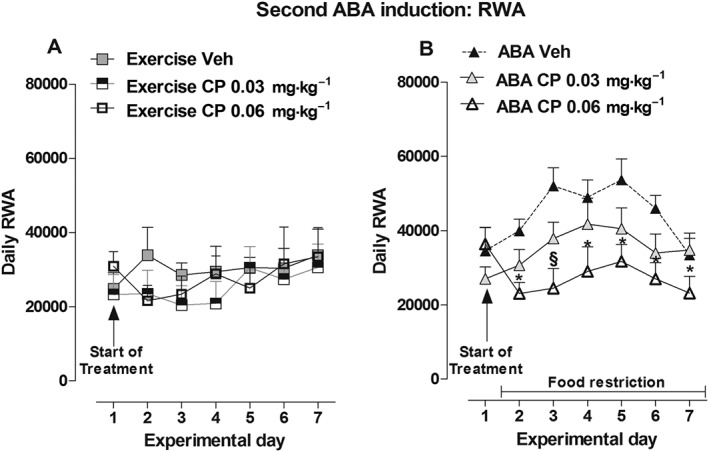
Effect of CP administration (0.03 and 0.06 mg·kg−1) on RWA in Exercise (A) and ABA (B) rats during the 6 days of the second ABA induction. Results are presented as the mean ± SEM (n = 7 rats per dose). Statistical analysis was performed by two‐way ANOVA followed by Bonferroni post hoc test (*and §P < 0.05 vs vehicle).
Plasma analysis of hormone levels
Effect of THC administration
As expected (de Rijke et al., 2005), comparison of vehicle‐treated rats of all four main groups showed significantly lower plasma leptin levels in ABA and Restricted rats with respect to Exercise and Control rats [one‐way ANOVA: F(3,24) = 124.1, P < 0.05]. Post hoc analysis also showed leptin plasma levels in Exercise rats were significantly lower than Controls (Figure 8A). A similar effect was observed when animals were treated with THC at both doses [one‐way ANOVA: THC 0.5 mg·kg−1 F(3,24) = 25.79, P < 0.05; THC 0.75 mg·kg−1 F(3,24) = 31.33, P < 0.05]. Moreover, intra‐group analysis revealed that leptin plasma levels in ABA rats were significantly affected by THC treatment. Compared with vehicle, one‐way ANOVA revealed a significant THC treatment effect between ABA subjects [F(2,18) = 6.76, P < 0.05], and post hoc analysis showed that THC 0.75 mg·kg−1 produced a significant increase in leptin plasma levels. There were no significant differences in leptin plasma levels between vehicle‐ and THC‐treated rats in the other main experimental groups [one‐way ANOVA: Restricted, F(2,18) = 1.80, P < 0.05; Exercise: F(2,18) = 0.25, P > 0.05; Control: F(2,18) = 1.69, P > 0.05]. However, as already demonstrated (Boersma et al. 2016), corticosterone plasma levels were significantly increased in vehicle‐treated ABA rats compared with the vehicle‐treated rats of the other main experimental groups [one‐way ANOVA: F(3,24) = 4.201, P < 0.05; post hoc analysis: P < 0.05; Figure 8B]. Likewise, ABA rats showed higher corticosterone levels when treated with 0.5 mg·kg−1 THC compared with the other main experimental groups [one‐way ANOVA: F(3,24) = 2.862, P < 0.05; post hoc analysis: P < 0.05]. No significant statistical difference was detected between the main groups with 0.75 mg·kg−1 of THC although there is a clear upward trend in ABA rats [one‐way ANOVA: F(3,24) = 1.141, P > 0.05].
Figure 8.
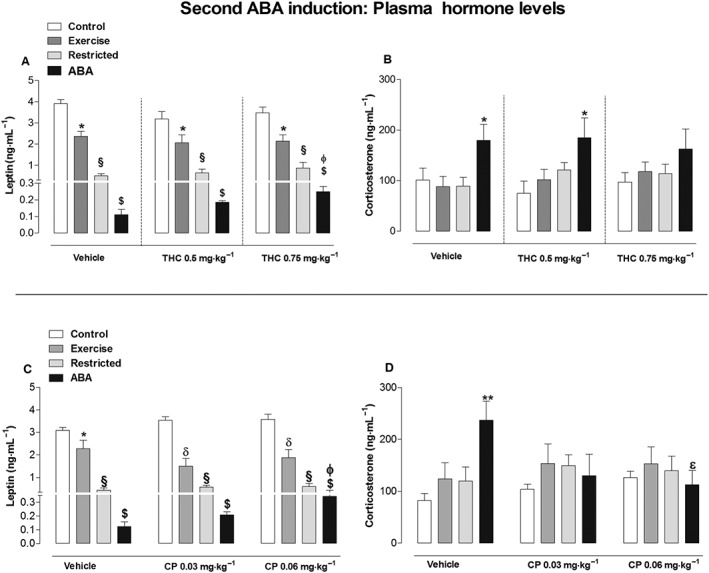
Effect of THC (0.5 and 0.75 mg·kg−1) (A, B) and CP (0.03 and 0.06 mg·kg−1) (C, D) administration on plasma leptin and corticosterone levels in Control, Exercise, Restricted and ABA groups. Results are presented as the mean ± SEM (n = 7 rats per dose). Statistical analysis was performed by one‐way ANOVA followed by Newman–Keuls post hoc test (leptin: *P < 0.05 vs Control, ɸP < 0.05 vs vehicle; corticosterone: *P < 0.05 vs Control, Exercise and Restricted rats; εP < 0.05 vs vehicle).
Effect of CP administration
Similar to THC, comparison of vehicle‐treated rats of all four main experimental groups showed plasma leptin levels were significantly lower in ABA and Restricted rats versus Exercise and Control rats [one‐way ANOVA: F(3,24) = 49.02, P < 0.05]. Additionally, leptin plasma levels in Exercise rats were significantly lower compared with Controls (Figure 8C). The same differences between main groups were also found with CP treatment by one‐way ANOVA [CP0.03 mg·kg−1: F(3,24) = 50.15, P < 0.05; CP0.06 mg·kg−1: F(3,24) = 40.25, P < 0.05]. Intra‐group analysis revealed that CP treatment increased plasma leptin levels in ABA rats which became significant when animals were treated with CP0.06 mg·kg−1 versus the vehicle [one‐way ANOVA: F(2,18) = 4.141, P < 0.05]. However, there were no significant differences in leptin plasma levels between the vehicle and CP‐treated rats in the other main groups [one‐way ANOVA: Restricted F(2,18) = 2.881, P > 0.05; Exercise: F(2,18) = 1.181, P > 0.05; Control: F(2,1) = 2.22, P > 0.05]. Moreover, vehicle‐treated ABA rats had higher corticosterone plasma levels compared with vehicle‐treated rats of the other main groups [one‐way ANOVA: F(2,18) = 6.984, P < 0.05; Figure 8D]. Conversely, there were no significant differences in corticosterone plasma levels between groups when animals were treated with either dose of CP [one‐way ANOVA: CP 0.03 mg·kg−1, F(3,24) = 0.5179, P > 0.05; CP 0.06 mg·kg−1, F(3,24) = 0.0952, P > 0.05]. Accordingly, intra‐group analysis of ABA rats treated with CP 0.06 mg·kg−1 showed a significant effect compared with vehicle‐treated ABA rats [one‐way ANOVA: F(2,18) = 3.580, P < 0.05]. Intra‐group analysis also revealed that there is no difference between vehicle‐ and CP‐treated rats of the other experimental groups via one‐way ANOVA [Restricted: F(2,18) = 0.347, P > 0.05; Exercise: F(2,18) = 0.8710, P = 0.4354; Control: F(2,18) = 3.40, P > 0.05].
Discussion
Using the ABA protocol, the present study evaluated whether positive pharmacological modulation of EC signalling could effectively modify weight loss, RWA and neuroendocrine changes in rats subjected to a repeated ABA regime (Chowdhury et al., 2014; Aoki et al., 2017). In agreement with previous reports, we confirmed that during ABA development (first ABA induction), rats subjected to a restricted feeding schedule in combination with free access to a running wheel presented a massive decline in body weight associated with a progressive increase in RWA (Routtenberg and Kuznesof, 1967; Routtenberg, 1968). In contrast, control rats on a restricted feeding schedule without running wheel (Restricted rats) exhibited marginal weight loss compared with ABA rats. Moreover, control ad libitum‐fed rats with continuous access to running wheel (Exercise rats) showed stable levels of RWA as well as an increase in food intake to compensate for their increased energy expenditure, compared with Control rats that did not have access to a running wheel and did not experience food restriction (Looy and Eikelboom, 1989; Scheurink et al., 1999).
During the recovery period, during which restricted rats were allowed to feed ad libitum, ABA rats showed a transient decline in RWA and a hyperphagia that persisted throughout the entire recovery phase. Even though their body weight was restored to baseline within 4 days, ABA rats weighed less than those of the other main experimental groups at the end of the recovery period. It is important to note that during recovery, ABA rats had continuous access to running wheels so it is possible that exercise availability influenced body weight recovery (Dwyer and Boakes, 1997; Ratnovsky and Neuman, 2011). In agreement with this observation, women with AN tend to maintain lower body mass indices than healthy women after recovery, and physical activity could be a possible contributing factor (Dellava et al., 2011). Indeed, to get back to a healthy body weight, people with AN require even larger amounts of food than healthy controls, and physical activity could also contribute to this increased caloric requirement (Kaye et al., 1988). Compared with ABA rats, weights of Restricted rats were restored to baseline levels within the first day of recovery, and their body weights were not statistically different from that of ad libitum‐fed rats at the end of the recovery period.
After the refeeding period, we re‐exposed the ABA rats to a second ABA protocol. Even though this fails to mimic a real relapse because there are no precipitating events that drive the animals into ABA (food restriction is imposed rather than voluntary), the double exposure to the ABA protocol might lead to a better understanding of the efficacy of pharmacological treatments on repeated ABA regimes and is reminiscent of the human condition, in which patients repeatedly undergo cycles of recovery and illness.
During the second ABA induction, we found that ABA rats treated with vehicle show a percentage of weight loss similar to that of the first induction, while RWA further increased. The excessive RWA of ABA model therefore mimics the physical hyperactivity that is frequently utilized by AN patients as a strategy to lose weight, and our results are in agreement to the notion that it appears to play a central role in the progression of the disorder (Gümmer et al., 2015; Achamrah et al., 2016). Physical hyperactivity also appears to be related to the higher relapse rates in AN following weight restoration and, thus, seems to be associated with a higher probability of chronic outcome of AN (Casper and Leslie, 1996; Strober et al., 1997; Carter et al., 2004).
Subchronic treatment with both the natural CB1/CB2 receptor partial agonist THC and the synthetic CB1/CB2 receptor agonist CP effectively prevented body weight loss in ABA compared with vehicle‐treated rats. In contrast, neither THC nor CP had an effect on body weight of Restricted rats or ad libitum‐fed rats, suggesting a specific effect on ABA rat body weight. Similar data were previously published by Verty et al. (2011), which showed the attenuating effect of THC on weight loss associated with ABA development in female rats. Thus, our results extend Verty's data confirming that THC is also effective after a repeated regime of ABA. In addition, we showed, for the first time, the efficacy of a full cannabinoid receptor agonist CP, in attenuating the body weight loss in ABA rats. Accordingly, a randomized controlled clinical trial showed that dronabinol (a synthetic form of THC) administration induced a small but significant weight gain in women with severe AN in the absence of adverse events (Andries et al., 2014). In line with the orexigenic effects of cannabinoid agonists in humans and rodents (Williams and Kirkham, 1999; Hart et al., 2002), both drugs increased food intake during the 1.5 h food access period in both ABA and Restricted rats, even if only on day 1 of the treatment that represented the start of ABA when the animals were no yet under the restricted feeding schedule. Again, Verty et al. (2011) also found that subchronic THC treatment significantly stimulated chow intake only on day 1 of treatment. Since neither THC nor CP modified chow consumption after the second day of treatment, we assume that food intake during the restriction period was already at its highest; therefore, the CB1/CB2 receptor agonists were not able to increase it further. In addition, it is well‐established that chronic cannabinoid administration can rapidly induce tolerance to behavioural and biochemical outcomes (Breivogel et al., 1999; Maldonado and Rodríguez de Fonseca, 2002). As a consequence, it is possible that the lack of effect on food intake observed in our study may be interpreted as development of tolerance to the orexigenic properties of THC and CP following repeated administration (Järbe and DiPatrizio, 2005). However, neither drug influenced the 24 h food intake in ad libitum‐fed rats, which is probably due to the fact that the 24 h interval between measurements was too long to observe an orexigenic effect.
In our study, both THC and CP were able to significantly reduce RWA in ABA rats without a tolerance effect. This suggests that attenuation of body weight loss in our ABA rats may be due to a decrease in physical activity. Notably, THC and CP treatments did not affect RWA in Exercise rats, suggesting this effect was specific for ABA rats rather than due to a general motor effect. As mentioned above, hyperactivity has been associated with higher relapse rates; therefore, reducing activity levels in AN patients might be crucial for therapeutic outcome (Kostrzewa et al., 2013; Maestro et al., 2014).
Herein, plasma analysis performed at the end of the second ABA induction showed, as also reported after a single exposure to the ABA protocol (Pardo et al., 2010), that the ABA group of rats had very low levels of leptin compared with all other experimental groups.
Importantly, our results demonstrate that both THC and CP treatments were able to significantly increase plasma leptin levels compared with vehicle‐treated ABA rats. To the best of our knowledge, this is the first report demonstrating the ability of cannabinoid agonist drugs to attenuate the effect of ABA induction on leptin levels. Leptin is a hormone mainly synthesized in adipocytes whose levels are highly correlated with body mass index and percent body fat (Heymsfield et al., 1999; Cammisotto et al., 2006). Indeed, serum leptin levels rise with increasing adiposity and drop as a result of loss of fat mass (Frederich et al., 1995; Maffei et al., 1995). Accordingly, low leptin levels are an endocrinological feature of acute AN (Hebebrand et al., 1997). Different studies support a potential link between decreased leptin signalling and the presence of physical hyperactivity in AN patients and rats (Hebebrand et al., 2003; Holtkamp et al., 2003). Thus, the observed effects of THC and CP on RWA in ABA rats could be attributed to the capacity of both cannabinoids to increase leptin signalling. In agreement with this, leptin treatment has been shown to reduce hyperactivity in ABA rat models (Exner et al., 2000; Hillebrand et al., 2005). Moreover, Verhagen et al. (2011) showed that leptin reduces locomotor activity in ABA models by acting at the level of the ventral tegmental area, where CB1 receptors are abundantly expressed as part of the mesolimbic reward system (Herkenham et al., 1990). Plasma analysis of our animals shows a decrease in leptin levels also in Restricted and Exercise rats compared with ad libitum‐fed rats without running wheel access. Thus, the two variables manipulated in the ABA model were able to alter leptin signalling when applied separately. In agreement with this result, leptin levels have been shown to decrease in response to starvation or exercise in both humans and rats (Ahima et al., 1996; Iwasa et al., 2016). It is very important to underline that both THC and CP treatments are able to significantly modify the reduced plasma leptin level exclusively in ABA rats that are exposed concomitantly to food restriction and exercise and not in Restricted and Exercise rats in which each variable is applied independently. Moreover, our ABA rats showed higher corticosterone levels compared with the other main groups. As previously discussed, the ABA regimen used herein resulted in activation of the HPA axis, with a subsequent increase in plasma corticosterone levels (Burden et al., 1993). As for leptin signalling, a link between higher HPA axis activation and increased RWA with food restriction has been postulated (Challet et al., 1995; Duclos et al., 2005). Likewise, wheel running induced by food restriction was shown to be absent in adrenalectomized, food‐restricted rats but increased in a dose‐related manner with corticosterone replacement (Duclos et al., 2009). Moreover, excessive physical activity seems to reinforce HPA axis over activation, as part of a vicious cycle, in some patients with AN (Klein et al., 2007). Our data show no significant effect on corticosterone levels of ABA rats after THC treatment even though with the higher dose we found a trend toward a decrease. On the contrary, we found that treatment with CP was able to significantly decrease corticosterone levels, suggesting that cannabinoid stimulation by CP may in part exert a moderating effect on RWA by reducing HPA axis activation. Accordingly, augmentation of the EC signalling has been shown to suppress HPA axis activity and reduce stress‐induced elevations in corticosterone levels (Ganon‐Elazar and Akirav, 2009). In addition, Andries et al. (2015) suggested that dronabinol treatment alleviates the increased HPA axis activity in women with severe and chronic AN. Moreover, both genetic disruption and pharmacological blockade of CB1 receptor signalling resulted in an increase in HPA axis activity under basal conditions and following exposure to acute stress (Hill and Tasker, 2012). The significant effect of CP versus THC in reducing the corticosterone levels in ABA rats could be ascribed to the full cannabinoid receptor agonism of this synthetic compound leading to greater activation of CB1 receptors (Castaneto et al., 2014).
Despite the growing knowledge of AN neurobiology, therapeutic interventions are still lacking, and the incidence of relapse remains high. At present, no medication has been approved for the treatment of AN, and there is a clear need for new, more effective AN treatments in addition to standard psychotherapy. As already mentioned in the introduction, a promising therapy for AN may be based on the use of drugs that target the EC system, which appears dysregulated in AN patients with increased plasma levels of AEA (Monteleone et al., 2005). The authors speculated that elevated AEA levels may represent an adaptive response aimed at counteracting their restrictive behaviour by elevating the drive to eat. However, the limitation of this work is that it is uncertain whether peripheral levels could reflect the ones found in the brain. However, it has been hypothesized that the EC system is hypoactive in anorexic conditions, which could explain the increased CB1 receptor availability seen in both human patients and rats subject to the ABA protocol (Gérard et al., 2011; Casteels et al., 2014). To date, no direct evidence on endocannabinoid levels have been reported in animal models of AN. However, Hanus et al. (2003) reported decreased levels of 2‐AG in the entire mouse brain, including hypothalamic and hippocampal regions, upon 12 days of food restriction in mice. Our findings show that cannabinoid agonists were able to attenuate RWA and, consequently, reduce the body weight loss associated with ABA, probably by increasing leptin signalling and stabilizing HPA axis activity through the activation of the EC system. In contrast, Lewis and Brett (2010) reported that the CB1 receptor antagonist AM251 did not restore body weight or food intake in the ABA model in C57/BL6 mice but rather decreased feeding and furthermore increased mortality at the highest dose. If analogous effects occur in humans, pharmacological manipulation of the EC system with cannabinoid agonists shows promise for treatment of AN, specifically in patients where physical hyperactivity plays a central role in the pathogenesis and progression of this disorder.
Author contributions
P.F. and W.F. conceived and designed this study. M.S., V.S, R.C. and M.F.B. performed the experiments. M.S, P.F. W.F and P.U. were involved in the discussions of the data. M.S. and P.F. wrote the manuscript. M.S., W.F. and P.F. reviewed and edited this manuscript. All the authors read and approved this manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was funded by Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 2010), by ‘Regione Autonoma della Sardegna, Assessorato alla Programmazione’ grants for basic research (Legge Regionale 7/2007), by Fondazione Banco di Sardegna (Prot.U627.2013/AI.551MGB) and by the Departmentof Biomedical Sciences Project (RICDIP_2012_Fratta_01) at the University of Cagliari.
Scherma, M. , Satta, V. , Collu, R. , Boi, M. F. , Usai, P. , Fratta, W. , and Fadda, P. (2017) Cannabinoid CB1/CB2 receptor agonists attenuate hyperactivity and body weight loss in a rat model of activity‐based anorexia. British Journal of Pharmacology, 174: 2682–2695. doi: 10.1111/bph.13892.
References
- Achamrah N, Coëffier M, Déchelotte P (2016). Physical activity in patients with anorexia nervosa. Nutr Rev 74: 301–311. [DOI] [PubMed] [Google Scholar]
- Adan RA, Hillebrand JJ, Danner UN, Cardona Cano S, Kas MJ, Verhagen LA (2011). Neurobiology driving hyperactivity in activity‐based anorexia. Curr Top Behav Neurosci 6: 229–250. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos‐Flier E et al. (1996). Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5™). American Psychiatric Association: Arlington, VA. [Google Scholar]
- Andries A, Frystyk J, Flyvbjerg A, Støving RK (2014). Dronabinol in severe, enduring anorexia nervosa: a randomized controlled trial. Int J Eat Disord 47: 18–23. [DOI] [PubMed] [Google Scholar]
- Andries A, Frystyk J, Flyvbjerg A, Støving RK (2015). Changes in IGF‐I, urinary free cortisol and adipokines during dronabinol therapy in anorexia nervosa: Results from a randomised, controlled trial. Growth Horm IGF Res 25: 247–252. [DOI] [PubMed] [Google Scholar]
- Aoki C, Chowdhury TG, Wable GS, Chen YW (2017). Synaptic changes in the hippocampus of adolescent female rodents associated with resilience to anxiety and suppression of food restriction‐evoked hyperactivity in an animal model for anorexia nervosa. Brain Res 1654 (Pt B): 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcelus J, Mitchell AJ, Wales J, Neilson S (2011). Mortality rates in patients with anorexia nervosa and other eating disorders. A meta‐analysis of 36 studies. Arch Gen Psychiatry 68: 724–731. [DOI] [PubMed] [Google Scholar]
- Bergh C, Callmar M, Danemar S, Hölcke M, Isberg S et al. (2013). Effective treatment of eating disorders: results at multiple sites. Behav Neurosci 127: 878–889. [DOI] [PubMed] [Google Scholar]
- Boersma GJ, Liang NC, Lee RS, Albertz JD, Kastelein A, Moody LA et al (2016). Failure to upregulate Agrp and Orexin in response to activity based anorexia in weight loss vulnerable rats characterized by passive stress coping and prenatal stress experience. Psychoneuroendocrinology 67: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden VR, White BD, Dean RG, Martin RJ (1993). Activity of the hypothalamic‐pituitary‐adrenal axis is elevated in rats with activity‐based anorexia. J Nutr 123: 1217–1225. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim‐Selley LJ (1999). Chronic delta9‐tetrahydrocannabinol treatment produces a time‐dependent loss of cannabinoid receptors and cannabinoid receptor‐activated G proteins in rat brain. J Neurochem 73: 2447–2459. [DOI] [PubMed] [Google Scholar]
- Cammisotto PG, Bukowiecki LJ, Deshaies Y, Bendayan M (2006). Leptin biosynthetic pathway in white adipocytes. Biochem Cell Biol 84: 207–214. [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Maccioni P, Gessa GL (2006). Efficacy of rimonabant and other cannabinoid CB1 receptor antagonists in reducing food intake and body weight: preclinical and clinical data. CNS Drug Rev 12: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JC, Blackmore E, Sutandar‐Pinnock K, Woodside DB (2004). Relapse in anorexia nervosa: a survival analysis. Psychol Med 34: 671–679. [DOI] [PubMed] [Google Scholar]
- Casper C, Leslie J (1996). An eight‐year follow‐up: outcome from adolescent compared to adult onset anorexia nervosa. J Youth Adolesc 25: 499–517. [Google Scholar]
- Casper RC, Sullivan EL, Tecott L (2008). Relevance of animal models to human eating disorders and obesity. Psychopharmacology (Berl) 199: 313–329. [DOI] [PubMed] [Google Scholar]
- Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA (2014). Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend 144: 12–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels C, Gérard N, van Kuyck K, Pottel L, Nuttin B, Bormans G et al. (2014). Small animal PET imaging of the type 1 cannabinoid receptor in a rodent model for anorexia nervosa. Eur J Nucl Med Mol Imaging 41: 308–321. [DOI] [PubMed] [Google Scholar]
- Challet E, Le Maho Y, Robin JP, Malan A, Cherel Y (1995). Involvement of corticosterone in the fasting‐induced rise in protein utilization and locomotor activity. Pharmacol Biochem Behav 50: 405–412. [DOI] [PubMed] [Google Scholar]
- Chowdhury TG, Ríos MB, Chan TE, Cassataro DS, Barbarich‐Marsteller NC, Aoki C (2014). Activity‐based anorexia during adolescence disrupts normal development of the CA1 pyramidal cells in the ventral hippocampus of female rats. Hippocampus 24: 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury TG, Chen YW, Aoki C (2015). Using the activity‐based anorexia rodent model to study the neurobiological basis of anorexia nervosa. J Vis Exp 105: e52927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Katzman DK, Kaptein S, Kirsh C, Brewer H, Kalmbach K et al. (1997). The prevalence of high‐level exercise in the eating disorders: etiological implications. Compr Psychiatry 38: 321–326. [DOI] [PubMed] [Google Scholar]
- Dellava JE, Hamer RM, Kanodia A, Reyes‐Rodríguez ML, Bulik CM (2011). Diet and physical activity in women recovered from anorexia nervosa: a pilot study. Int J Eat Disord 44: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rijke CE, Hillebrand JJ, Verhagen LA, Roeling TA, Adan RA (2005). Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel‐running rats. J Mol Endocrinol 35: 381–390. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I (2005). Endocannabinoid control of food intake and energy balance. Nat Neurosci 8: 585–589. [DOI] [PubMed] [Google Scholar]
- Di Marzo V (2008). Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov 7: 438–455. [DOI] [PubMed] [Google Scholar]
- Di Marzo V (2009). The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res 60: 77–84. [DOI] [PubMed] [Google Scholar]
- Dixon DP, Ackert AM, Eckel LA (2003). Development of, and recovery from, activity‐based anorexia in female rats. Physiol Behav 80: 273–279. [DOI] [PubMed] [Google Scholar]
- Dodd GT, Stark JA, McKie S, Williams SR, Luckman SM (2009). Central cannabinoid signaling mediating food intake: a pharmacological‐challenge magnetic resonance imaging and functional histology study in rat. Neuroscience 163: 1192–1200. [DOI] [PubMed] [Google Scholar]
- Duclos M, Bouchet M, Vettier A, Richard D (2005). Genetic differences in hypothalamic‐pituitary‐adrenal axis activity and food restriction‐induced hyperactivity in three inbred strains of rats. J Neuroendocrinol 17: 740–752. [DOI] [PubMed] [Google Scholar]
- Duclos M, Gatti C, Bessière B, Mormède P (2009). Tonic and phasic effects of corticosterone on food restriction‐induced hyperactivity in rats. Psychoneuroendocrinology 34: 436–445. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Boakes RA (1997). Activity‐based anorexia in rats as failure to adapt to a feeding schedule. Behav Neurosci 111: 195–205. [PubMed] [Google Scholar]
- Exner C, Hebebrand J, Remschmidt H, Wewetzer C, Ziegler A, Herpertz S et al. (2000). Leptin suppresses semi‐starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol Psychiatry 5: 476–481. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS (1995). Leptin levels reflect body lipid content in mice: evidence for diet‐induced resistance to leptin action. Nat Med 1: 1311–1314. [DOI] [PubMed] [Google Scholar]
- Frieling H, Albrecht H, Jedtberg S, Gozner A, Lenz B, Wilhelm J et al. (2009). Elevated cannabinoid 1 receptor mRNA is linked to eating disorder related behavior and attitudes in females with eating disorders. Psychoneuroendocrinology 34: 620–624. [DOI] [PubMed] [Google Scholar]
- Ganon‐Elazar E, Akirav I (2009). Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci 29: 11078–11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard N, Pieters G, Goffin K, Bormans G, Van Laere K (2011). Brain type 1 cannabinoid receptor availability in patients with anorexia and bulimia nervosa. Biol Psychiatry 70: 777–784. [DOI] [PubMed] [Google Scholar]
- Gümmer R, Giel KE, Schag K, Resmark G, Junne FP, Becker S et al. (2015). High levels of physical activity in anorexia nervosa: a systematic review. Eur Eat Disord Rev 23: 333–344. [DOI] [PubMed] [Google Scholar]
- Gutierrez E (2013). A rat in the labyrinth of anorexia nervosa: contributions of the activity‐based anorexia rodent model to the understanding of anorexia nervosa. Int J Eat Disord 46: 289–301. [DOI] [PubMed] [Google Scholar]
- Hanus L, Avraham Y, Ben Shushan D, Zolotarev O, Berry EM, Mechoulam R (2003). Short‐term fasting and prolonged semistarvation have opposite effects on 2‐AG levels in mouse brain. Brain Res 983: 144–151. [DOI] [PubMed] [Google Scholar]
- Hart L, Ward AS, Haney M, Comer SD, Foltin R, Fishman MW (2002). Comparison of smoked marijuana and oral D9‐tetrahydrocannabinol in humans. Psychopharmacology (Berl) 164: 407–415. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Blum WF, Barth N, Coners H, Englaro P, Juul A et al. (1997). Leptin levels in patients with anorexia nervosa are reduced in the acute stage and elevated upon short‐term weight restoration. Mol Psychiatry 2: 330–334. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Exner C, Hebebrand K, Holtkamp C, Casper RC, Remschmidt H et al. (2003). Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiol Behav 79: 25–37. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T et al. (1999). Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose‐escalation trial. JAMA 282: 1568–1575. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR et al. (1990). Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87: 1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Tasker JG (2012). Endocannabinoid signaling, glucocorticoid‐mediated negative feedback, and regulation of the hypothalamic–pituitary–adrenal axis. Neuroscience 204: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand JJ, Koeners MP, de Rijke CE, Kas MJ, Adan RA (2005). Leptin treatment in activity‐based anorexia. Biol Psychiatry 58: 165–171. [DOI] [PubMed] [Google Scholar]
- Holtkamp K, Herpertz‐Dahlmann B, Mika C, Heer M, Heussen N, Fichter M et al. (2003). Elevated physical activity and low leptin levels co‐occur in patients with anorexia nervosa. J Clin Endocrinol Metab 88: 5169–5174. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC (2007). The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa T, Matsuzaki T, Yano K, Munkhzaya M, Tungalagsuvd A, Yiliyasi M et al. (2016). Developmental changes in the hypothalamic mRNA expression levels of brain‐derived neurotrophic factor and serum leptin levels: Their responses to fasting in male and female rats. Int J Dev Neurosci 54: 1–5. [DOI] [PubMed] [Google Scholar]
- Jager G, Witkamp RF (2014). The endocannabinoid system and appetite: relevance for food reward. Nutr Res Rev 27: 172–185. [DOI] [PubMed] [Google Scholar]
- Järbe TU, DiPatrizio NV (2005). Delta9‐THC induced hyperphagia and tolerance assessment: interactions between the CB1 receptor agonist delta9‐THC and the CB1 receptor antagonist SR‐141716 (rimonabant) in rats. Behav Pharmacol 16: 373–380. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Gwirtsman HE, Obarzanek E, George DT (1988). Relative importance of calorie intake needed to gain weight and level of physical activity in anorexia nervosa. Am J Clin Nutr 47: 989–994. [DOI] [PubMed] [Google Scholar]
- Keshaviah A, Edkins K, Hastings ER, Krishna M, Franko DL, Herzog DB et al. (2014). Re‐examining premature mortality in anorexia nervosa: a meta‐analysis redux. Compr Psychiatry 55: 1773–1784. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DA, Mayer LE, Schebendach JE, Walsh BT (2007). Physical activity and cortisol in anorexia nervosa. Psychoneuroendocrinology 32: 539–547. [DOI] [PubMed] [Google Scholar]
- Kostrzewa E, van Elburg AA, Sanders N, Sternheim L, Adan RA, Kas MJ (2013). Longitudinal changes in the physical activity of adolescents with anorexia nervosa and their influence on body composition and leptin serum levels after recovery. PLoS One 8: e78251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looy H, Eikelboom R (1989). Wheel running, food intake, and body weight in male rats. Physiol Behav 45: 403–405. [DOI] [PubMed] [Google Scholar]
- Lewis DY, Brett RR (2010). Activity‐based anorexia in C57/BL6 mice: effects of the phytocannabinoid, Delta9‐tetrahydrocannabinol (THC) and the anandamide analogue, OMDM‐2. Eur Neuropsychopharmacol 20: 622–631. [DOI] [PubMed] [Google Scholar]
- Maestro S, Scardigli S, Brunori E, Calderoni S, Curzio O, Denoth F (2014). Anorexia nervosa and hyperactivity in adolescence: psychiatric and internal medicine features. Minerva Pediatr 66: 237–248. [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y et al. (1995). Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight‐reduced subjects. Nat Med 1: 1155–1161. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodríguez de Fonseca F (2002). Cannabinoid addiction: behavioral models and neural correlates. J Neurosci 22: 3326–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzola E, Kaye WH (2015). Anorexia nervosa. World Rev Nutr Diet 111: 169–173. [DOI] [PubMed] [Google Scholar]
- Mattar L, Thiébaud MR, Huas C, Cebula C, Godart N (2012). Depression, anxiety and obsessive‐compulsive symptoms in relation to nutritional status and outcome in severe anorexia nervosa. Psychiatry Res 200: 513–517. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler PS, Brown C (2015). Anorexia nervosa – medical complications. J Eat Disord 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Klibanski A (2014). Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol 2: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Matias I, Martiadis V, De Petrocellis L, Maj M, Di Marzo V (2005). Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge‐eating disorder, but not in bulimia nervosa. Neuropsychopharmacology 30: 1216–1221. [DOI] [PubMed] [Google Scholar]
- Pardo M, Roca‐Rivada A, Al‐Massadi O, Seoane LM, Camiña JP, Casanueva FF (2010). Peripheral leptin and ghrelin receptors are regulated in a tissue‐specific manner in activity‐based anorexia. Peptides 31: 1912–1919. [DOI] [PubMed] [Google Scholar]
- Ratnovsky Y, Neuman P (2011). The effect of pre‐exposure and recovery type on activity‐based anorexia in rats. Appetite 56: 567–576. [DOI] [PubMed] [Google Scholar]
- Routtenberg A (1968). “Self‐starvation” of rats living in activity wheels: adaptation effects. J Comp Physiol Psychol 66: 234–238. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Kuznesof AW (1967). Self‐starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol 64: 414–421. [DOI] [PubMed] [Google Scholar]
- Scheurink AJ, Ammar AA, Benthem B, Van Dijk G, Sodersten PA (1999). Exercise and the regulation of energy intake. Int J Obes Relat Metab Disord 23: S1–S6. [DOI] [PubMed] [Google Scholar]
- Smink FR, van Hoeken D, Hoek HW (2012). Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep 14: 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober M, Freeman R, Morrell W (1997). The long‐term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10‐15 years in a prospective study. Int J Eat Disord 22: 339–360. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V (2003). The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol 480: 133–150. [DOI] [PubMed] [Google Scholar]
- Verhagen LA, Luijendijk MC, Adan RA (2011). Leptin reduces hyperactivity in an animal model for anorexia nervosa via the ventral tegmental area. Eur Neuropsychopharmacol 21: 274–281. [DOI] [PubMed] [Google Scholar]
- Verty AN, Evetts MJ, Crouch GJ, McGregor IS, Stefanidis A, Oldfield BJ (2011). The cannabinoid receptor agonist THC attenuates weight loss in a rodent model of activity‐based anorexia. Neuropsychopharmacology 36: 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC (1999). Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 143: 315–317. [DOI] [PubMed] [Google Scholar]


