Abstract
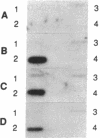
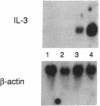
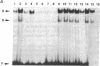
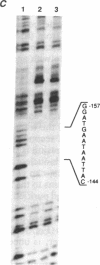
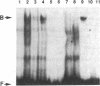
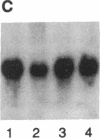
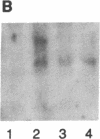
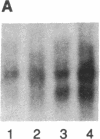
Interleukin 3 (IL-3 or multi-colony-stimulating factor) plays an important role in the hematopoietic response to inflammatory stimuli through its action on both immature and mature blood cells. Like other lymphokines, IL-3 is produced in response to activation of the T-cell receptor and protein kinase C pathways. By using nuclear run-on assays of quiescent and stimulated T-cell lines, we demonstrate that IL-3 gene expression is controlled, at least in part, at the level of transcription. Functional reporter gene analysis was used to delineate two regions of the IL-3 5' flanking sequence responsible for transcriptional stimulation. DNA binding proteins that potentially mediate these responses were then recognized by mobility-shift and DNase footprinting assays. One region responsible for transcriptional enhancement was localized to the sequence GATGAATAAT, the cognate site of a transcription factor, here termed NF-IL3-A. A second region of functional activity and protein binding was localized to a single transcription factor AP-1 site. In addition three functionally inhibitory regions were identified. These results, along with the further characterization of NF-IL3-A, will contribute to the understanding of IL-3 gene regulation in stimulated T cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohmann D., Tjian R. Biochemical analysis of transcriptional activation by Jun: differential activity of c- and v-Jun. Cell. 1989 Nov 17;59(4):709–717. doi: 10.1016/0092-8674(89)90017-2. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D. B., Bush M. R., Morgan J. G., Weiss A., Crabtree G. R. A 275 basepair fragment at the 5' end of the interleukin 2 gene enhances expression from a heterologous promoter in response to signals from the T cell antigen receptor. J Exp Med. 1987 Feb 1;165(2):395–407. doi: 10.1084/jem.165.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. Control of granulocyte-macrophage colony-stimulating factor production in normal endothelial cells by positive and negative regulatory elements. J Immunol. 1989 Oct 15;143(8):2525–2529. [PubMed] [Google Scholar]
- Kaushansky K., O'Hara P. J., Berkner K., Segal G. M., Hagen F. S., Adamson J. W. Genomic cloning, characterization, and multilineage growth-promoting activity of human granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3101–3105. doi: 10.1073/pnas.83.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., O'Hara P. J., Hart C. E., Forstrom J. W., Hagen F. S. Role of carbohydrate in the function of human granulocyte-macrophage colony-stimulating factor. Biochemistry. 1987 Jul 28;26(15):4861–4867. doi: 10.1021/bi00389a038. [DOI] [PubMed] [Google Scholar]
- Kimoto M., Kindler V., Higaki M., Ody C., Izui S., Vassalli P. Recombinant murine IL-3 fails to stimulate T or B lymphopoiesis in vivo, but enhances immune responses to T cell-dependent antigens. J Immunol. 1988 Mar 15;140(6):1889–1894. [PubMed] [Google Scholar]
- Kobayashi M., Van Leeuwen B. H., Elsbury S., Martinson M. E., Young I. G., Hapel A. J. Interleukin-3 is significantly more effective than other colony-stimulating factors in long-term maintenance of human bone marrow-derived colony-forming cells in vitro. Blood. 1989 May 15;73(7):1836–1841. [PubMed] [Google Scholar]
- Lamph W. W., Wamsley P., Sassone-Corsi P., Verma I. M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988 Aug 18;334(6183):629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., To L. B., Yang Y. C., Gamble J. R., Shannon M. F., Burns G. F., Dyson P. G., Juttner C. A., Clark S., Vadas M. A. Stimulation of proliferation, differentiation, and function of human cells by primate interleukin 3. Proc Natl Acad Sci U S A. 1987 May;84(9):2761–2765. doi: 10.1073/pnas.84.9.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T., Sakura H., Kanei-Ishii C., Sudo T., Yoshimura T., Fujisawa J., Yoshida M., Ishii S. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO J. 1989 Jul;8(7):2023–2028. doi: 10.1002/j.1460-2075.1989.tb03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Andrews N. C., Murphy H. S., Kreissman S. G., Nathan D. G. Positive and negative elements regulate human interleukin 3 expression. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5046–5050. doi: 10.1073/pnas.87.13.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner H. A., Yamasaki K., Jamal N., Minden M. M., Yang Y. C., Wong G. G., Clark S. C. Growth of human hemopoietic colonies in response to recombinant gibbon interleukin 3: comparison with human recombinant granulocyte and granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6765–6769. doi: 10.1073/pnas.84.19.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Muegge K., Williams T. M., Kant J., Karin M., Chiu R., Schmidt A., Siebenlist U., Young H. A., Durum S. K. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science. 1989 Oct 13;246(4927):249–251. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- Niemeyer C. M., Sieff C. A., Mathey-Prevot B., Wimperis J. Z., Bierer B. E., Clark S. C., Nathan D. G. Expression of human interleukin-3 (multi-CSF) is restricted to human lymphocytes and T-cell tumor lines. Blood. 1989 Mar;73(4):945–951. [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M. E., Owen W. F., Jr, Silberstein D. S., Woods J., Soberman R. J., Austen K. F., Stevens R. L. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988 Jun;81(6):1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon M. F., Gamble J. R., Vadas M. A. Nuclear proteins interacting with the promoter region of the human granulocyte/macrophage colony-stimulating factor gene. Proc Natl Acad Sci U S A. 1988 Feb;85(3):674–678. doi: 10.1073/pnas.85.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Valent P., Schmidt G., Besemer J., Mayer P., Zenke G., Liehl E., Hinterberger W., Lechner K., Maurer D., Bettelheim P. Interleukin-3 is a differentiation factor for human basophils. Blood. 1989 May 15;73(7):1763–1769. [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]