Abstract
Drug addiction is a severe psychiatric disorder characterized by the compulsive pursuit of drugs of abuse despite potential adverse consequences. Although several decades of studies have revealed that psychostimulant use can result in extensive alterations of neural circuits and physiology, no effective therapeutic strategies or medicines for drug addiction currently exist. Changes in neuronal connectivity and regulation occurring after repeated drug exposure contribute to addiction-like behaviors in animal models. Among the involved brain areas, including those of the reward system, the striatum is the major area of convergence for glutamate, GABA, and dopamine transmission, and this brain region potentially determines stereotyped behaviors. Although the physiological consequences of striatal neurons after drug exposure have been relatively well documented, it remains to be clarified how changes in striatal connectivity underlie and modulate the expression of addiction-like behaviors. Understanding how striatal circuits contribute to addiction-like behaviors may lead to the development of strategies that successfully attenuate drug-induced behavioral changes. In this review, we summarize the results of recent studies that have examined striatal circuitry and pathway-specific alterations leading to addiction-like behaviors to provide an updated framework for future investigations.
Keywords: addiction-like behaviors, circuit-specific modulation, drug addiction, striatal circuits
INTRODUCTION
Drug addiction involves perseverant and compulsive drug seeking and attempts to obtain and consume drugs despite aversive consequences. One leading circuit-level hypothesis for how addiction arises is that maladaptive neuroadaptations are caused by reward circuits because the dopamine system is usurped by the addictive substances (Everitt and Robbins, 2005; Wise, 1998). The main brain areas composing the reward circuits are distributed across multiple areas and include the basal ganglia (including the striatum), the limbic system (including the amygdala and the hippocampus), and the prefrontal cortex (PFC). Among these regions, the striatum is the core input nucleus and plays key roles in reward-related learning as well as in addictive behaviors. The acquisition and maintenance of addiction-like behaviors appear to arise from a series of molecular and cellular adaptations in striatal circuits (Gerfen and Surmeier, 2011; Hyman et al., 2006).
In fact, the striatum is composed of several subregions that exhibit distinct connectivity and consequently different functional roles. In rodents, the dorsomedial striatum (DMS) and the dorsolateral striatum (DLS) receive excitatory inputs from the limbic and sensorimotor cortices, respectively, while the intermediate region is activated by axons from the association cortex (Crittenden and Graybiel, 2011). The ventral region of the striatum includes the nucleus accumbens (NAc), which consists of the core and shell subregions. The NAc is innervated by the basolateral amygdala (BLA), hippocampus, and medial PFC (Alexander et al., 1986; Haber, 2003). Importantly, the striatum receives abundant dopaminergic innervation from the midbrain. The NAc receives dopaminergic inputs from the ventral tegmental area (VTA), whereas the dorsal striatum receives dopaminergic inputs mainly from the substantia nigra pars compacta (SNpc) (Amalric and Koob, 1993).
Thus, the striatum is considered an area of convergence for various inputs from multiple cortical areas and midbrain structures (Bolam et al., 2000; Kincaid et al., 1998; Smith et al., 1994) (Fig. 1). Within striatal circuits, the integration of various synaptic contacts has been described: gamma-aminobutyric acid (GABA)-ergic innervation has been observed (Brown et al., 2012) along with glutamatergic synapses located on the heads of spines on striatal medium spiny neurons (MSNs) and dopaminergic synapses on the necks of spines (Freund et al., 1984). Therefore, the striatum likely enables expression through activation and integration of distinct neuronal signals, and defining the role of each pathway will substantially aid in our understanding for addictive behaviors.
Fig. 1.
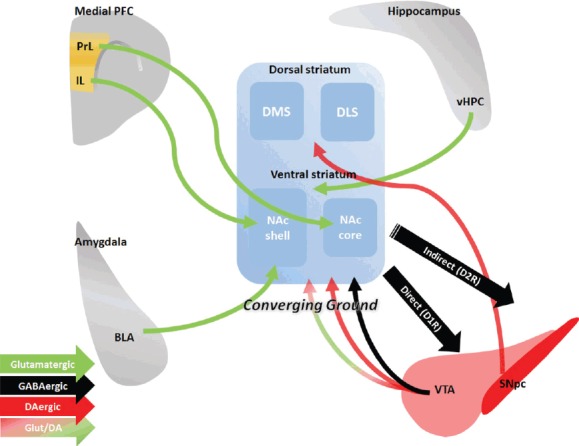
Diverse afferent and efferent connectivity in the striatum.
Medial PFC, medial prefrontal cortex; PrL, prelimbic cortex; IL, infralimbic cortex; vHPC, ventral hippocampus; DMS, dorsomedial striatum; DLS, dorsolateral striatum; NAc, nucleus accumbens; BLA, basolateral amygdala; D1R, dopamine receptor type 1; D2R, dopamine receptor type 2; VTA, ventral tegmental area; SNpc, substantia nigra pars compacta; Glut/DA, glutamate and dopamine co-transmission (not discussed in this review).
In addition to the striatal connectome, the unique composition of the striatal neuronal populations must also be addressed. Striatal neurons comprise mainly GABAergic MSNs but also a small population of various types of interneurons. The MSNs, which exhibit low firing rates and high spine densities, are further divided into two subtypes: dopamine receptor type 1 (D1R)-expressing and D2R-expressing MSNs (Gerfen et al., 1990). The striatal interneuron population includes fast-spiking parvalbumin-positive interneurons, low threshold-spiking somatostatin-positive interneurons, and tonically active cholinergic interneurons (ChINs). Although dynamic regulation of synaptic plasticity at individual pathways appears to play a pivotal role in the expression of distinct addiction-like behavioral phenotypes, it remains unknown which striatal circuits are implicated and modulate specific forms of the behaviors.
Along with other accumulating knowledge, emerging methods, such as optogenetics and chemogenetics further increase our understanding of addiction-related striatal circuits (Ferguson and Neumaier, 2015; Tye and Deisseroth, 2012). Using these molecular and cellular approaches, we have just begun to characterize the causal brain regions and related circuits playing distinct roles in addiction-like behaviors. In here, we summarize recent studies examining pathway-specific regulation of inbound and outbound striatal circuits and also provide conceptual bases for future investigations.
MESO-STRIATAL CIRCUIT
Dopamine released in the target brain areas controls and shapes the neural circuits and addictive behaviors. A majority of dopaminergic neurons in the brain are located in the VTA and the SNpc, which project to the ventral and dorsal striatum, respectively. Psychostimulants, including cocaine and amphetamine, elevate dopamine concentrations in these target brain areas by blocking reuptake of dopamine at the axon terminal (Giorgetti et al., 2001; Kalivas and Duffy, 1993). As a result, accumulation of extracellular dopamine by drug intake may induce abnormal dopamine-dependent plasticity (Gerfen and Surmeier, 2011). Indeed, single or repeated exposure to addictive drugs induces long-term synaptic plasticity that can persist for months (Borgland et al., 2004). Such observations have supported the view that addictive drugs hijack dopamine pathways and may account for long-lasting remodeling of synaptic transmission (Wise and Koob, 2014).
A physiological consequence of increased excitatory inputs to VTA dopamine neurons is the heighted activation of the mesolimbic pathway, which may in turn contribute to addiction states (Saal et al., 2003; Ungless et al., 2001). These findings have been substantiated by recent studies using optogenetic manipulation mimicking the activity of dopamine neurons and acting as a positive reinforcer (Steinberg et al., 2014). For example, activation of dopamine neurons supports operant responding, which represents reward-seeking behaviors (Adamantidis et al., 2011; Pascoli et al., 2015), and conditioned place preference (CPP), which represents reward learning (Tzschentke, 1998), both of which are paralleled by an elevation of dopamine (Tsai et al., 2009; Witten et al., 2011). Thus, activation of the mesostriatal dopaminergic pathway could determine dopamine-induced plasticity that is critical for setting up and maintaining drug addiction.
The NAc receives not only dopaminergic but also GABAergic inputs from the mesolimbic pathway (Brown et al., 2012). However, it is not well understood how inhibitory transmission is provided by the long-range GABAergic projections from the VTA, and whether or not the pathway modulates drug-seeking behavior. The VTA GABAergic projections synapse on the soma and proximal dendrites of ChINs in the NAc (Brown et al., 2012). ChINs express D2Rs and also control dopamine release; thus activation of ChINs could modulate spontaneous dopamine release (Alcantara et al., 2003; Cachope et al., 2012; Yorgason et al., 2017). Moreover, collateral dopaminergic and GABAergic projections from the VTA to the NAc heterosynaptically induce long-term depression (LTD) in inhibitory transmission (Ishikawa et al., 2013). Interestingly, this LTD is occluded after withdrawal from cocaine exposure (Ishikawa et al., 2013). Thus, the physiological roles of the accumbal ChINs could contribute to the altered emotional and motivational states that occur during drug (Warner-Schmidt et al., 2012). However, it is still unclear whether and how this cholinergic regulation is involved in controlling addiction-like behaviors.
CORTICO-STRIATAL CIRCUIT
The corticostriatal pathway has been extensively characterized, and its physiological relevance has long been emphasized as a part of the cortico–striato–thalamic circuit that is implicated in cognitive hierarchies (Haber, 2003; Kalivas, 2009). Specifically, the PFC participates in modulating goal-directed behaviors by re-evaluation of drug-associated instrumental response contingency (Dalley et al., 2004; Killcross and Coutureau, 2003; Ostlund and Balleine, 2005). Neuronal information from the PFC is conveyed to the striatum, which may result in habit learning (Yin and Knowlton, 2006). Indeed, synaptic potentiation is observed in the medial PFC–striatal circuits of drug-seeking mice after sustained withdrawal. This increased synaptic strength may suggest the potential role of the medial PFC–striatal pathway for cue-induced drug-seeking responses (Pascoli et al., 2014). The medial PFC can be further divided into the prelimbic cortex (PrL) and infralimbic cortex (IL), preferentially projecting to the NAc core and shell, respectively. The PrL and IL putatively exhibit opposite roles in drug addiction, especially when being subjected to changing environmental contingencies during and after extinction training. Consistent with this notion, inactivation of the PrL prevents reinstatement of drug memory (Fuchs et al., 2007; Kalivas and McFarland, 2003; Stefanik et al., 2013), whereas inactivation of the IL facilitates reinstatement of drug-seeking behavior (Peters et al., 2008). However, there are incongruent studies indicating functional roles of the medial PFC in incubation of drug craving (Bossert et al., 2011; Koya et al., 2009; Ma et al., 2014). Therefore, it is worth investigating how distinct corticostriatal pathways control and sculpt the learning and expression of goal-directed instrumental behavior, ultimately updating the value of drug-seeking behavior.
AMYGDALO–ACCUMBAL CIRCUIT
Addictive drugs or psychostimulants modulate emotional states, and recreational drug use can induce positive reinforcement and advance the progression of the addiction stages. The amygdala, which plays pivotal roles in emotional learning and memory, also appears to be involved in addiction-like behavior. Principal neurons in the BLA project to the NAc, and the functional role of this pathway has been initially addressed by disconnection studies. For example, selective lesion of the BLA or NAc core results in impaired acquisition of drug-seeking behavior (Fuchs et al., 2002; Whitelaw et al., 1996). The BLA–NAc pathway was recently shown to mediate behaviors associated with positive or negative valences (Kim et al., 2016; Paton et al., 2006; Stuber et al., 2011). Applying optical stimulation to this pathway promotes motivated behavior, which requires D1R-expressing but not D2R-expressing MSNs (Stuber et al., 2011). Stuber and colleagues (2011) demonstrated that intracranial self-stimulation of the amygdala projection, but not the cortical inputs, to the NAc induces positive reinforcement. The data are consistent with other studies that indicate significant alteration of the D1R-expressing MSNs after repeated drug exposure and the previous observation that the amygdala–striatal circuits are critical for selectively strengthening the innervation of D1R-expressing MSNs in the NAc (Lee et al., 2013; Pascoli et al., 2014). Furthermore, synaptic alterations in only the BLA–NAc circuit are sufficient to control locomotor sensitization (MacAskill et al., 2014), CPP expression, and craving behavior through the maturation of silent synapses and recruitment of calcium-permeable AMPA receptors (Brown et al., 2011; Lee et al., 2013; Shukla et al., 2017). The hM4Di-mediated chemogenetic modulation of Gi/o signaling in the amygdala–striatal circuit attenuates locomotor sensitization to drug exposure, but does not affect basal locomotion (MacAskill et al., 2014). Taken together, these findings suggest that the BLA–NAc circuit plays necessary and critical roles for reinforcement learning, and putatively addiction-like, behaviors.
HIPPOCAMPAL–STRIATAL CIRCUIT
The ventral hippocampus (vHPC) is another major source of glutamatergic inputs to the NAc, especially to the medial shell (Zhu et al., 2016). Indeed, vHPC neurons activate NAc MSNs, with stronger inputs on D1R-expressing rather than D2R-expressing MSNs. This vHPC–NAc pathway is also affected by cocaine exposure. After repeated non-contingent injections of cocaine, the bias in the amplitude of excitatory currents in D1R- and D2R-MSNs is abolished, suggesting that the vHPC–NAc pathway is capable of mediate drug-induced synaptic plasticity (MacAskill et al., 2014). Indeed, lesions of the dorsal subiculum result in hyperactivity, whereas lesions of the ventral subiculum reduce locomotor responses to amphetamine and impair acquisition of cocaine self-administration (Caine et al., 2001; Rogers and See, 2007). Interestingly, the vHPC–striatal pathway is potentiated after drug exposure (Britt et al., 2012) and supports discrimination of drug-associated actions in the operant chamber (Pascoli et al., 2014). Thus, hippocampal inputs to the NAc, especially to the shell, would be highly involved in both the psychomotor stimulant effect and information processing of the contextual values. The preponderance of evidence suggests that the hippocampus is necessary for the expression of drug addiction-like behaviors.
STRIATAL DIRECT AND INDIRECT PATHWAYS
As described above, GABAergic MSNs constitute either the direct or indirect pathway based on their projection targets. The direct pathway comprises D1R-expressing MSNs that directly project to basal ganglia output nuclei, such as the substantia nigra or subthalamic nucleus. By contrast, the indirect pathway is composed of D2R-expressing MSNs that project to other basal ganglia nuclei that subsequently innervate output nuclei (e.g., the globus pallidus externa) (Gerfen and Surmeier, 2011). The D1R is a Gs/a protein-coupled receptor whose activation results in stimulation of adenylyl cyclase, whereas the D2R is a Gi/a protein-coupled receptor whose activation inhibits adenylyl cyclase (Neve et al., 2004). Chemogenetic inhibition of D1R-MSNs in the dorsal striatum suppresses locomotor sensitization, while inhibition of D2R-MSNs promotes locomotor activity after amphetamine exposure (Ferguson et al., 2011). Furthermore, dorsal striatal D1R-MSNs likely mediate acquisition of reinforced behavior and place preference behavior, whereas D2R-MSNs play a sufficient role for place aversion (Kravitz et al., 2012). Chemogenetic inhibition of striatal D2R-MSNs increases motivation for cocaine (Bock et al., 2013).
Expression of D1R is necessary to produce cocaine self-administration behavior (Caine et al., 2007). By contrast, D2R is not essential for self-administration behavior (Caine et al., 2002), but the activation of striatal D2R-MSNs rather impairs locomotor sensitization (Lobo et al., 2010). Furthermore, the ablation of striatal D2R-expressing MSNs results in increased amphetamine CPP (Durieux et al., 2009), suggesting that D2R-expressing MSNs in the NAc play an inhibitory role in addiction-like behaviors. Taken together, this evidence suggests that the expression of addiction-like behaviors is controlled by the balanced activity of D1Rs and D2Rs, which are differentially expressed in distinct subtypes of projection neurons in the striatum. However, it still remains challenging to conclusively establish differential roles for each MSN type in addiction-like behaviors.
Axons from both D1R-MSNs and D2R-MSNs in the NAc innervate the ventral pallidum (VP) (Creed et al., 2016). These pathways appear to encode the overall direction of the behavioral outputs. Normalization of cocaine-induced plasticity at NAc–VP synapses by optogenetic modulation of the direct pathway indicates that the collateral NAc–VP pathway composed of D1R-MSNs is necessary for locomotor sensitization and maintenance of motivation for cocaine seeking (Creed et al., 2016). Interestingly and also in agreement with the optogenetic results, drug-induced (i.e., amphetamine) sensitization is blocked by Gs-coupled receptor activation of the adenosine A2a receptor, a marker of D2R-MSNs, expressing neurons (Farrell et al., 2013). Thus, activation of D2R-MSNs seems to lead to lateral inhibition of the D1R-MSNs in the NAc to control reward-related behaviors. Exposure to cocaine suppresses this lateral inhibition, which thus promotes behavioral sensitization (Dobbs et al., 2016).
ADDITIONAL COMPONENTS UNDERLYING ADDICTION-LIKE BEHAVIORS
In the progression of drug addiction, relapse is the recurrence of addiction that had advanced to recovery or remission. Stress is a major priming stimulus for triggering relapse (Kalivas and McFarland, 2003), and addictive drugs that have hedonic effects may help cope with the stressful conditions. There is ample evidence that stress increases the occurrence of relapse, but the cellular and molecular mechanisms have just begun to be addressed. For example, activation of extracellular signal-regulated kinase by brain-derived neurotrophic factor (BDNF) in the mesostriatal pathway is required for acquisition of drug-induced sensitization and CPP (Lobo et al., 2010). BDNF-mediated dopamine neuron activation is controlled by corticotropin-releasing factor (CRF; also known as corticotropin-releasing hormone), which is released under stressful conditions (Walsh et al., 2014). CRF signaling, which arises from the extended amygdala structures, including the central amygdala, may contribute to the priming of drug seeking in stressful conditions (Shaham et al., 2000).
Another factor that needs to be addressed in drug addiction is the connectivity between neural ensembles that arise from the association between sensory inputs and the hedonic effect of drugs. Considering that drug-induced plasticity occurs at a small subset of activated striatal neurons (Koya et al., 2012), neuronal connectivity would change between drug-recruited neurons and the other neuronal components, which would sculpt the acquisition and expression of drug-related memory. Additional research dedicated to this line of study will benefit further understanding of circuit-mediated addictive behavior.
CONCLUSION
The aim of circuit-wide and circuit-specific investigations for addiction-like behavior is to elucidate addiction mechanisms and offer successful therapeutic intervention for addiction. Accumulated data indicate that the striatum is a key brain area involved in drug addiction, as striatal circuits play critical roles in setting-up of addiction-like behaviors and are critically involved in all stages of addiction progression, from initial exposure to relapse. Studies using optogenetic and chemogenetic strategies have revealed distinct neuronal circuits relevant to the progression of addiction and shared circuits with common behavioral consequences after exposure to various psychostimulants (Fig. 2). Striatal circuit-selective activation–inactivation or potentiation–depotentiation precedes the significant alteration of addiction-like behaviors, substantiating the net effect of an individual circuit on the progression of addiction. After an exposure to psychostimulant drugs, motor activity is controlled by inputs to the striatum from the vHPC and the amygdala and via the direct and indirect pathways to increase striatal dopamine levels. These pathways are also necessary for encoding components of addictive drug-related learning and memories after repeated use. Furthermore, relapse to psychostimulant drugs after abstinence largely involves the PFC, which projects to the ventral striatum, for the expression of craving or compulsive drug-seeking behaviors. Among the striatal circuits involved in the progression of addiction, activation of the IL–NAc shell and striatal D2R-MSN indirect pathways are effective for inhibiting related behavioral expression. Indeed, natural protective mechanisms of the striatal indirect pathway have been described (Bock et al., 2013), and striatal circuit-selective restoration of synaptic transmission has been shown to normalize circuit functions and rescue animal behaviors (Lüscher et al., 2015). Therefore, circuit-specific modulations provide a promising key solution for the development of effective therapeutic interventions that ameliorate (or even cure) addiction at each step of the addiction processes.
Fig. 2.
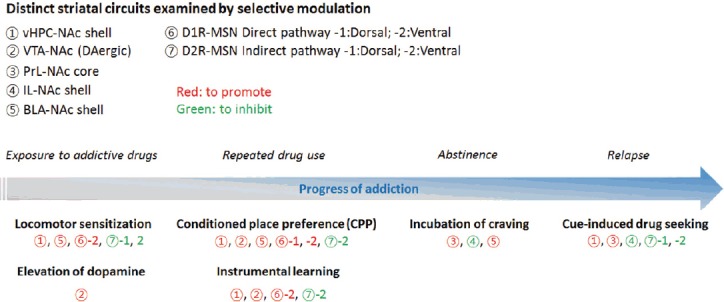
Distinct striatal circuits involved in the progression of addiction-like behaviors.
Each pathway (represented by numbers) has been examined using optogenetic or chemogenetic modulation to determine its physiological contribution to the various addiction progression phases.
ACKNOWLEDGMENTS
This work was supported by grants from the National Research Foundation of Korea (2014051826 and NRF-2017R1 A2B2004122) to J.-H.K.
REFERENCES
- Adamantidis A.R., Tsai H.C., Boutrel B., Zhang F., Stuber G.D., Budygin E.A., Tourino C., Bonci A., Deisseroth K., de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara A.A., Chen V., Herring B.E., Mendenhall J.M., Berlanga M.L. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 2003;986:22–29. doi: 10.1016/s0006-8993(03)03165-2. [DOI] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amalric M., Koob G.F. Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog Brain Res. 1993;99:209–226. doi: 10.1016/s0079-6123(08)61348-5. [DOI] [PubMed] [Google Scholar]
- Bock R., Shin J.H., Kaplan A.R., Dobi A., Markey E., Kramer P.F., Gremel C.M., Christensen C.H., Adrover M.F., Alvarez V.A. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam J.P., Hanley J.J., Booth P.A.C., Bevan M.D. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland S.L., Malenka R.C., Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert J.M., Stern A.L., Theberge F.R., Cifani C., Koya E., Hope B.T., Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt J.P., Benaliouad F., McDevitt R.A., Stuber G.D., Wise R.A., Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.T., Tan K.R., O’Connor E.C., Nikonenko I., Muller D., Lüscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Brown T.E., Lee B.R., Mu P., Ferguson D., Dietz D., Ohnishi Y.N., Lin Y., Suska A., Ishikawa M., Huang Y.H., et al. A silent synapse-based mechanism for cocaine-Induced locomotor sensitization. J Neurosci. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R., Mateo Y., Mathur B.N., Irving J., Wang H.L., Morales M., Lovinger D.M., Cheer J.F. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine S.B., Humby T., Robbins T.W., Everitt B.J. Behavioral effects of psychomotor stimulants in rats with dorsal or ventral subiculum lesions : locomotion, cocaine self-administration, and prepulse inhibition of startle. Behav Neurosci. 2001;115:880–894. doi: 10.1037//0735-7044.115.4.880. [DOI] [PubMed] [Google Scholar]
- Caine S.B., Negus S.S., Mello N.K., Patel S., Bristow L., Kulagowski J., Vallone D., Saiardi A., Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine S.B., Thomsen M., Gabriel K.I., Berkowitz J.S., Gold L.H., Koob G.F., Tonegawa S., Zhang J., Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M., Ntamati N.R., Chandra R., Lobo M.K., Lüscher C. Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron. 2016;92:214–226. doi: 10.1016/j.neuron.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden J.R., Graybiel A.M. Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:1–25. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J.W., Cardinal R.N., Robbins T.W. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dobbs L.K., Kaplan A.R., Lemos J.C., Matsui A., Rubinstein M., Alvarez V.A. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron. 2016;90:1100–1113. doi: 10.1016/j.neuron.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux P.F., Bearzatto B., Guiducci S., Buch T., Waisman A., Zoli M., Schiffmann S.N., de Kerchove d’Exaerde A. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Farrell M.S., Pei Y., Wan Y., Yadav P.N., Daigle T.L., Urban D.J., Lee H.M., Sciaky N., Simmons A., Nonneman R.J., et al. A Gαs DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology. 2013;38:854–862. doi: 10.1038/npp.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S.M., Neumaier J.F. Using DREADDs to investigate addiction behaviors. Curr Opin Behav Sci. 2015;2:69–72. doi: 10.1016/j.cobeha.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S.M., Eskenazi D., Ishikawa M., Wanat M.J., Phillips P.E., Dong Y., Roth B.L., Neumaier J.F. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund T.F., Powell J.F., Smith A.D. Freund Tyrosine hydroxylase immunoreactive boutons in synaptic contact with identified striatonigral neurons with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Fuchs R.A., Weber S.M., Rice H.J., Neisewander J.L. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Fuchs R.A., Eaddy J.L., Su Z.I., Bell G.H. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Gerfen C.R., Surmeier D.J. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C.R., Engber T.M., Mahan L.C., Susel Z., Chase T.N., Monsma F.J., Jr, Sibley D.R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Giorgetti M., Hotsenpiller G., Ward P., Teppen T., Wolf M.E. Amphetamine-induced plasticity of AMPA receptors in the ventral tegmental area: effects on extracellular levels of dopamine and glutamate in freely moving rats. J Neurosci. 2001;21:6362–6369. doi: 10.1523/JNEUROSCI.21-16-06362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hyman S.E., Malenka R.C., Nestler E.J. NEURAL MECHANISMS OF ADDICTION: The Role of Reward-Related Learning and Memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Otaka M., Huang Y.H., Neumann P.A., Winters B.D., Grace A.A., Schlu O.M., Dong Y. Dopamine Triggers Heterosynaptic Plasticity. J Neurosci. 2013;33:6759–6765. doi: 10.1523/JNEUROSCI.4694-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P.W. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P.W., McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Killcross S., Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kim J., Pignatelli M., Xu S., Itohara S., Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci. 2016;19:1636–1646. doi: 10.1038/nn.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid A.E., Zheng T., Wilson C.J. Connectivity and convergence of single corticostriatal axons. J Neurosci. 1998;18:4722–4731. doi: 10.1523/JNEUROSCI.18-12-04722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E., Uejima J.L., Wihbey K.A., Bossert J.M., Hope B.T., Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56:177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E., Cruz F.C., Ator R., Golden S.A., Hoffman A.F., Lupica C.R., Hope B.T. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15:1556–1562. doi: 10.1038/nn.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz A.V., Tye L.D., Kreitzer A.C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.R., Ma Y.Y., Huang Y.H., Wang X., Otaka M., Ishikawa M., Neumann P.A., Graziane N.M., Brown T.E., Suska A., et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo M.K., Covington H.E., 3rd, Chaudhury D., Friedman A.K., Sun H., Damez-Werno D., Dietz D.M., Zaman S., Koo J.W., Kennedy P.J., et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C., Pascoli V., Creed M. Optogenetic dissection of neural circuitry: From synaptic causalities to blue prints for novel treatments of behavioral diseases. Curr Opin Neurobiol. 2015;35:95–100. doi: 10.1016/j.conb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Ma Y.Y., Lee B.R., Wang X., Guo C., Liu L., Cui R., Lan Y., Balcita-Pedicino J.J., Wolf M.E., Sesack S.R., et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill A.F., Cassel J.M., Carter A.G. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17:1198–1207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve K.A., Seamans J.K., Trantham-Davidson H. Dopamine Receptor Signaling. J Recept Signal Transduct. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Ostlund S.B., Balleine B.W. Lesions of Medial Prefrontal Cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci. 2005;25:7763–7770. doi: 10.1523/JNEUROSCI.1921-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V., Terrier J., Espallergues J., Valjent E., O’Connor E.C., Lüscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Pascoli V., Terrier J., Hiver A., Lu C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron. 2015;88:1054–1066. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Paton J.J., Belova M.A., Morrison S.E., Salzman C.D. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Vallone J., Laurendi K., Kalivas P.W. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 2008;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Rogers J.L., See R.E. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D., Dong Y., Bonci A., Malenka R.C. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shukla A., Beroun A., Panopoulou M., Neumann P.A., Grant S.G., Olive M.F., Dong Y., Schlüter O.M. Calcium–permeable AMPA receptors and silent synapses in cocaine–conditioned place preference. EMBO J. 2017;36:458–474. doi: 10.15252/embj.201695465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y., Bennett B.D., Bolam J.P., Parent A., Sadikot A.F. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344:1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- Stefanik M.T., Moussawi K., Kupchik Y.M., Smith K.C., Miller R.L., Huff M.L., Deisseroth K., Kalivas P.W., Lalumiere R.T. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg E.E., Boivin J.R., Saunders B.T., Witten I.B., Deisseroth K., Janak P.H. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS One. 2014;9:e94771. doi: 10.1371/journal.pone.0094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber G.D., Sparta D.R., Stamatakis A.M., van Leeuwen W.A., Hardjoprajitno J.E., Cho S., Tye K.M., Kempadoo K.A., Zhang F., Deisseroth K., et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.C., Zhang F., Adamantidis A., Stuber G.D., Bonci A., de Lecea L., Deisseroth K. Phasic Firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye K.M., Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke T.M. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Ungless M.A., Whistler J.L., Malenka R.C., Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Walsh J.J., Friedman A.K., Sun H., Heller E.A., Ku S.M., Juarez B., Burnham V.L., Mazei-Robison M.S., Ferguson D., Golden S.A., et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt J.L., Schmidt E.F., Marshall J.J., Rubin A.J., Arango-Lievano M., Kaplitt M.G., Ibañez-Tallon I., Heintz N., Greengard P. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci USA. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw R.B., Markou A., Robbins T.W., Everitt B.J. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcememt. Psychopharmacology. 1996;127:213–224. [PubMed] [Google Scholar]
- Wise R.A. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Wise R.A., Koob G.F. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten I.B., Steinberg E.E., Lee S.Y., Davidson T.J., Zalocusky K.A., Brodsky M., Yizhar O., Cho S.L., Gong S., Ramakrishnan C., et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.H., Knowlton B.J. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yorgason J.T., Zeppenfeld D.M., Williams J.T. Cholinergic interneurons underlie spontaneous dopamine release in nucleus accumbens. J Neurosci. 2017;37:2086–2096. doi: 10.1523/JNEUROSCI.3064-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Wienecke C.F., Nachtrab G., Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530:219–222. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]


