Abstract
The primary cilium is a non-motile microtubule-based organelle that protrudes from the surface of most human cells and works as a cellular antenna to accept extracellular signals. Primary cilia assemble from the basal body during the resting stage (G0 phase) and simultaneously disassemble with cell cycle re-entry. Defective control of assembly or disassembly causes diverse human diseases including ciliopathy and cancer. To identify the effective compounds for studying primary cilium disassembly, we have screened 297 natural compounds and identified 18 and 17 primary cilium assembly and disassembly inhibitors, respectively. Among them, the application of KY-0120, identified as Brefeldin A, disturbed Dvl2-Plk1-mediated cilium disassembly via repression of the interaction of CK1ɛ-Dvl2 and the expression of Plk1 mRNA. Therefore, our study may suggest useful compounds for studying the cellular mechanism of primary cilium disassembly to prevent ciliopathy and cancer.
Keywords: brefeldin A, Dvl2, inhibitor, Plk1, primary cilium disassembly
INTRODUCTION
Primary cilia are microtubule-based cellular sensing structures important for transducing extracellular signals and observed in most stromal and epithelial cells (Satir and Christensen, 2008; Seeley and Nachury, 2010). They are assembled in the G0 or G1 phase of the cell cycle and are disassembled as the cell cycle progresses to mitosis (Santos and Reiter, 2008; Seeley and Nachury, 2010). Numerous signaling receptors are present in the primary cilium, which transmit numerous external signals into the cell (Goetz and Anderson, 2010; Rohatgi et al., 2007). Despite many studies, research on the assembly and disassembly of primary cilia are only at the beginning. We found a primary cilium disassembly pathway regulated by the Wnt5a-casein kinase 1ɛ (CK1ɛ)-Dvl2-Plk1 axis in our previous study (Lee et al., 2012). Wnt5a stimulation activates CK1ɛ, phosphorylating the S143 and T224 residues of Dvl2, and Plk1 binds to each of these two residues, triggering primary cilium disassembly. We confirmed that the binding of phosphorylated Dvl2 to Plk1 is the most important factor of primary cilium disassembly (Lee et al., 2012). Abnormalities in the mechanisms of assembly or disassembly of primary cilia lead to diseases called ciliopathy. The pleiotropic effect of ciliopathy has been revealed by defects in multiple organs: eyes (retinitis pigmentosa), skeleton (polydactyly), kidney (polycystic kidney disease, PKD), adipose tissue (obesity), pancreas (diabetes), and CNS (Goetz and Anderson, 2010; Mockel et al., 2011; Nachury et al., 2007; Park et al., 2006). Recently, abnormality in the assembly and disassembly of primary cilia is also involved in carcinogenesis (Gradilone et al., 2013; Han et al., 2009; Kim et al., 2011; Moser et al., 2009; Wong et al., 2009). Over the years, various factors involved in the assembly and disassembly of primary cilia have been identified (Goetz and Anderson, 2010; Lee et al., 2012; Pugacheva et al., 2007), but research on identifying the underlying mechanism has only started now.
Brefeldin A is a small hydrophobic fungal metabolite that was initially isolated as an antibiotic from toxic fungi and that induces the redistribution of Golgi apparatus into the endoplasmic reticulum (ER) (Klausner et al., 1992). BFA inhibits ADP-ribosylation factor (Arf) and subsequently induces the collapse of Golgi-derived protein secretion. Therefore, BFA has recently been used as a therapeutic inhibitor of Arf-related viral/bacterial diseases (Colanzi et al., 2013; Tseng et al., 2014). In this study, we screened 297 fungus-derived natural compounds to isolate inhibitors of primary cilium disassembly and found KY-0120, identified as Brefeldin A (BFA), as an effective inhibitor of Dvl2-Plk1-mediated primary cilium disassembly. Therefore, our study may provide a novel pharmaceutical inhibitor to prevent ciliopathy and cancer.
MATERIAL AND METHODS
Plasmid construction
A BamHI-EcoRI fragment of mouse Dvl2 S594E, T595E, and S597E mutant (3E) was sub-cloned into pcDNA-Flag (Invitrogen, USA). The BamHI-EcoRI fragment of mouse Dvl23E was generated by polymerase chain reaction (PCR) using pcDNA-Flag-Dvl2 wild-type (WT) as a template. The construction of pcDNA Flag-Dvl2 WT has been described previously (Lee et al., 2012).
Serum-starvation based ciliogenesis assay (the first round of screening), serum-restimulation assay (the second round of screening), and Immunofluorescence analyses
For serum-starvation based ciliogenesis assay, hTERT-RPE cells were seeded on cover slips in 24 culture wells, cultured for 24 h and then serum-starved for 24 h. Compounds [KY-compounds or DMSO (Sigma, USA)] were treated simultaneously with serum-starvation. For serum-restimulation assay, less than 50% cells were seeded on cover slips in 24 culture wells, cultured for 24 h and then serum-starved for 48 h. The resulting cells were then re-stimulated with serum to induce primary cilia disassembly for 24 h. For the serum-restimulation assay, compounds [KY-compounds, DMSO (Sigma), BFA (Sigma), or Monensin (Sigma)] were treated 30 min before serum-restimulation. Resulting cells were fixed with 4% paraformaldehyde for 10 min at room temperature and immunostained with mouse anti-acetylated α-tubulin (Sigma, 1:200) and rabbit anti-γ-tubulin (Sigma, 1:200) antibodies. The number of cells bearing cilia was counted manually under a microscope. To measure the length of primary cilia, images were acquired by Zeiss Axi-oObserver. Z1 microscope at 1388 X 1040 pixels and 12-bit resolution, and analyzed by ZEN v2.1 (Carl Zeiss) software.
GST pull-down assay and immunoblotting analysis
To perform GST pull-down assay, bead-associated GST-CK1ɛ WT was purified from bacteria (BL21) and incubated with total cell lysates of Flag-Dvl2 overexpressed HEK293T cells for 5 hours. DMSO or BFA was added to the incubation mixture. The precipitates were washed with TBSN buffer [20 mM Tris-Cl (pH8.0), 150 mM NaCl, 1.5 mM EDTA, 0.5% NP-40, 5 mM EGTA, 0.5 mM Na3VO4, and 20 mM p-nitrophenyl phosphate] 4 times and subjected to immunoblotting analysis with indicated antibodies. All the antibodies used in immunoblotting analyses are summarized in Supplementary Table S2.
Immunoprecipitation (IP)-kinase assays
For IP-kinase assays, HEK 293T cells transfected with Myc-tagged CK1ɛ (a gift of Jeffrey S. Rubin, NIH/NCI, MD) were harvested at 24 h after transfection and subjected to immunoprecipitation with anti-Myc antibody (Santa Cruz). Immunoprecipitates were reacted in a kinase cocktail [50 mM Tris-Cl (pH 7.5), 10 mM MgCl2, 2 mM EGTA, 5 mM dithiothreitol, 0.5 mM Na3VO4, and 20 mM p-nitrophenyl phosphate] in the presence of 10 μM ATP (10 μCi of [γ-32P]ATP) for 30 min at 30°C. Either DMSO, BFA, or D4476 (20 μM) was treated to in-vitro kinase reaction mixture.
In vivo ubiquitination assay
HEK293T cells were co-transfected with HA-ubiquitin (Ub) and Flag-Plk1 construct (a gift of Kyung S. Lee, NIH/NCI, MD). The cells were then treated with either DMSO or BFA for 18 h before harvest. To prevent proteasomal degradation of Plk1, cells were also treated with 10 μM of MG132 for 3 h before harvest. After 24 h of transfection, cells were harvested and used for immunoprecipitation with anti-Flag antibody (Sigma).
Semi-quantitative conventional RT-PCR and Quantitative real-time RT-PCR analysis
Total RNA was extracted from hTERT-RPE cells treated with DMSO or BFA using RNeasyR mini kit (Qiagen). DMSO or BFA was treated 30 min before serum-restimulation at the indicated concentrations. cDNA was generated by SuperScript III Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. Conventional RT-PCR was performed using a ProFlex™ Base Thermal Cycler (Applied Bio-systems) with the conditions of 95°C for 20 s, 62°C for 30 s, and 72°C for 45 s for a total of 25 cycles for Plk1, Dvl2, and GAPDH, followed by a 10 min final extension at 72°C. PCR products were electrophoresed in 3% agarose gel, stained with EcoDye™ Nucleic Acid Staining Solution (BIOFACT, Korea), and photographed. Real-time RT-PCR was performed in a final volume of 20 μl with 2 μl of cDNA, 10 pmol forward and 10 pmol reverse primer in 1X power SYBG green PCR master Mix (Applied Biosystems, USA) with the condition of 95°C for 15 s for denaturation, 55°C for 1 min for annealing and 72°C for 15 s extension using an QuantStudio™ 3 Real-Time PCR System (Applied Biosystems). The expression value of each gene was normalized by that of GAPDH. Final values were calculated using the ΔΔCt method. The results were analyzed using QuantStudio™ design & Analysis software v1.4 (Applied Biosystems). All the primers used in these experiments are summarized in Supplementary Table S3.
Flow cytometry (FACS) analyses
The same condition as serum-restimulation assay was used for FACS analyses. Resulting cells were trypsinized and subjected to propidium iodide staining using BD cycletest™ Plus DNA reagent kit (BD Biosciences, USA). FACS analyses were carried out using BD FACSCalibur™ Flow Cytometer (BD Biosciences) and data were analyzed by the CellQuest Pro v6.0 (BD Biosciences).
Chemical structure elucidation
The structure elucidation of isolated Brefeldin A (collection No. KY-0120) was achieved by spectroscopic data measurements (1H and 13C NMR and MS). The NMR spectra were recorded on a Bruker AVANCE HD 800 NMR spectrometer (800 MHz for 1H and 200 MHz for 13C) at Korea Basic Science Institute (KBSI) in Ochang. Chemical shift values were referenced to the residual solvent signal (δH 3.31 and δC 49.15). ESI-MS data were obtained using a Thermo Scientific LTQ XL ion trap mass spectrometer.
RESULTS
Compound library screening for inhibitors of primary cilium disassembly
We previously identified the Wnt5a-induced primary cilium disassembly pathway (Lee et al., 2012). Therefore, we attempted to isolate effective compounds for regulating the Wnt5a-CK1ɛ-Dvl2-Plk1-mediated primary cilium disassembly pathway. We used an in-house chemical library, KY compounds, which contains 297 natural compounds, as described in Supplementary Table S1. To investigate the effect of compounds on ciliogenesis, we first employed a serum-starvation-based ciliogenesis assay using hTERT-immortalized retinal pigment epithelial (hTERT-RPE) cells treated with each compound. hTERT-RPE cells were treated with 10 μM compound simultaneously with serum-starvation for 24 h. Because this condition leads to 70% ciliation when treated with the DMSO control, <60% or >90% ciliation was defined as assembly inhibition or disassembly inhibition (it can be assembly promotion), respectively. From the first round of screening, 18 primary cilium assembly inhibitors and 17 disassembly inhibitors/assembly promotors were identified according to the number of ciliated cells (Fig. 1A and Supplementary Table S1). As we have identified the Plk1-mediated primary cilium disassembly pathway (Lee et al., 2012), we were interested in a primary cilium disassembly blocker. Therefore, we attempted to clarify the most powerful inhibitors of primary cilium disassembly in the second round of screening (Fig. 1B). For this purpose, a serum-restimulation assay was applied to the 17 candidates of primary cilium disassembly inhibitor that were obtained from the first round of screening. hTERT-RPE cells were serum-starved for 48 h to allow robust ciliation and then serum was added (serum-restimulation assay) to induce cilium disassembly. Cells were treated with 17 isolated compounds for 30 min before serum-restimulation and cells were fixed 24 h after serum-restimulation. Because of this experiment, we were able to identify KY-0120 as the most powerful cilium disassembly inhibitor in hTERT-RPE cells (Fig. 1B). Treatment with KY-0120 showed a remarkable inhibitory effect on primary cilium disassembly, even with low-concentration treatment (1μM) represented 30% ciliated cells. Aside from KY-0120, the other candidates showed a similar effect as the DMSO control on the inhibition of cilium disassembly (Fig. 1B). Next, we performed immunoblotting analyses, examining whether the amount of proteins in the cilium disassembly pathway can be changed by the 17 isolated candidates of primary cilium disassembly inhibitor. Therefore, we applied the same experimental scheme as in the second round of screening, as shown in Fig. 1B. After 24 h of serum-restimulation and compound treatment (24.5 h), cells were harvested and subjected to immunoblotting analyses. Among the monitored proteins, Dvl2 and Plk1 levels were significantly changed by KY-0120 treatment in the primary cilium disassembly condition, whereas the other candidates did not affect the protein level (Fig. 1C). Treatment with KY-0120 in cilium disassembly conditioned-hTERT-RPE cells induced a fast-migrating Dvl2 form and significant reduction in Plk1 levels. These results constitute strong evidence that KY-0120 acts as a blocker of primary cilium disassembly by modulating the activity of Dvl2 and Plk1. Therefore, we focused on KY-0120 in further analyses.
Fig. 1.
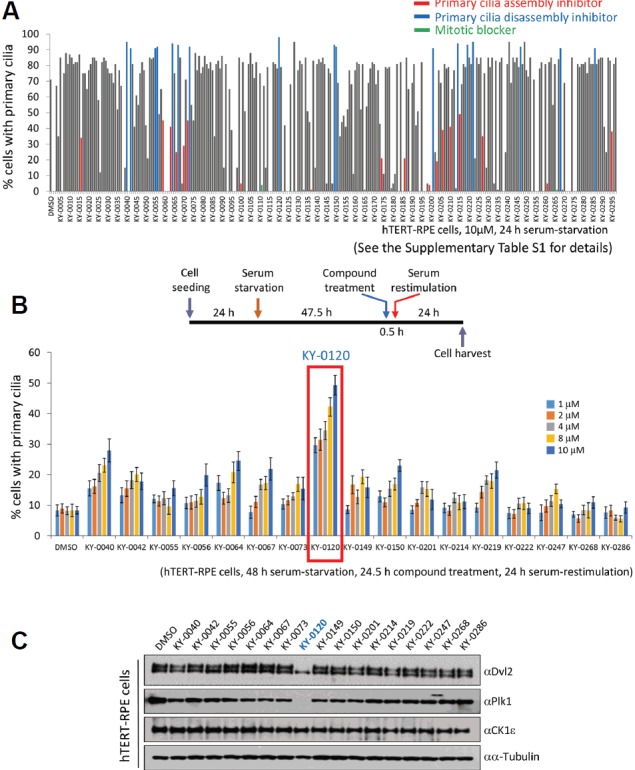
Compound library screening for inhibitors of primary cilium disassembly.
(A) The first round of compound library screening. A total of 297 fungal metabolites were screened for ciliogenesis. hTERT-RPE cells were serum-depleted for 24 h, and 10 μM of each compound was applied to hTERT-RPE cells simultaneously with serum-depletion. After 24 h of serum-starvation, cells were fixed and subjected to immunocytochemistry. (See Supplementary Table S1 for details). (B) The second round of compound library screening for inhibitors of primary cilium disassembly. At 48 h after serum-starvation, the cells were restimulated with serum. Each compound was applied to cells 30 min before serum-restimulation. After 24 h of serum-restimulation, cells were fixed and subjected to immunocytochemistry. The schedule of serum-restimulation assay is drawn (upper). Cells bearing primary cilia were counted. Data are mean ± SD from three independent experiments. >300 cells were counted for each sample. (C) KY-0120 changes the status of Dvl2 and the amount of Plk1 during primary cilium disassembly. The same cells as in Fig. 1B were subjected to immunoblotting analysis with the indicated antibodies. α-tubulin was used as a loading control.
Brefeldin A inhibits primary cilium disassembly
We attempted to characterize KY-0120 and identified its structure as Brefeldin A (BFA) by NMR analysis (Fig. 2A and Supplementary Fig. S1). We, therefore, purchased and tested commercial BFA to determine whether it has the same activity as KY-0120, which we isolated from fungus, with respect to the inhibition of cilium disassembly. To this end, we first investigated the inhibitory effect of BFA on primary cilium disassembly at various concentrations using the same experimental scheme as for treatment with KY-0120 in Fig. 1B. Because of treatment with BFA, cilium disassembly was strongly blocked. Even at a low concentration (50 nM) of BFA, its application resulted in a remarkable inhibitory effect on primary cilium disassembly in terms of both the size of the cilium-containing population and cilium length (Figs. 2B–2E). Upon treatment at 50 nM, BFA increased the proportion of cells with primary cilia in the cilium disassembly condition to more than six-fold and substantially increased the length of primary cilia to more than seven-fold of that after treatment with the DMSO control. In addition, treatment with 1 μM BFA caused an average length of primary cilia of approximately 9 μm; in contrast, in the DMSO control, the corresponding value was 1 μm. Therefore, BFA causes remarkable blockage of ciliary reabsorption, as KY-0120 did.
Fig. 2.
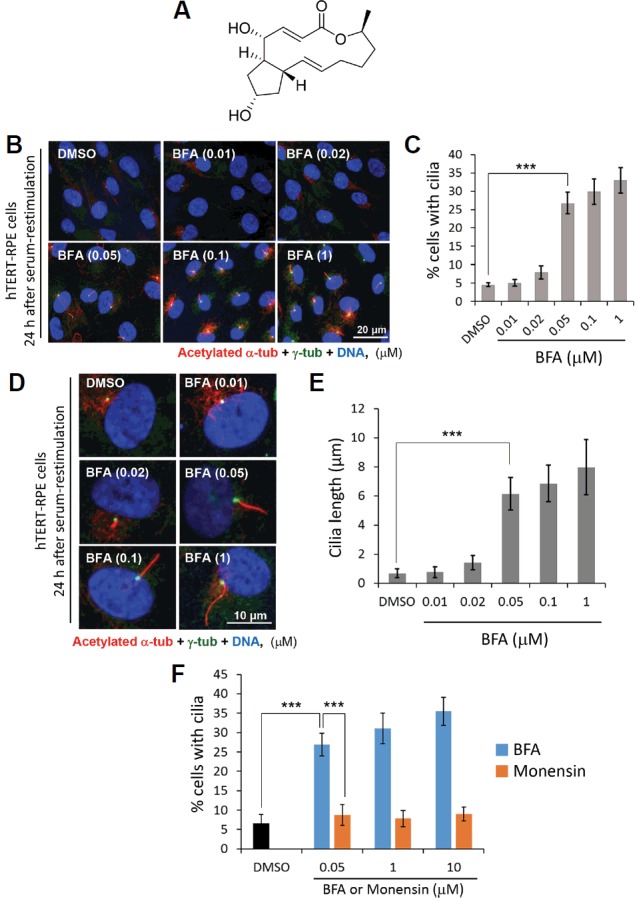
Brefeldin A inhibits primary cilium disassembly.
(A) Structural elucidation of KY-0120. The structure of KY-0120 was defined by NMR analyses (See Supplementary Fig. S1 for details). (B–F) hTERT-RPE cells serum-depleted for 48 h were treated with either the DMSO control, BFA, or menensin, 30 min prior to serum-restimulation. Cells were fixed at 24 h after serum-restimulation and subjected to immunocytochemistry. The cells with primary cilia were counted (C, F) and the lengths of primary cilia were measured (E). Data are mean ± SD from three independent experiments. >300 cells were counted for each sample. Statistical analysis: ***P < 0.001 (unpaired two-tailed t-test).
BFA specific blockage of ciliary disassembly was further confirmed by using other Golgi-destroying drug, monensin (Nylander and Kalies, 1999; Rosa et al., 1992). Since monensin is widely used drug that inhibits protein secretion by Golgi disruption, we tested monensin for comparison. Surprisingly, monensin did not show the inhibitory effect on primary cilia disassembly which was seen by BFA. Even in high dose treatment of monensin, 100 μM, primary cilia disassembly was not blocked in comparison to BFA treatment (Fig. 2F). This result probably originated from the different mode of actions between these two drugs. In fact, BFA inhibits protein transport from the cis/medial Golgi complex to the ER, while monensin prevents protein secretion by inhibition of trans-Golgi function (Nylander and Kalies, 1999; Rosa et al., 1992).
BFA inhibits the CK1ɛ - Dvl2 interaction
To understand the mechanism underlying BFA’s inhibition of primary cilium disassembly, we applied the same strategy as that used for the isolation of KY-0120 shown in Fig. 1C. Treatment with BFA, similar to that with KY-0120, caused marked fast-migrating form of Dvl2 and reduction in Plk1 levels in the cilium disassembly condition (Fig. 3A). To clarify this, we used two different approaches for the two different proteins Dvl2 and Plk1. For Dvl2, we closely investigated phosphorylation regulation by CK1ɛ. Previous studies have shown that CK1-induced phosphorylation of Dvl2 generates a slow-migrating band, whereas dephosphorylation results in a fast-migrating form of Dvl2 (Bryja et al., 2007; Lee et al., 2012). Therefore, we first investigated whether BFA inhibits the activity of CK1ɛ directly. To this end, we performed an IP-kinase assay in the presence of DMSO control, CK1 inhibitor (D4476) (Rena et al., 2004), or BFA, as described in the Figure Legends and Materials & Methods section. As a result, the autophosphorylation signal of CK1ɛ was significantly decreased by treatment with D4476, a CK1 inhibitor, compared with that by treatment with the DMSO control. However, no difference was observed between treatment with the DMSO control and BFA (Fig. 3B). No significant difference was observed in both low- (0.1 μM) and high-concentration (100 μM) treatments with BFA in comparison to treatment with the DMSO control. Therefore, we changed the strategy to monitor the effect of BFA on the binding of CK1ɛ to Dvl2. For this, we performed both a GST pull-down assay (Fig. 3C) and an immunoprecipitation assay (Fig. 3D) in the presence of the DMSO control or the indicated concentrations of BFA. Compared with treatment with the DMSO control, treatment with BFA significantly reduced the binding of GST-CK1ɛ to Flag-Dvl2 (Fig. 3C). In line with this observation, the immunoprecipitation assay also showed the inhibitory effect of BFA on the binding between CK1ɛ and Dvl2 (Fig. 3D). These results suggest that BFA inhibits the phosphorylation of Dvl2 by interfering with the interaction between CK1ɛ and Dvl2, although BFA does not affect the activity of CK1ɛ itself.
Fig. 3.

BFA inhibits the interaction of CK1ɛ - Dvl2.
(A) BFA reduces the phosphorylation of Dvl2 and the amount of Plk1 during primary cilium disassembly. hTERT-RPE cells were treated with either the DMSO control or BFA as described in Fig. 2. Cells were harvested and subjected to immunoblotting analysis with the indicated antibodies. α-tubulin was used as a loading control. (B) BFA does not inhibit the activity of CK1ɛ. HEK293T cells transfected with Myc-tagged CK1ɛ were harvested at 24 h after transfection and subjected to immunoprecipitation with anti-Myc antibody. Precipitates were subjected to in-vitro kinase assay in the presence of γ-32P ATP. The DMSO control, BFA, or D4476 was treated to in-vitro kinase assay mixture. (C) BFA inhibits the binding of CK1ɛ to Dvl2. Bacterially purified GST-CK1ɛ proteins were incubated with Flag-Dvl2-expressing HEK293T cell lysates in the presence of the DMSO control or BFA and GST pull-down assay was performed. (D) BFA interferes with the binding between CK1ɛ and Dvl2 in in-vivo conditions. HEK293T cells co-transfected with Myc-tagged CK1ɛ and Flag-tagged Dvl2 were harvested at 48 h after transfection and subjected to immunoprecipitation with anti-Myc antibody. Precipitates were subjected to immunoblotting analysis with the indicated antibodies. BFA was applied to cells either 6 h or 24 h prior to cell harvest. Note that exogenous Dvl2 does not make a double band, only endogenous Dvl2 makes a double band.
BFA inhibits the expression of Plk1 mRNA during cilium disassembly period
For Plk1, we focused on the activation of the Plk1 degradation machinery or the inhibition of its expression. Because Plk1 was directly ubiquitinated by specific E3s and degraded by the ubiquitin-proteasome system (Charles et al., 1998; Shirayama et al., 1998), we then investigated whether Plk1 ubiquitination was enhanced by BFA. To this end, we employed an in-vivo ubiquitination assay in the presence or absence of BFA. We could not find significant differences in Plk1 ubiquitin ladders between treatment with the DMSO control and BFA. BFA could not enhance the ubiquitination of Plk1 both with and without treatment with MG132 (Fig. 4A). In contrast, the expression of Plk1 mRNA was dramatically reduced by BFA in both conventional RT-PCR and quantitative real-time RT-PCR analyses (Figs. 4B and 4C). Treatment with BFA severely reduced the expression of Plk1 mRNA in conventional RT-PCR analysis. Upon treatment with 50 nM BFA, the expression of Plk1 mRNA was reduced to less than half of that after treatment with the DMSO control and Plk1 mRNA almost completely disappeared upon treatment with 10 μM BFA (Fig. 4B). In line with this observation, quantitative real-time RT-PCR analysis revealed exactly the same results as conventional RT-PCR analysis (Fig. 4C). Therefore, we concluded that BFA reduces Plk1 not by post-translational modification but by transcriptional repression. To confirm direct effect of BFA on Plk1 or Dvl2 in blockage of primary cilia disassembly, we performed rescue experiments by using overexpression of either constitutively active form of Plk1 (Plk1T210D, a gift of Kyung S. Lee, NIH/NCI) or phosphomimetic form of Dvl2 (Dvl23E) or both. To generate Dvl2 phosphomimetic mutant (Dvl23E), we chose three candidate sites (Ser594, Thr595, and Ser597) which are responsible for CK1-induced Dvl2 band shift (González-Sancho et al., 2013). As a result, overexpression of either Plk1T210D or Dvl23E rescued BFA-induced blockage of primary cilia disassembly efficiently (Fig. 4D). Moreover, co-transfection of both plasmids rescued more efficiently than single overexpression. These data strongly support the conclusion that BFA inhibits primary cilia disassembly through the disruption of Dvl2-Plk1 axis directly.
Fig. 4.
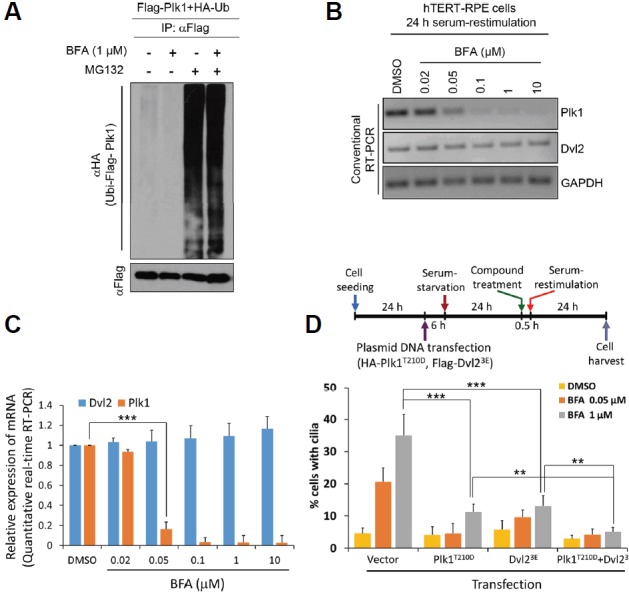
BFA inhibits the expression of Plk1 mRNA.
(A) BFA does not work in the ubiquitination of Plk1. HEK293T cells were co-transfected with HA-Ub and Flag-Plk1. The DMSO control or BFA was applied for 18 h. To prevent protein degradation, MG132 was applied to cells 3 h prior to cell harvest. Flag-Plk1 was immunoprecipitated with anti-Flag antibody and precipitates were subjected to immunoblotting analysis with the indicated antibodies. (B, C) BFA reduces the expression of Plk1 mRNA. hTERT-RPE cells were treated with BFA as indicated in Fig. 2. Total RNAs were subjected to semi-quantitative conventional RT-PCR analysis (B) and quantitative real-time RT-PCR analysis (C). (D) Overexpression of Plk1T210D and Dvl23E rescues BFA-induced defect of primary cilia disassembly. The schedule of transfection and serum-restimulation assay is drawn (upper). Data are mean ± SD from three independent experiments. Statistical analysis: ***P < 0.001, **P < 0.01 (unpaired two-tailed t-test). > 300 cells were counted for each sample (D).
BFA arrests serum restimulation-induced cell cycle progression
We further confirmed the arrest of cell cycle progression by BFA during serum-starvation following serum-restimulation. FACS analyses revealed the blocking of cell cycle progression from the G1-phase to the S-phase of the cell cycle in the serum-restimulated condition by treatment with BFA (Fig. 5A). Additionally, in line with this observation, treatment with BFA increased cyclin E1 and decreased cyclin A, B1, and D1 levels (Fig. 5B). Presumably, the prevention of cilia disassembly by BFA inhibits cell cycle progression in serum-restimulation condition.
Fig. 5.
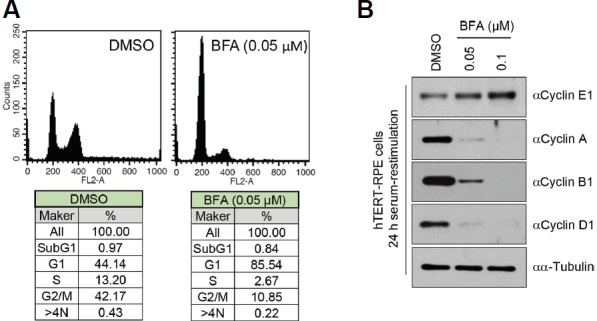
BFA arrests serum restimulation-induced cell cycle progression.
BFA increases the G1 population of the cells. The same cells as in Fig. 2 were harvested and subjected to flow cytometry analysis (A) and immunoblotting analysis with the indicated anti-cyclin antibodies (B). α-tubulin was used as a loading control.
DISCUSSION
It has been reported that the mechanisms of primary cilium assembly and disassembly are closely associated with ciliopathy and cancer development (Goetz and Anderson, 2010; Gradilone et al., 2013; Han et al., 2009; Kim et al., 2011; Moser et al., 2009; Nachury et al., 2007; Park et al., 2006; Wong et al., 2009). Although the underlying mechanism of how those events are regulated remains unclear, the importance of the regulation of primary cilia for treating ciliopathy and cancer has recently emerged (Peluso et al., 2014; Xiang et al., 2014). Therefore, primary cilium regulation-based drug development is just the beginning. In this study, we identified an effective compound, KY-0120 (identified as BFA), from our natural compound library as a primary cilium disassembly inhibitor. BFA drastically reduced the phosphorylation of Dvl2 and the expression of Plk1. Additionally, BFA blocked cell cycle progression after serum-restimulation. Therefore, we speculate that the combination of those events by BFA may exert an inhibitory effect on primary cilium disassembly in the Wnt5a-CK1ɛ-Dvl2-Plk1-dependent primary cilium disassembly pathway. In our previous study, we demonstrated that the activation of CK1ɛ by Wnt5a stimulation phosphorylates S143 and T224 of Dvl2, and Plk1 binds to the phosphorylated residues, which eventually results in primary cilium disassembly (Lee et al., 2012). In this study, BFA was shown to interfere with the formation of the CK1ɛ-Dvl2 complex, which inhibits phosphorylation of Dvl2 by CK1ɛ and also inhibits the expression of Plk1 mRNA. Ultimately, this may result in the collapse of the primary cilium disassembly pathway induced by the Dvl2-Plk1 complex. Consistent with previous report that the blockage of cilia disassembly pathway caused longer cilia than normal condition (Lee et al., 2012), BFA treatment caused primary cilia elongation. The cilia elongation by BFA treatment is presumably due to the breakdown of the force balance between assembly and disassembly mechanism. BFA is known as an inhibitor of anterograde transport from ER to the Golgi apparatus, and the ER-Golgi system has been shown to have important functions in ciliogenesis (Hoffmeister et al., 2011). Therefore, we cannot rule out the possibility of BFA controlling ciliogenesis, by regulation of the ER-Golgi system, as well as primary cilium disassembly inhibition, by regulation of the Dvl2-Plk1 complex, as identified in this study. Since the transcriptional repressions by blockage of Golgi-dependent vesicular transport have been reported (Kingsbury and Cardenas, 2016; Pahl and Baeuerle, 1995; Wyrozumska et al., 2014), we speculate that BFA may inhibit the Plk1 mRNA expression via the blockage of vesicular trafficking-induced cytoplasmic-nuclear translocation of some transcription factor which induces Plk1 expression. However, the exact mechanism is needed to be elucidated in further study.
Plk1 has been reported to play various roles in mitosis, such as in mitotic entry, G2/M checkpoint regulation, spindle assembly, chromosome segregation, and cytokinesis (Barr et al., 2004; Petronczki et al., 2008; von Schubert et al., 2015). Therefore, the inhibitory effect of BFA on the expression of Plk1 during cilia disassembly period, which is confirmed in this study, may be able to function not only in primary cilium disassembly but also in mitotic regulation. In particular, the overexpression of Plk1 has been identified as a major cause of cancer (Knecht, 1999; Strebhardt and Ullrich, 2006), suggesting that the inhibition of the expression of Plk1 by treatment with BFA may be applied as a therapeutic option against the development of Plk1-induced cancers. Dvl2 is known as a scaffold protein that plays an important role in both Wnt canonical and non-canonical pathways (MacDonald et al., 2009; Singh et al., 2010). Several kinases that regulate Dvl2 function have been reported, among which phosphorylation of Dvl2 by the CK1 family has been shown to be an important mechanism for controlling Wnt signaling (Kishida et al., 2001; Peters et al., 1999). These findings suggest the possibility that the inhibition of binding between CK1ɛ and Dvl2 by BFA may not only result in deficiency of primary cilium disassembly but also result in regulatory effects on Wnt canonical and Wnt-PCP signaling pathways.
We also confirmed that treatment with BFA arrested the progression of the cell cycle from the G1-phase to the S-phase during the serum-restimulation period. This suggests the inhibition of primary cilium disassembly by blocking G1-phase to S-phase progression and implies two possibilities: BFA directly acts on cell cycle progression or it indirectly inhibits cell cycle progression by regulating Dvl2 or Plk1. Presumably, it is possible that the cells treated with BFA in the absence of serum fail to leave the G0 phase even after serum restimulation. Our findings may provide a powerful tool for studying ciliogenesis and suggest a pharmaceutical inhibitor to treat cilium-related diseases including cancer.
Supplementary data
ACKNOWLEDGMENTS
We thank Sean B. Lee, Jeffrey S. Rubin, and Kyung S. Lee for kindly providing plasmid DNA. This work was supported by the World Class Institute (WCI 2009-002), Bio & Medical Technology Development Program (NRF-2014M3A9B5073938), KRIBB Research Initiative Programs funded by the Ministry of Science, ICT and Future Planning (MSIP) of Republic of Korea, and the R & D Convergence Program (CAP-16-03-KRIBB) of NST (National Research Council of Science & Technology) of Republic of Korea.
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Barr F.A., Silljé H.H., Nigg E.A. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- Bryja V., Schulte G., Rawal N., Grahn A., Arenas E. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci. 2007;120:586–595. doi: 10.1242/jcs.03368. [DOI] [PubMed] [Google Scholar]
- Charles J.F., Jaspersen S.L., Tinker-Kulberg R.L., Hwang L., Szidon A., Morgan D.O. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr Biol. 1998;8:497–507. doi: 10.1016/s0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- Colanzi A., Grimaldi G., Catara G., Valente C., Cericola C., Liberali P., Ronci M., Lalioti V.S., Bruno A., Beccari A.R., et al. Molecular mechanism and functional role of brefeldin A-mediated ADP-ribosylation of CtBP1/BARS. Proc Natl Acad Sci USA. 2013;110:9794–9799. doi: 10.1073/pnas.1222413110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S.C., Anderson K.V. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Sancho J.M., Greer Y.E., Abrahams C.L., Takigawa Y., Baljinnyam B., Lee K.H., Lee K.S., Rubin J.S., Brown A.M. Functional consequences of Wnt-induced dishevelled 2 phosphorylation in canonical and noncanonical Wnt signaling. J Biol Chem. 2013;288:9428–9437. doi: 10.1074/jbc.M112.448480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilone S.A., Radtke B.N., Bogert P.S., Huang B.Q., Gajdos G.B., LaRusso N.F. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259–2270. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.G., Kim H.J., Dlugosz A.A., Ellison D.W., Gilbertson R.J., Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister H., Babinger K., Gürster S., Cedzich A., Meese C., Schadendorf K., Osten L., de Vries U., Rascle A., Witzgall R. Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. J Cell Biol. 2011;192:631–645. doi: 10.1083/jcb.201007050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Dabiri S., Seeley E.S. Primary cilium depletion typifies cutaneous melanoma in situ and malignant melanoma. PLoS One. 2011;6:e27410. doi: 10.1371/journal.pone.0027410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury J.M., Cardenas M.E. Vesicular trafficking systems impact TORC1-controlled transcriptional programs in Saccharomyces cerevisiae. G3 (Bethesda) 2016;6:641–652. doi: 10.1534/g3.115.023911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida M., Hino S.I., Michiue T., Yamamoto H., Kishida S., Fukui A., Asashima M., Kikuchi A. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase Iε. J Biol Chem. 2001;276:33147–33155. doi: 10.1074/jbc.M103555200. [DOI] [PubMed] [Google Scholar]
- Klausner R.D., Donaldson J.G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht R., Elez R., Oechler M., Solbach C., von Ilberg C., Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794–2797. [PubMed] [Google Scholar]
- Lee K.H., Johmura Y., Yu L.R., Park J.E., Gao Y., Bang J.K., Zhou M., Veenstra T.D., Kim B.Y., Lee K.S. Identification of a novel Wnt5a-CK1ε-Dvl2-Plk1- mediated primary cilia disassembly pathway. EMBO J. 2012;31:3104–3117. doi: 10.1038/emboj.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockel A., Perdomo Y., Stutzmann F., Letsch J., Marion V., Dollfus H. Retinal dystrophy in Bardet-Biedl syndrome and related syndromic ciliopathies. Prog Retin Eye Res. 2011;30:258–274. doi: 10.1016/j.preteyeres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Moser J.J., Fritzler M.J., Rattner J.B. Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer. 2009;488:1–12. doi: 10.1186/1471-2407-9-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury M.V., Loktev A.V., Zhang Q., Westlake C.J., Peränen J., Merdes A., Slusarski D.C., Scheller R.H., Bazan J.F., Sheffield V.C., et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nylander S., Kalies I. Brefeldin A, but not monensin, completely blocks CD69 expression on mouse lymphocytes: efficacy of inhibitors of protein secretion in protocols for intracellular cytokine staining by flow cytometry. J Immunol Methods. 1999;224:69–76. doi: 10.1016/s0022-1759(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Pahl H.L., Baeuerle P.A. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T.J., Haigo S.L., Wallingford J.B. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Peluso M.O., Campbell V.T., Harari J.A., Tibbitts T.T., Proctor J.L., Whitebread N., Conley J.M., White K.F., Kutok J.L., Read M.A., McGovern K., Faia K.L. Impact of the Smoothened inhibitor, IPI-926, on smoothened ciliary localization and Hedgehog pathway activity. PLoS One. 2014;9:e90534. doi: 10.1371/journal.pone.0090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J.M., McKay R.M., McKay J.P., Graff J.M. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- Petronczki M., Lénárt P., Peters J.M. Polo on the Rise-from Mitotic Entry to Cytokinesis with Plk1. Dev Cell. 2008;14:646–659. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Pugacheva E.N., Jablonski S.A., Hartman T.R., Henske E.P., Golemis E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G., Bain J., Elliott M., Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5:60–65. doi: 10.1038/sj.embor.7400048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R., Milenkovic L., Scott M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Rosa P., Mantovani S., Rosboch R., Huttner W.B. Monensin and brefeldin A differentially affect the phosphorylation and sulfation of secretory proteins. J Biol Chem. 1992;267:12227–12232. [PubMed] [Google Scholar]
- Santos N., Reiter J.F. Building it up and taking it down: the regulation of vertebrate ciliogenesis. Dev Dyn. 2008;237:1972–1981. doi: 10.1002/dvdy.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P., Christensen S.T. Structure and function of mammalian cilia, Histochem. Cell Biol. 2008;129:687–693. doi: 10.1007/s00418-008-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley E.S., Nachury M.V. The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci. 2010;123:511–518. doi: 10.1242/jcs.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M., Zachariae W., Ciosk R., Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Yanfeng W.A., Grumolato L., Aaronson S.A., Mlodzik M. Abelson family kinases regulate Frizzled planar cell polarity signaling via Dsh phosphorylation. Genes Dev. 2010;24:2157–2168. doi: 10.1101/gad.1961010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebhardt K., Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- Tseng C.N., Hong Y.R., Chang H.W., Yu T.J., Hung T.W., Hou M.F., Yuan S.S., Cho C.L., Liu C.T., Chiu C.C., et al. Brefeldin A reduces anchorage-independent survival, cancer stem cell potential and migration of MDA-MB-231 human breast cancer cells. Molecules. 2014;19:17464–17477. doi: 10.3390/molecules191117464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schubert C., Cubizolles F., Bracher J.M., Sliedrecht T., Kops G.J., Nigg E.A. Plk1 and Mps1 cooperatively regulate the spindle assembly checkpoint in human cells. Cell Rep. 2015;12:66–78. doi: 10.1016/j.celrep.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Wong S.Y., Seol A.D., So P.L., Ermilov A.N., Bichakjian C.K., Epstein E.H., Jr, Dlugosz A.A., Reiter J.F. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;9:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrozumska P., Ashley J.W., Ramanadham S., Liu Q., Garvey W.T., Sztul E. Novel effects of Brefeldin A (BFA) in signaling through the insulin receptor (IR) pathway and regulating FoxO1-mediated transcription. Cell Logist. 2014;4:e27732. doi: 10.4161/cl.27732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W., Jiang T., Guo F., Gong C., Yang K., Wu Y., Huang X., Cheng W., Xu K. Hedgehog pathway inhibitor-4 suppresses malignant properties of chondrosarcoma cells by disturbing tumor ciliogenesis. Oncol Rep. 2014;32:1622–1630. doi: 10.3892/or.2014.3372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


