Abstract
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease characterized by lack of insulin and high glucose levels. T2DM can cause bone loss and fracture, thus leading to diabetic osteoporosis. Promoting osteogenic differentiation of osteoblasts may effectively treat diabetic osteoporosis. We previously reported that Sirtuin 1 (Sirt1), a NAD+-dependent deacetylase, promotes osteogenic differentiation through downregulation of peroxisome proliferator-activated receptor (PPAR) γ. We also found that miR-132 regulates osteogenic differentiation by downregulating Sirt1 in a PPARβ/δ-dependent manner. The ligand-activated transcription factor, PPARα, is another isotype of the peroxisome proliferator-activated receptor family that helps maintain bone homeostasis and promot bone formation. Whether the regulatory role of PPARα in osteogenic differentiation is mediated via Sirt1 remains unclear. In the present study, we aimed to determine this role and the underlying mechanism by using high glucose (HG) and free fatty acids (FFA) to mimic T2DM in MC3T3-E1 cells. The results showed that HG-FFA significantly inhibited expression of PPARα, Sirt1 and osteogenic differentiation, but these effects were markedly reversed by PPARα overexpression. Moreover, siSirt1 attenuated the positive effects of PPARα on osteogenic differentiation, suggesting that PPARα promotes osteogenic differentiation in a Sirt1-dependent manner. Luciferase activity assay confirmed interactions between PPARα and Sirt1. These findings indicate that PPARα promotes osteogenic differentiation via the Sirt1-dependent signaling pathway.
Keywords: diabetic osteoporosis, MC3T3-E1, Sirt 1, osteogenic differentiation, PPARα
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is the most common type of diabetes mellitus. It accounts for more than 90% of diabetic patients and usually develops after 35–40 years of age. T2DM is characterized by hyperglycemia and redundant fatty acid secretion due to insulin resistance and decreased insulin sensitivity (Schwartz, 2016). Diabetic osteoporosis is a major complication of T2DM that originates mainly from alterations in the bone microenvironment (Zhang et al., 2016), thus leading to subsequent bone loss, mineral density reduction and fractures (Schwartz, 2016).
High glucose (HG) and free fatty acids (FFA) reportedly inhibit osteogenic differentiation (You et al., 2014) and induce apoptosis of osteoblasts (Feng et al., 2011). Bone metabolic homeostasis relies on the balance between osteoblast-induced bone formation and osteoclast-induced bone resorption (Raisz, 2005). Diabetic osteoporosis primarily results from the disequilibrium between osteoblast and osteoclast, as osteoblast activity is significantly decreased by HG and FFA in T2DM (Rakel et al., 2008). This imbalance leads to reduced bone formation, impairment in bone mineral density and bone strength, and destruction of bone microarchitecture (Blakytny et al., 2011). Reinforcing and recovering osteogenic differentiation of osteoblasts and rebalancing the number of osteoblasts and osteoclasts may help to treat diabetic osteoporosis.
Peroxisome proliferator-activated receptors (PPARs) are ligand-inducible nuclear hormone receptors that are ubiquitously expressed in many biological processes (Chang et al., 2007; Desvergne and Wahli, 1999; Issemann and Green, 1990). Among the three PPAR subtypes in mammals, PPARα, PPARβ/δ, and PPARγ, PPARα plays a positive role in osteogenic differentiation. For example, Takano et al. (2012) reported that PPARα promotes the osteoblastic differentiation induced by bone morphogenetic proteins 4 by enhancing their receptor signaling. Other evidence indicates that, in addition to affecting osteogenic differentiation, PPARα regulates bone metabolism by providing energy through fatty acid oxidation and by controlling hematopoietic cell lineage fate rather than just simply affecting osteogenic differentiation (Lecka-Czernik, 2010). The PPARα agonist fenofibrate also increases bone mineral density and plays a positive role in skeletal homeostasis (Syversen et al., 2009). Stunes et al. (2011) reported that administration of the PPARα agonist fenofibrate can maintain bone quality in an ovariectomized rat model. However, the role of PPARα in diabetic osteoporosis and its underlying mechanism remain largely unknown.
The Sirtuin family (Sirt1–7) includes evolutionarily highly conserved nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases that can deacetylate and hence regulate multiple transcription factors, such as p53, peroxisomal proliferator-activated receptor gamma coactivator-1α (PGC-1α), p300, Forkhead box protein O1, and nuclear factor-kappaB (NF-κB) (Choi and Kemper, 2013; Finkel et al., 2009). Sirt1 is the most widely studied molecule that reportedly participates in resisting obesity, diabetes, cardiovascular diseases, and neurodegenerative disease (Haigis and Sinclair, 2010). Moreover, Sirt1 functions in the resistance against stress and inflammatory responses (Yao and Rahman, 2012). Sirt1 also facilitates osteogenesis. For instance, Sirt1 promotes the differentiation of mesenchymal stem cells into osteoblasts rather than adipocytes (Backesjo et al., 2006). Resveratrol, a natural specific agonist of Sirt1 (Borra et al., 2005), increases osteoblastogenesis and reduces adipogenesis (Backesjo et al., 2006). Our previous results showed that mouse pre-osteoblast MC3T3-E1 cells cultured under osteogenic medium (OM) demonstrated strong osteogenic differentiation and that HG-FFA reversed this trend (Gong et al., 2016). Sirt1 overexpression also significantly promoted the osteogenic differentiation of MC3T3-E1 cells, but Sirt1 expression notably increased during osteogenic differentiation (Qu et al., 2016). These results prompted us to investigate whether PPARα regulates osteogenic differentiation via the Sirt1-dependent signaling pathway.
In the present study, MC3T3-E1 cells were cultured in OM supplemented with HG and FFA to mimic diabetic conditions. The expression of PPARα was evaluated. We focused on the role of PPARα overexpression in the osteogenic differentiation of MC3T3-E1 cells and the underlying mechanism. Because Sirt1 promotes osteogenic differentiation, the interactions between PPARα and Sirt1 were also explored using the luciferase assay. Taken together, this study illustrates the effects of PPARα on osteogenic differentiation under diabetic conditions and reveals the molecular mechanism through which PPARα functions, which may benefit the treatment of diabetic osteoporosis.
MATERIALS AND METHODS
Cell culture and treatment with HG-FFA
MC3T3-E1 cells and 293T cells were cultured as previously described (Gong et al., 2016; Qu et al., 2016). Briefly, MC3T3-E1 cells obtained from ATCC (USA) were cultured inα-MEM (Hyclone, USA) supplemented with 10% fetal bovine serum (Hyclone) and 100 U/ml penicillin-streptomycin (Hyclone) at 37°C with 5% CO2. 293T cells were cultured in DMEM (Hyclone). For osteogenic differentiation, MC3T3-E1 cells were incubated in osteogenic medium (OM, additionally supplemented with 20 mM β-glycerophosphate and 100μg/ml ascorbic acid; Sigma, USA) for 0, 2, 6, 10, and 14 days. To mimic T2DM in MC3T3-E1 cells, HG and FFA were used as previously described (Gong et al., 2016) concurrently with osteogenic induction.
PPARα plasmid transfection
For PPARα overexpression, the recombinant plasmid pLasP-PARα was constructed by GenePharma (China), and then 4μg of pLasPPARα was transfected into MC3T3-E1 cells using Lipofectamine 2000 Reagent (Invitrogen, USA). The stably transfected cells were selected with G418 (Sigma). MC3T3-E1 cells transfected with empty plasmid served as a negative control (pLasNC). Plasmid transfection was performed prior to osteogenic induction.
Sirt1 siRNA transfection
To silence Sirt1, Sirt1 siRNA (siSirt, 5′-GCGTACGGCAATGGC TTTA-3′) was synthesized by GenePharma (Shanghai) and transfected into MC3T3-E1 cells using Lipofectamine 2000 (Invitrogen). Cells transfected with scrambled siRNA (Gene-Pharma, 5′-GCGCGGCGTAATCGATTTA-3′) served as a negative control (siNC). The 48 h later, Sirt1 expression was evaluated using quantitative PCR and Western blot. Sirt1 siRNA transfection was performed prior to osteogenic induction, but after stable transfection with PPARα plasmid.
Alkaline phosphatase activity assay
Alkaline phosphatase (ALP) activity was assessed using an Alkaline Phosphatase Diethanolamine Activity Kit (Sigma). Briefly, the cell lysates were resuspended in phosphate buffer solution (PBS) and incubated with p-nitrophenol phosphate (pNPP, Ameresco, USA) for 1 h at 37°C. Subsequently, the reaction was stopped with 2 M NaOH (provided with the kit) and absorbance at 405 nm was detected using a spectrophotometer (Bio-Rad, USA).
Alizarin Red staining
Briefly, MC3T3-E1 cells were washed twice with PBS and then fixed in 95% ethanol for 10 min at room temperature. After washing with sterilized water, cells were stained with 0.1% Alizarin Red-Tris-HCl (pH 8.3) at 37°C for 30 min. Orange and red bodies were thought to be mineralized nodules. 10% cetylpyridinium chloride was then used to dissolve the nodules, and absorbance was examined at 562 nm using a spectrophotometer (Bio-Rad).
Quantitative PCR
Total RNA was extracted using TRIzolR Reagent (Invitrogen) and reverse-transcribed into cDNA using PrimeScript™ RT reagent Kit (TaKaRa Biotechnology, China). Quantitative PCR was performed using SYBRR Premix Ex Taq (Takara Biotechnology) and a CFX96 touch qPCR system (Bio-Rad). All procedures followed the manufacturer’s instructions. The relative fold to reference gene, GAPDH, was calculated using the 2−ΔΔCt method. The primer sequences are shown in Table 1. The mRNA expression of Runx2 and Col1α1 was evaluated 14 days after osteogenic differentiation.
Table 1.
The primer sequences used in quantitative PCR
| Gene | Primer Sequences (5′-3′) | Product (bp) |
|---|---|---|
| PPARα | Forward: TGCAGCCTCAGCCAAGTTGAA Reverse: TTCCCGAACTTGACCAGCCA |
77 |
| Runx2 | Forward: TTCTCAGCTTTAGCGTCGTCA Reverse: GACAGATCTGGAGCCTGCGG |
195 |
| Col1α1 | Forward: GGGGCAAGACAGTCATCGAA Reverse: GAGGGAACCAGATTGGGGTG |
159 |
| Sirt1 | Forward: CGGCTACCGAGGTCCATATAC Reverse: ACAATCTGCCACAGCGTCAT |
135 |
| PPARγ | Forward: CCTCAGGTCAGAGTCGCCC Reverse: CCTTGTCGTCACACTCGGTC |
123 |
| PGC-1α | Forward: AGACAGGTGCCTTCAGTTCAC Reverse: TGGTCGCTACACCACTTCAA |
291 |
| GAPDH | Forward: CAGGTTGTCTCCTGCGACTT Reverse: GCCTCTCTTGCTCAGTGTCC |
218 |
Western blot
Proteins were extracted using RIPA buffer (Sigma), and 20μg of proteins were subjected to 10% SDS-PAGE and then transferred to nitrocellulose membrane (Merck Millipore, USA). After blocking with 10% skim milk in TBS-T (Sigma), the membrane was incubated with the primary antibodies overnight at 4°C. Primary antibodies are shown in Table 2. The membrane was then incubated with horseradish peroxidase-conjugated secondary antibodies (catalog No. 31466 or 31433 or 31480, 1:5000, Invitrogen) for 2 h at room temperature. Bands were detected using an enhanced chemiluminescence kit (Invitrogen). Densitometry analysis was performed using Image-Pro Plus 6.0 (Media Cybernetics, USA). The protein expression of Runx2 and Col1α1 was evaluated 14 days after osteogenic differentiation.
Table 2.
The primary antibodies used in Western blot
| Antibody | Host | Cat. No. | Dilution | Supplier |
|---|---|---|---|---|
| PPARα | Rabbit | PA1-822A | 1:1000 | Invitrogen |
| Runx2 | Rabbit | PA5-14816 | 1:1000 | Invitrogen |
| Col1α1 | Rabbit | PA5-29569 | 1:1000 | Invitrogen |
| Sirt1 | Rabbit | PA5-17074 | 1:500 | Invitrogen |
| PPARγ | Rabbit | PA1-824 | 1:500 | Invitrogen |
| PGC-1α | Rabbit | PA5-38022 | 1:1000 | Invitrogen |
| GAPDH | Rabbit | PA1-988 | 1:1000 | Invitrogen |
Luciferase assay
The luciferase assay was performed as previously described (Qu et al., 2016) to verify the regulatory role of PPARα in the activity of Sirt1. Briefly, an internal ribosome entry site was amplified and cloned into the BglII site of pGL6 plasmids (Beyotime, China). The PPARγ and PGC-1α promoter regions were amplified from mouse genomic DNA and cloned into the XhoI site of pGL6 plasmids, respectively. Subsequently, 100 ng of pGL6 constructs, 100 ng of pLasPPARα and siSirt1 constructs, and 2 ng of the Renilla luciferase plasmid pRL-TK expressing Renilla luciferase as an internal reference (Promega, USA) were co-transfected into 293T cells using Lipofectamine 2000 (Invitrogen). 24 hours later, luciferase activities were measured using the Dual Luciferase Reporter Gene Assay Kit (Beyotime) and SmartSpec Plus Spectrophotometer (Bio-Rad). All values were normalized to pRL-TK.
Statistical analysis
Values are presented as mean ± standard deviation (SD). Statistical analyses were performed using SPSS 19.0 software (SPSS Inc., USA) with Student’s t-test and one-way analysis of variance. P < 0.05 indicated statistical significance.
RESULTS
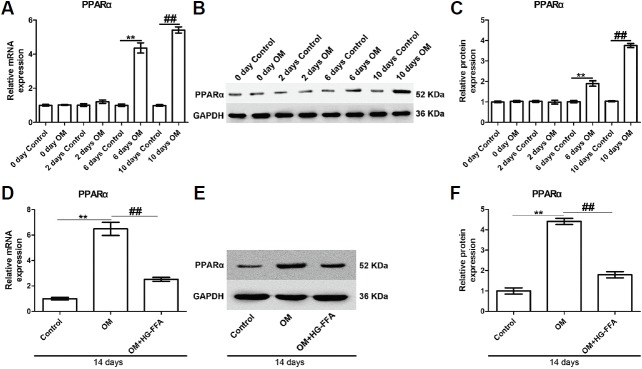
PPARα expression induced by OM is significantly down-regulated by HG-FFA
MC3T3-E1 cells were cultured in OM to induce osteogenic differentiation, and incubated in 25 mM of glucose and 1 mM of FFA to mimic T2DM for 0, 2, 6, 10, and 14 days. As shown in Figs. 1A–1C, PPARα expression gradually increased along with induction time and was significantly up-regulated from 6 days after osteogenic induction, compared with the respective control group, reaching a peak at day 14. Considering the degree of osteogenic differentiation, day 14 was selected as the induction time in the following experiments. Noteworthily, PPARα expression at day 14 was reciprocally down-regulated by HG-FFA treatment at both mRNA (Fig. 1D) and protein (Figs. 1E and 1F) levels. These results suggest that PPARα may play a role in the development of diabetic osteoporosis.
Fig. 1.
HG-FFA treatment markedly down-regulates PPARα expression induced by OM.
(A–C) PPARα expression gradually increased along with induction time and peaked at day 14. The significant up-regulation appeared at day 6 after osteogenic induction. (D–F) At day 14, HG-FFA treatment decreased PPARα expression significantly. (A, D) Quantitative PCR results. (B, E) Western blot bands. (C, F) The densitometry analysis of protein bands. Quantitative PCR and Western blot were performed 0, 2, 6, 10, and 14 days after osteogenic induction and HG-FFA treatment. **and ## indicate P < 0.01. N = 3.
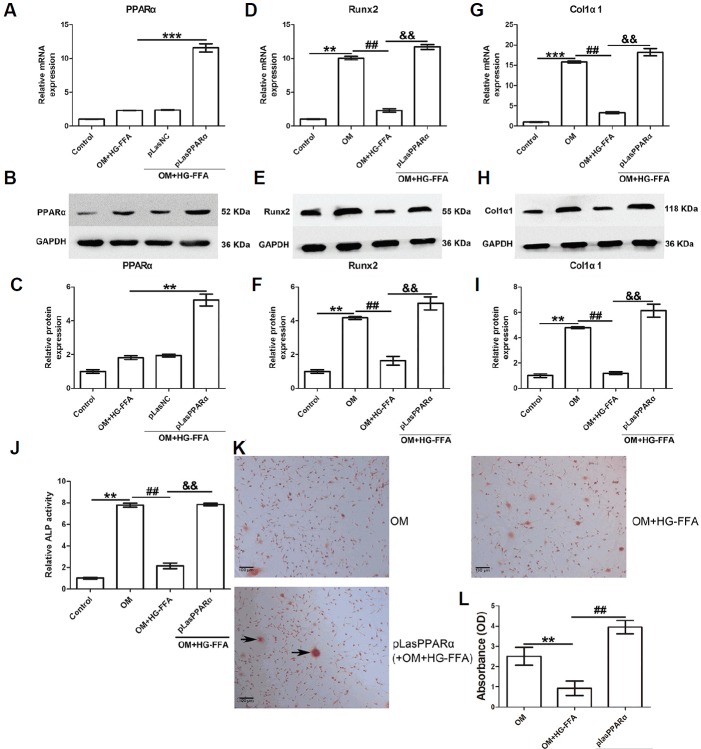
PPARα overexpression promotes osteogenic differentiation
Next, we aimed to determine the role of PPARα in osteogenic differentiation. As shown in Figs. 2A–2C, PPARα was over-expressed in MC3T3-E1 cells through pLasPPARα transfection in the presence of incubation for 14 days with OM and HG-FFA. The expression of Runx2 and Col1α1 was evaluated 14 days after osteogenic induction and HG-FFA treatment. Runx2 and Col1α1 expression, as shown in Figs. 2D–2I, was significantly induced by osteogenic induction, but HG-FFA treatment abolished their expression. Notably, PPARα over-expression significantly restored the expression of Runx2 (Figs. 2D–2F) and Col1α1 (Figs. 2G–2I). Compared to the OM with HG-FFA group, PPARα overexpression also markedly improved ALP activity (an approximately 3.7-fold increase) under HG-FFA conditions (Fig. 2J). The Alizarin Red staining results confirmed that PPARα overexpression increased the number of mineralized nodules (Fig. 2K) and therefore enhanced absorbance (Fig. 2L), indicating that PPARα contributed to promoting osteogenic differentiation.
Fig. 2.
PPARα overexpression facilitates osteogenic differentiation.
The expression of PPARα, Runx2, and Col1α1 was evaluated 14 days after osteogenic induction and HG-FFA treatment. (A–C) PPARα was overexpressed through pLasPPARα transfection. PPARα overexpression significantly restored Runx2 (D–F), Col1α1 (G–I) expression, and ALP activity (J) compared to the OM with HG-FFA treatment group. The Alizarin Red staining results demonstrated that PPARα overexpression induced more mineralized nodules (K) and therefore increased absorbance (L) under HG-FFA conditions, compared to the OM with HG-FFA treatment only. (C, F, and I) The densitometry analysis of Western blot results. *** indicates P < 0.001; **, ## and && indicate P < 0.01. N = 3. Scale bars = 100 μm.
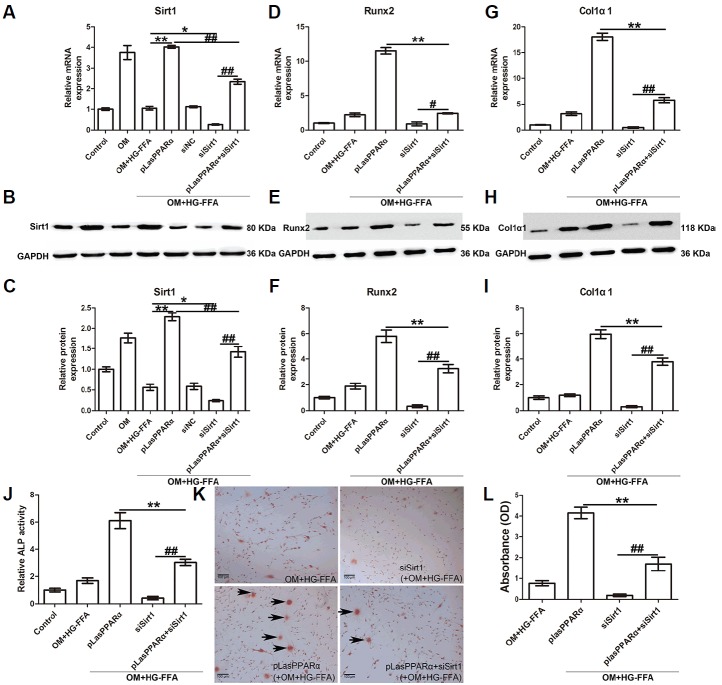
Knockdown of Sirt1 reverses the promotive effects of PPARα on osteogenic differentiation
We explored whether PPARα facilitates bone differentiation by regulating Sirt1 expression. The expression of Sirt1, Runx2, and Col1α1 was evaluated 14 days after osteogenic induction and HG-FFA treatment. As shown in Figs. 3A–3C, OM treatment significantly increased Sirt1 expression, whereas Sirt1 expression was reversed by OM+HG-FFA. Moreover, Sirt1 expression was remarkably up-regulated in the presence of PPARα overexpression, confirming their positive relationship, and siSirt1 transfection silenced the expression of Sirt1. When cells were co-transfected with pLasPPARα and siSirt1, Sirt1 expression was significantly restored, compared with siSirt1 transfection only (Figs. 3A–3C).
Fig. 3.
Silencing of Sirt1 reverses the promotive effects of PPARα on osteogenic differentiation.
The expression of Sirt1, Runx2, and Col1α1 was assessed 14 days after osteogenic induction and HG-FFA treatment. (A–C) Sirt1 expression was induced by OM and significantly promoted by PPARα overexpression under HG-FFA conditions. Sirt1 silencing by siSirt1 transfection reversed the effects of PPARα, thus significantly reducing Runx2 expression (D–F), Col1α1 expression (G–I), ALP activity (J), mineralized nodules (K) and absorbance (L) assessed by Alizarin Red staining. (C, F, and I) The densitometry analysis of Western blot results. **and ## indicate P < 0.01; *and # indicate P < 0.05. N = 3. Scale bars = 100 μm.
We subsequently investigated the effects of siSirt1 on osteogenic differentiation. The results showed that silencing of Sirt1 observably reversed the promotive effects of PPARα overexpression on the expression of Runx2 (Figs. 3D–3F) and Col1α1 (Figs. 3G–3I), and on ALP activity (Fig. 3J). Fewer mineralized nodules were observed when Sirt1 expression was silenced (Fig. 3K), accompanied by a significant decline in absorbance (Fig. 3L). These results suggest that Sirt1 is the effector of PPARα in the promotion of osteogenic differentiation of MC3T3-E1 cells. Non-transfected cells incubated in OM for only 2 days were also harvested for evaluation of the expression of Sirt1, Runx2, and Col1α1. As a result, expression of these three genes did not notably change compared with cells without OM treatment (Supplementary Fig. 1).
PPARα functions via the Sirt1-dependent signaling pathway
Finally, we aimed to confirm the interactions between PPARα and Sirt1 using a luciferase reporter assay. Because PPARγ and PGC-1α are both downstream molecules of Sirt1, the promoter regions of PPARγ and PGC-1α were amplified from mouse genomic DNA and cloned into the XhoI site of pGL6 plasmids, respectively. The recombinant plasmids were co-transfected into 293T cells, and the luciferase activity of recombinant cells was then evaluated. As shown in Fig. 4A, PPARα overexpression notably reduced the luciferase activity of PPARγ and PGC-1α promoters, accompanied by decreased mRNA (Fig. 4B) and protein (Figs. 4C and 4D) expression of PPARγ and PGC-1α. On the contrary, knockdown of Sirt1 significantly improved the relative luciferase activity, which was notably reversed by PPARα overexpression (Fig. 4A). PPARα overexpression also markedly reduced siSirt1 transfection-induced mRNA (Fig. 4B) and protein (Figs. 4C and 4D) expression of PPARγ and PGC-1α. These results indicate that PPARα facilitates osteogenic differentiation via the Sirt1-dependent signaling pathway.
Fig. 4.
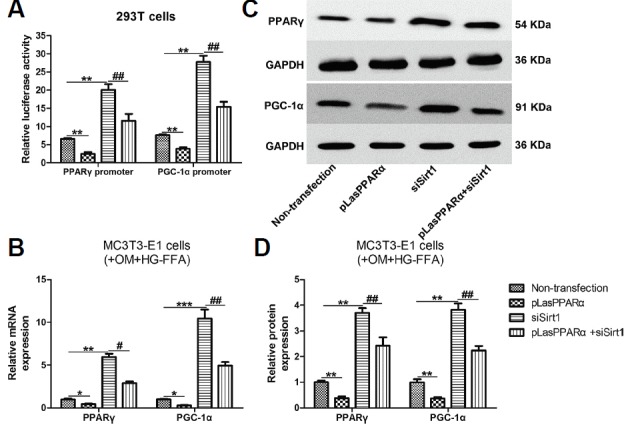
Interactions between PPARα and Sirt1.
First, 100 ng of pGL6 constructs, 100 ng of pLasPPARα and siSirt1 constructs, and 2 ng of the Renilla luciferase plasmid pRL-TK were co-transfected into 293T cells. The relative luciferase activity was then assessed using a Spectrophotometer. MC3T3-E1 cells transfected with pLasPPARα and siSirt1 were incubated in OM and HG-FFA for 14 days for evaluation of the expression of PPARγ and PGC-1α. (A) PPARα overexpression significantly decreased the relative luciferase activity of both PPARγ and PGC-1α promoters. Silencing of Sirt1 increased the relative luciferase activity, whereas PPARα overexpression reversed this activity significantly. PPARα overexpression markedly reduced and reversed the mRNA (B) and protein (C, D) expression of PPARγ and PGC-1α induced by Sirt1 silencing. (D) The densitometry analysis of Western blot results. *** indicates P < 0.001; ** and ## indicate P < 0.01; * and # indicate P < 0.05. N = 3.
DISCUSSION
In the present study, PPARα expression was found to be significantly up-regulated in MC3T3-E1 cells cultured under osteogenic conditions, but its expression was inhibited by HG-FFA treatment. These results indicate that PPARα may play a regulatory role in bone metabolism under T2DM conditions. Next, PPARα was overexpressed through plasmid transfection. High expression of PPARα markedly promoted osteogenic differentiation, as evaluated by ALP activity, Runx2 and Col1α1 expression, and Alizarin Red staining. However, silencing of Sirt1 remarkably reversed the promotive effects of PPARα, suggesting that PPARα regulates osteogenic differentiation via the Sirt1-dependent signaling pathway, which was further confirmed by the interactions between PPARα and Sirt1 assessed by luciferase assay. The Sirt1 downstream molecules PPARγ and PGC-1α were also found to be notably altered by the ectopic expression of PPARα, whereas their expression was reversed when Sirt1 was silenced by siSirt1 transfection. Collectively, our results suggest that PPARα contributes to bone differentiation in a Sirt1-dependent manner, which may benefit the development of new therapeutic targets.
Sirt1 plays a significant role in the modulation of multiple transcription factors through their deacetylation, thus inhibiting apoptosis, relieving senescence, and prolonging lifespan (Wang et al., 2011). In the present study, we found that PPARα overexpression significantly up-regulated the expression of Sirt1 (Figs. 3A–3C). Because Sirt1 is a known activator of bone formation (Backesjo et al., 2006; Cohen-Kfir et al., 2011), we therefore speculated that PPARα facilitates osteogenic differentiation through targeting Sirt1. The results showed that high expression of PPARα markedly suppressed the activity and expression of PPARγ and PGC-1α (Fig. 4), both of which are reportedly downstream molecules and effectors of Sirt1. To be specific, Sirt1 was reported to increase PPARγ expression in brown adipose tissues (Qiang et al., 2012) but decreased PPARγ expression in white adipose tissues (Picard et al., 2004). Moreover, knockout of Sirt1 in chronically obese mice promoted PPARγ activity (Mayoral et al., 2015). During osteogenic differentiation, we previously found that Sirt1 promotes osteoblast differentiation through down-regulation of PPARγ (Qu et al., 2016). In glucose homeostasis, Sirt1 modulated and deacetylated PGC-1α (Rodgers et al., 2005), a key regulator in the liver of diabetic mice that contributes to glyconeogenesis (Yoon et al., 2001). In brief, our results demonstrated notably inhibitory effects of PPARα on both the activity and expression of PPARγ and PGC-1α, indicating that PPARα regulates osteogenic differentiation in a Sirt1-dependent manner and that these two proteins could interact. In addition, the fact that PPARγ activation induces the differentiation of mesenchymal stem cells into adipocytes over osteoblasts (Ali et al., 2005) suggests that PPARα could block PPARγ signaling and therefore restore the differentiation into osteoblasts, which may benefit the treatment of diabetic osteoporosis with inhibition of PGC-1α and gluconeogenesis.
Until now, we have investigated the roles of all three PPARs subtypes (PPARα, PPARβ/δ, and PPARγ) in the osteogenic differentiation of MC3T3-E1 cells. We found that Sirt1 promotes osteogenic differentiation through down-regulating PPARγ expression (Qu et al., 2016) and that PPARβ/δ is necessary for the regulatory role of miR-132 by targeting Sirt1 in osteogenic differentiation of MC3T3-E1 cells (Gong et al., 2016). The present study indicates that PPARα has a positive effect on bone differentiation in a Sirt1-dependent manner. In conclusion, these studies provide compelling evidence that PPARs molecules interact with Sirt1, all of which could serve as therapeutic targets for the treatment of osteoporosis, especially diabetic osteoporosis. Further investigation and verification using in vivo studies would be worthwhile.
Supplementary data
ACKNOWLEDGMENTS
This work was supported by the Projects of Science and Technology of Sichuan Province, China (2015SZ0167).
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Ali A.A., Weinstein R.S., Stewart S.A., Parfitt A.M., Manolagas S.C., Jilka R.L. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- Backesjo C.M., Li Y., Lindgren U., Haldosen L.A. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res. 2006;21:993–1002. doi: 10.1359/jbmr.060415. [DOI] [PubMed] [Google Scholar]
- Blakytny R., Spraul M., Jude E.B. Review: The diabetic bone: a cellular and molecular perspective. Int J Low Extrem Wounds. 2011;10:16–32. doi: 10.1177/1534734611400256. [DOI] [PubMed] [Google Scholar]
- Borra M.T., Smith B.C., Denu J.M. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Chang F., Jaber L.A., Berlie H.D., O’Connell M.B. Evolution of peroxisome proliferator-activated receptor agonists. Ann Pharmacother. 2007;41:973–983. doi: 10.1345/aph.1K013. [DOI] [PubMed] [Google Scholar]
- Choi S.E., Kemper J.K. Regulation of SIRT1 by microRNAs. Mol Cells. 2013;36:385–392. doi: 10.1007/s10059-013-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kfir E., Artsi H., Levin A., Abramowitz E., Bajayo A., Gurt I., Zhong L., D’Urso A., Toiber D., Mostoslavsky R., et al. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology. 2011;152:4514–4524. doi: 10.1210/en.2011-1128. [DOI] [PubMed] [Google Scholar]
- Desvergne B., Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Feng Z., Deng H., Du J., Chen D., Jiang R., Liang X. Lentiviral-mediated RNAi targeting p38MAPK ameliorates high glucose-induced apoptosis in osteoblast MC3T3-E1 cell line. Indian J Exp Biol. 2011;49:94–104. [PubMed] [Google Scholar]
- Finkel T., Deng C.X., Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong K., Qu B., Liao D., Liu D., Wang C., Zhou J., Pan X. MiR-132 regulates osteogenic differentiation via downregulating Sirtuin1 in a peroxisome proliferator-activated receptor beta/delta-dependent manner. Biochem Biophys Res Commun. 2016;478:260–267. doi: 10.1016/j.bbrc.2016.07.057. [DOI] [PubMed] [Google Scholar]
- Haigis M.C., Sinclair D.A. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B. PPARs in bone: the role in bone cell differentiation and regulation of energy metabolism. Curr Osteoporos Rep. 2010;8:84–90. doi: 10.1007/s11914-010-0016-1. [DOI] [PubMed] [Google Scholar]
- Mayoral R., Osborn O., McNelis J., Johnson A.M., Oh D.Y., Izquierdo C.L., Chung H., Li P., Traves P.G., Bandyopadhyay G., et al. Adipocyte SIRT1 knockout promotes PPARgamma activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity. Mol Metab. 2015;4:378–391. doi: 10.1016/j.molmet.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Machado De Oliveira R., Leid M., McBurney M.W., Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L., Wang L., Kon N., Zhao W., Lee S., Zhang Y., Rosenbaum M., Zhao Y., Gu W., Farmer S.R., et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu B., Ma Y., Yan M., Gong K., Liang F., Deng S., Jiang K., Ma Z., Pan X. Sirtuin1 promotes osteogenic differentiation through downregulation of peroxisome proliferator-activated receptor gamma in MC3T3-E1 cells. Biochem BiophysRes Commun. 2016;478:439–445. doi: 10.1016/j.bbrc.2016.06.154. [DOI] [PubMed] [Google Scholar]
- Raisz L.G. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakel A., Sheehy O., Rahme E., LeLorier J. Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab. 2008;34:193–205. doi: 10.1016/j.diabet.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Schwartz A.V. Efficacy of osteoporosis therapies in diabetic patients. Calcif Tissue Int. 2016;100:165–173. doi: 10.1007/s00223-016-0177-8. [DOI] [PubMed] [Google Scholar]
- Stunes A.K., Westbroek I., Gustafsson B.I., Fossmark R., Waarsing J.H., Eriksen E.F., Petzold C., Reseland J.E., Syversen U. The peroxisome proliferator-activated receptor (PPAR) alpha agonist fenofibrate maintains bone mass, while the PPAR gamma agonist pioglitazone exaggerates bone loss, in ovariectomized rats. BMC Endocr Disord. 2011;11:11. doi: 10.1186/1472-6823-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syversen U., Stunes A.K., Gustafsson B.I., Obrant K.J., Nordsletten L., Berge R., Thommesen L., Reseland J.E. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr Disord. 2009;9:10. doi: 10.1186/1472-6823-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M., Otsuka F., Matsumoto Y., Inagaki K., Takeda M., Nakamura E., Tsukamoto N., Miyoshi T., Sada K.E., Makino H. Peroxisome proliferator-activated receptor activity is involved in the osteoblastic differentiation regulated by bone morphogenetic proteins and tumor necrosis factor-alpha. Mol Cell Endocrinol. 2012;348:224–232. doi: 10.1016/j.mce.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liang Y., Vanhoutte P.M. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett. 2011;585:986–994. doi: 10.1016/j.febslet.2010.11.047. [DOI] [PubMed] [Google Scholar]
- Yao H., Rahman I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol. 2012;84:1332–1339. doi: 10.1016/j.bcp.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C.R., Granner D.K., et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC–1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- You L., Gu W., Chen L., Pan L., Chen J., Peng Y. MiR-378 overexpression attenuates high glucose-suppressed osteogenic differentiation through targeting CASP3 and activating PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 2014;7:7249–7261. [PMC free article] [PubMed] [Google Scholar]
- Zhang W.L., Meng H.Z., Yang R.F., Yang M.W., Sun G.H., Liu J.H., Shi P.X., Liu F., Yang B. Melatonin suppresses autophagy in type 2 diabetic osteoporosis. Oncotarget. 2016;7:52179–52194. doi: 10.18632/oncotarget.10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.