Abstract
Interferon-γ-inducible protein 10 (IP-10), also known as chemokine C-X-C motif ligand (CXCL) 10, is closely associated with antiviral immunity and the progression of chronic hepatitis B (CHB). However, the value of baseline serological and histological IP-10 expression levels in predicting the efficacy of the antiviral response to nucleoside/nucleotide analogues (NAs) is still unknown. In our research, intrahepatic and peripheral IP-10 expression levels were systemically examined before and after treatment with entecavir (ETV). Baseline serological and histological IP-10 expression levels were significantly increased in patients with CHB, particularly in patients with higher degrees of liver inflammation and liver fibrosis. Moreover, higher baseline intrahepatic IP-10 levels indicated better prognoses in patients with CHB after entecavir therapy. The baseline IP-10 level was also positively associated with several clinical parameters, including baseline levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), hepatitis B virus (HBV) DNA, and hepatitis B surface antigen (HBsAg), and with the decrease in HBsAg levels after treatment. In addition, monocyte-derived IP-10 was expressed at higher levels in patients with CHB than in patients with liver cirrhosis (LC) and healthy controls (HC). According to the results of our in vitro experiments, IP-10 directly promoted hepatocyte apoptosis. Based on these findings, baseline serological and histological IP-10 levels might predict CHB severity and the decrease in HBsAg levels after entecavir therapy.
Keywords: chronic hepatitis B, entecavir, IP-10, prediction
INTRODUCTION
Chronic hepatitis B (CHB) is a potentially life-threatening liver disease caused by hepatitis B virus (HBV). Approximately 2 billion people worldwide are estimated to be infected with HBV, and more than 240 million people have a chronic liver infection (Wang et al., 2014). The major feature of CHB is the infiltration of inflammatory cells, including neutrophils, monocytes, natural killer cells and lymphocytes, into the liver (Gong et al., 2015). Only an effective HBV-specific immune response can arrest or slow disease progression. Although immunological control of chronic HBV infection appears to be essential for HBV suppression, an ideal immunological marker that predicts the outcomes of antiviral treatments has yet to be identified.
Serum interferon-γ-inducible protein (IP-10) has been discussed as a valuable predictor of the patient’s response to treatment with interferon-α (IFN-α) (Jaroszewicz et al., 2011) in hepatitis C virus (HCV) therapy. IP-10 has recently been suggested to predict responses to nucleoside/nucleotide analogues (NAs) and interferon therapy in patients with viral hepatitis, and high serum IP-10 levels may help to identify patients who have achieved a decrease in hepatitis B surface antigen (HBsAg) levels during treatment with tenofovir disoproxil fumarate and IFN-α (Jaroszewicz et al., 2011). IP-10 expression is increased in patients with CHB, severe hepatitis, and hepatocellular carcinoma (Sonneveld et al., 2013). However, no relationship was observed between hepatic IP-10 expression and disease progression.
As antiviral therapy becomes increasingly individualized, IP-10 may be a useful predictive maker of treatment outcomes, but the levels of IP-10 expression in patients with CHB and varying stages of inflammation and fibrosis and the predictive value of IP-10 are unknown. Most studies of patients with CHB have focused on serological studies, and thus, the dynamic changes in intrahepatic IP-10 levels after long-term individualized antiviral treatment remain unclear. Many clinical studies have reported a difference between serological and histological indices, and the effects mainly depend on the hepatic pathology. Therefore, the present study focused on the dynamic changes in serological and histological IP-10 levels after antiviral therapy. We first measured IP-10 levels in the peripheral blood and within the liver and then assessed dynamic changes in IP-10 expression after antiviral therapy. Next, the relationships between IP-10 expression and clinical characteristics were analysed. Finally, we identified the mechanism by which IP-10 induces liver damage in vitro.
MATERIALS AND METHODS
Patients
Sixty-nine patients with CHB and varying grades of liver disease were included before and after treatment with entecavir, in addition to 14 patients with liver cirrhosis (LC) and 17 healthy controls (HC). Individuals with concurrent HCV, hepatitis G virus, or human immunodeficiency virus (HIV)-1 infections or autoimmune liver diseases, along with patients who met the clinical or biological criteria for bacterial or fungal infections were excluded. These patients were diagnosed according to the diagnostic criteria of the 2010 Viral Hepatitis Management Scheme issued by the Chinese Medical Association of Hepatology (Zhang et al., 2010). All individuals were negative for antibodies to hepatitis A virus (HAV), HCV, hepatitis D virus (HDV), and HIV. Liver biopsies from 40 patients with CHB undergoing diagnosis, 4 patients with CHB complicated with LC, and 7 healthy liver transplant donors were included in the immunohistochemical analysis. The degree of hepatic inflammation was graded according to the modified histological activity index (HAI) described by Scheuer (1991). Paraffin sections were collected from 8 patients with CHB before and after treatment; of these patients, 4 had good prognoses (a decrease in the HBV DNA levels > 105), and 4 had poor prognoses (a decrease in the HBV DNA levels < 104). In this study, HBsAg/HBeAg (HBsAg/hepatitis B e antigen) levels, the decrease in the HBsAg/HBeAg levels, the HBV DNA load, and serum and intrahepatic IP-10 levels were assessed in patients with CHB at baseline and after treatment with 0.5 mg/day entecavir (Peglntron, Schering-Plough, USA). The clinical characteristics of the patients are listed in Table 1.
Table 1.
Clinical characteristics of the enrolled subjects
| Characteristic | Blood | Liver biopsy tissue | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HC | LC | CHB | HC | LC | CHB | |
| Case | 10 | 10 | 64 | 7 | 4 | 43 |
| Age (years) | 29 ± 5 | 44 ± 8 | 42 ± 6 | 27 ± 10 | 42 ± 7 | 41 ± 21 |
| Sex (male/female) | 5/5 | 7/3 | 46/18 | 2/5 | 3/1 | 30/13 |
| Baseline ALT (U/l) | ND | 55.0 ± 18.0 | 606.5 ± 593.5 | ND | 42.0 ± 26.0 | 606.0 ± 594.0 |
| Baseline AST (U/l) | ND | 47.0 ± 21 | 194.5 ± 172.5 | ND | 38.0 ± 22.0 | 697.0 ± 681.0 |
| Log10 baseline HBV DNA (IU/ml) | ND | 5.31 ± 1.65 | 7.1 ± 2.39 | ND | 6.31 ± 2.64 | 6.22 ± 2.73 |
| Baseline serum HBsAg (IU/ml) | ND | ND | 4.68 ± 4.62 | ND | ND | 4.68 ± 4.62 |
| Baseline serum IP-10 (pg/ml) | ND | ND | 866.03 ± 756.41 | ND | ND | 844.69 ± 795.48 |
| Intrahepatic IP-10 mRNA (lg cDNA) | ND | ND | ND | −2.18 ± 1.06 | −1.8 ± 0.21 | −1.48 ± 0.96 |
Abbreviations: HC, healthy controls; LC, liver cirrhosis; CHB, chronic hepatitis B; ALT, alanine ransaminase; AST, aspartate transaminase; HBsAg, hepatitis B surface antigen; IP-10, interferon (IFN)-γ-induced protein 10; ND, not detected. Data are shown as numbers ± s.d.
Plasma measurements
Plasma levels of IP-10 (eBioscience, USA), HBsAg (Alpha Diagnostic International, USA) and HBeAg (Abnova, Taiwan) were detected using commercial ELISA kits. The serum concentrations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were detected using a quantitative chemiluminescent immunoassay. Serum HBV DNA levels were quantified using a commercial real-time PCR kit (PG Biotech, China) according to the manufacturer’s instructions.
RNA extraction and reverse transcriptase polymerase chain reaction
Liver biopsy tissues were obtained from 35 patients with CHB, 4 patients with LC and 7 HCs, and RNA was extracted using the RNeasy Mini Kit (Qiagen, USA). Reverse transcription was performed using 5 μg of RNA and the First Strand cDNA Synthesis Kit (Thermo Scientific, USA). The expression of the IP-10 gene in liver biopsy tissues was analysed using real-time quantitative PCR (Power SYBR Green PCR Master Mix, USA). Real-time PCR (20-μl amplification mixtures) was performed as follows: 94°C for 5 min followed by 30 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 45 s. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. The primer sequences (Sangon Biotech, Shanghai, China) were designed as follows: IP-10 forward: 5′-CAAAATTGGCTTGCAGG AAT-3′; IP-10 reverse: 5′-TGGCAT TCAAGGAGTACCTCTC-3′; GAPDH forward: 5′-ACC ACA GTC CAT GCC ATC ACT-3′; and GAPDH reverse: 5′-TCC ACC ACC CTG TTG CTG TA-3′. Relative mRNA levels were calculated using the 2−ΔΔCt method.
Immunohistochemical staining
Liver tissue samples were fixed with 10% formalin, embedded in paraffin, and serially sectioned (5 μm) to examine monocyte-derived IP-10 expression in the liver. Briefly, immunohistochemical (IHC) staining was performed using the following steps: antigen retrieval via pressure cooking for 15 min in citrate buffer (pH 6.0), blocking of endogenous peroxidase activity with 3% H2O2 for 20 min, blocking with 5% bovine serum albumin (BSA) for 20 min at room temperature, incubation with a monoclonal rabbit anti-human IP-10 antibody (1:1,000 dilution) overnight at 4°C, and incubation with a goat anti-rabbit secondary antibody (Zhongshan Biotech, China) for 30 min at 37°C. After washing with phosphate-buffered saline, the sections were visualized using an ACE substrate kit (Zhongshan Biotech, China), followed by counterstaining with haematoxylin. After staining with haematoxylin, red cellular staining indicated areas that expressed IP-10, and cell nuclei were stained blue. As non-specific staining was observed, the percentage of the area with IP-10-positive staining was quantified using ImageJ software (Media Cybernetics, USA) (Zhang et al., 2015).
Cell culture and cell stimulation
HepG2 cells were maintained in our lab and cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% foetal bovine serum (Invitrogen, USA) (Xu et al., 2013). The cells were seeded in five 6-well plates at a concentration of 350,000 cells per well and were then stimulated with varying concentrations of IP-10 (Peprotech, USA). Apoptotic HepG2 cells were identified by measuring Annexin V and 7-aminoactinomycin-D (7-AAD, SouthernBiotech, USA) using FACS.
FACS analysis
All antibodies were purchased from Biolegend (Pacific Heights Blvd, USA). Surface and intracellular staining was performed using previously described protocols (Xu et al., 2013). For CD14 surface staining, fresh heparinized peripheral blood (300 μl) was incubated with the CD14-APC antibody at room temperature for 20 min, after which 2 ml of haemolysin (BD Biosciences, USA) were added. The cells were incubated at room temperature for 5 min and centrifuged at 1,200 rpm for 5 min, after which the supernatant was discarded. For intracellular staining, 300 μl of permeabilization solution (BD Biosciences, USA) were added, incubated at room temperature for 30 min, and then 1 ml of 1× wash buffer was added. After centrifugation at 1,200 rpm for 5 min, 10 μl of IP-10-phycoerythrin (IP-10-PE) was added, and the cells were incubated at room temperature for 20 min. IP-10 expression was detected in vitro by centrifuging the cells, resuspending them in 1× Annexin V buffer, and then successively incubating them with 5 μl of Annexin V for 15 min at room temperature and with 10 μl of 7-AAD for 5 min at room temperature. Cells were pelleted and analysed using the FACSCalibur system and CELL Quest software to assess apoptosis. Corresponding isotype controls were added according to the manufacturer’s instructions.
Statistical analysis
Data were analysed with SPSS version 13.0 software (SPAA Inc., USA) and are expressed as means ± standard deviations (s.d.). Statistically significant differences between pairs of groups were determined using the Mann-Whitney non-parametric u-test. Comparisons of data from the same individual were performed using the Wilcoxon matched-pairs t-test. Correlation analyses were conducted using the Spearman rank correlation test; p < 0.05 was considered statistically significant.
RESULTS
Main patient characteristics
Seventy-two patients with CHB, 17 HCs, and 14 patients with LC were included in this study. We collected both blood samples and liver biopsy samples. Blood samples were collected from 84 participants, including 10 HCs, 10 patients with LC and 64 patients with CHB. Liver biopsy specimens were obtained from 54 participants, including 7 HCs, 4 patients with LC and 43 patients with CHB. Paraffin sections were obtained from 8 patients with CHB. The patients ranged in age from 17 to 62 years and included 45 women and 88 men. Baseline ALT levels ranged from 16 U/l to 219 U/l, baseline AST levels ranged from 16 U/l to 233 U/l, baseline HBV DNA levels ranged from 2.7 × 106 to 2.4 × 108, and baseline serum HBsAg levels ranged from 3.3 IU/ml to 3.5 IU/ml. The average baseline serum IP-10 level was 493 pg/ml. Liver tissue IP-10 mRNA expression ranged from −2.3 lg cDNA to −1.6 lg cDNA. Patients with CHB received a 0.5-mg/d entecavir treatment for 48 weeks. The baseline demographic, clinical, biochemical and virological characteristics of the patients are shown in Table 1.
Baseline serum IP-10 levels were increased in patients with CHB who presented more severe liver inflammation and fibrosis
We first measured the baseline serum IP-10 concentrations in patients with CHB and observed significantly higher levels in patients with CHB rated as G > 2 than in patients rated as G ≤ 2 (p = 0.015, Fig. 1A). Moreover, when patients were grouped according to the liver fibrosis stage, the baseline serum IP-10 concentration was higher in patients with CHB rated as S > 3 than in patients rated as S1–2 (p = 0.014, Fig. 1B) and was higher in patients rated as S3 than in patients rated as S1–2 (p = 0.005, Fig. 1B). Thus, the baseline serum IP-10 concentration was associated with liver inflammation and fibrosis.
Fig. 1.
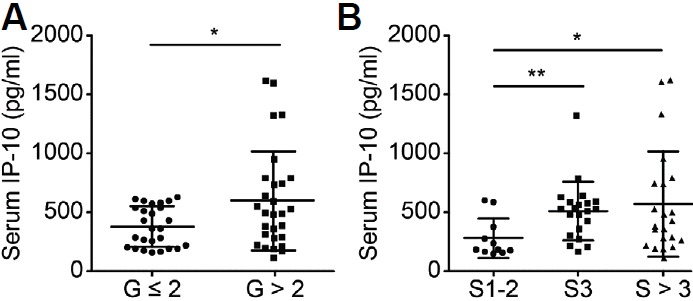
The baseline serum IP-10 concentration observed in patients with CHB was associated with liver inflammation and fibrosis.
(A) The baseline serum IP-10 concentration was significantly higher in patients with CHB rated as G > 2 (n = 26) than in patients with CHB rated as G ≤ 2 (n = 28). (B) When patients with CHB were grouped according to the severity of liver fibrosis, the baseline serum IP-10 concentration was lower in patients with CHB rated as S1–2 (n = 11) than in patients with CHB rated as S3 (n = 21) or S > 3 (n = 22). Each circle represents an individual. *p < 0.05; **<0.01.
IP-10 levels were significantly correlated with the clinical characteristics of patients with CHB
We investigated the correlations between IP-10 levels and ALT levels, AST levels, and HBV DNA load using the Spearman rank correlation test to analyse the relationship between the serum IP-10 levels and the clinical markers of liver injury. Baseline IP-10 levels were positively correlated with the levels of liver injury markers, such as ALT (r = 0.508, p < 0.0001, Fig. 2A) and AST (r = 0.587, p < 0.0001, Fig. 2B), as well as with the HBV DNA load (r = 0.473, p=0.0003, Fig. 2C). When we assessed the relationship between IP-10 and HBsAg levels, baseline IP-10 levels were positively correlated with not only baseline HBsAg levels (r = 0.486, p = 0.0002, Fig. 2D) but also with the decrease in HBsAg levels observed after antiviral treatment (r = 0.586, p < 0.0001, Fig. 2E). Furthermore, the decreases in IP-10 and HBsAg levels after antiviral treatment were positively correlated (r = 0.69, p < 0.0001, Fig. 2F). In contrast, no correlation was observed between the serum IP-10 concentration and baseline HBeAg levels or the decrease in HBeAg levels.
Fig. 2.
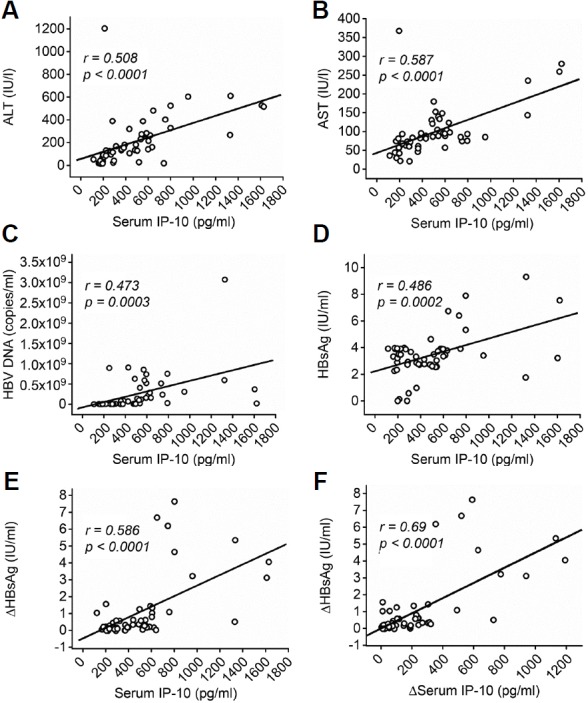
The baseline serum IP-10 concentration was positively correlated with the patients’ clinical characteristics.
The baseline serum IP-10 concentration was positively correlated with (A) serum ALT levels (n = 54), (B) serum AST levels (n = 54), (C) the HBV DNA load (n = 54), and (D) serum HBsAg levels (n = 54). (E) The decrease in serum HBsAg levels observed after antiviral therapy was also positively correlated with the baseline serum IP-10 concentration (n = 54). (F) The decrease in the serum IP-10 concentration observed after antiviral therapy was positively correlated with the decrease in the HBsAg levels observed after antiviral therapy (n = 54). Each circle represents an individual.
Intrahepatic expression of the IP-10 mRNA was increased in patients with CHB
In addition to serum levels of IP-10, we also assessed IP-10 production in the liver. Intrahepatic IP-10 mRNA levels were increased in patients with CHB compared with the LC (p = 0.003, Fig. 3A) and HC (p = 0.002, Fig. 3A) groups. When patients were grouped by the severity of liver inflammation and fibrosis, patients with higher degrees of fibrosis (S ≥ 3) expressed higher levels of the IP-10 mRNA than patients with S1–2 fibrosis (p = 0.047, Fig. 3B). Moreover, patients with CHB who presented higher grades of disease (G3–4) expressed higher levels of the IP-10 mRNA than patients with lower-grade (G1–2) disease (p = 0.001, Fig. 3C). Moreover, the Spearman rank correlation test revealed a positive correlation between serum IP-10 levels and intrahepatic IP-10 mRNA expression (r = 0.49, p = 0.003, Fig. 3D). Similar to the serum IP-10 levels, intrahepatic IP-10 production was positively correlated with the decrease in HBsAg levels after treatment (r = 0.34, p = 0.045, Fig. 3E).
Fig. 3.
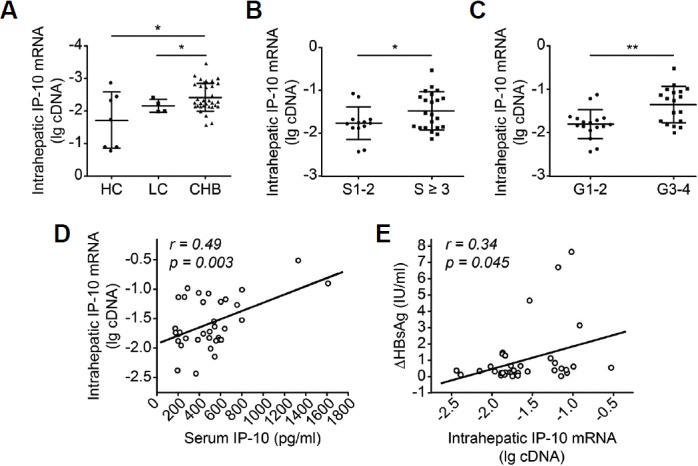
Intrahepatic IP-10 expression was increased in patients with CHB.
(A) Intrahepatic expression of the IP-10 mRNA was increased in patients with CHB (n = 35) compared with patients in the LC (n = 4) and HC (n = 7) groups. (B) Intrahepatic IP-10 mRNA levels were higher in patients with CHB rated as S ≥ 3 (n = 22) than in patients with CHB rated as S1–2 (n = 13). (C) Patients with G3–4 CHB (n = 18) expressed higher intrahepatic levels of the IP-10 mRNA than patients with G1–2 disease (n = 17). (D) Serum IP-10 levels were positively correlated with intrahepatic IP-10 mRNA levels (n = 35). (E) Intrahepatic IP-10 mRNA levels were positively correlated with the decrease in the HBsAg levels (n = 35). Each circle represents an individual. *p < 0.05; **p < 0.01.
Intrahepatic IP-10 levels predicted the outcomes of entecavir therapy in patients with CHB
We measured intrahepatic IP-10 expression in 4 patients with CHB who had good prognoses (a decrease in the HBV DNA levels >105) and 4 patients with CHB who had poor prognoses (a decrease in the HBV DNA levels <104) using specimens collected both before and after treatment to explore the dynamic changes in intrahepatic IP-10 levels during entecavir treatment. The areas with positive IP-10 staining were quantified using ImageJ software (Zhang et al., 2015). These results are shown in Table 2. As shown in Fig. 4A, IP-10 was expressed at higher levels in both lobular and portal areas in patients with good prognoses than in patients with poor prognoses, and the decrease in the IP-10 levels observed after entecavir therapy was more pronounced in patients with CHB who had good prognoses than in patients with poor prognoses. In lobular areas, IP-10 expression in patients with CHB who had good prognoses was significantly decreased after treatment (p = 0.001, Fig. 4B), whereas an obvious decrease was not observed in patients with poor prognoses. This difference was only observed in lobular areas and not in portal areas (data not shown). In subsequent analyses, patients with good prognoses had a higher baseline IP-10 level in lobular areas compared with patients with poor prognoses (p = 0.003, Fig. 4C), but this difference was not observed in portal areas (data not shown). Finally, patients with CHB who had good prognoses exhibited a more pronounced decrease in the IP-10 levels in both lobular (p = 0.001, Fig. 4D) and portal areas (p = 0.043, Fig. 4E) after entecavir therapy compared with patients with poor prognoses.
Table 2.
IP-10-positive area
| Lobular area | Portal area | |||
|---|---|---|---|---|
|
|
|
|||
| Pretreatment | After treatment | Pretreatment | After treatment | |
| Patient 1 | 44% | 22% | 46% | 21% |
| Patient 2 | 39% | 20% | 49% | 31% |
| Patient 3 | 37% | 26% | 36% | 27% |
| Patient 4 | 47% | 36% | 21% | 17% |
| Patient 5 | 25% | 38% | 19% | 35% |
| Patient 6 | 29% | 31% | 33% | 24% |
| Patient 7 | 16% | 23% | 18% | 19% |
| Patient 8 | 28% | 37% | 23% | 42% |
Fig. 4.

Intrahepatic expression of IP-10 mRNA was associated with the outcomes of entecavir therapy in patients with CHB.
(A) Representative histochemical images of the lobular and portal areas in tissue samples from patients with CHB who had good and poor prognoses before and after treatment. (B) IP-10 expression in lobular areas decreased significantly after treatment in patients with CHB who had good prognoses. (C) Patients with CHB who had good prognoses (n = 4) displayed higher baseline IP-10 levels in lobular areas compared with patients with CHB who had poor prognoses (n = 4). (D) Patients with CHB who had good prognoses (n = 4) exhibited a more pronounced decrease in the IP-10 levels in lobular areas after entecavir therapy compared with patients with CHB who had poor prognoses (n = 4). (E) Patients with CHB who had good prognoses (n = 4) exhibited a more pronounced decrease in the IP-10 levels in portal areas after entecavir therapy compared with patients with CHB who had poor prognoses (n = 4). Each circle represents an individual. *p < 0.05; **p < 0.01.
CD14+ monocyte-derived IP-10 levels were increased in patients with CHB
In addition to assessing IP-10 levels in patients with CHB and their correlation with the outcome of entecavir treatment, we also analysed monocyte-derived IP-10 levels in the peripheral blood. As shown in Fig. 5A, patients with CHB displayed higher levels of CD14+ monocyte-derived IP-10 in the peripheral blood than patients with LC and HCs. Patients with CHB exhibited a higher percentage of CD14+IP-10+ cells than patients with LC and HCs (p < 0.001, Fig. 5B), and this percentage was also higher in patients with LC than in HCs (p < 0.001, Fig. 5B).
Fig. 5.

CD14+ monocyte-derived IP-10 levels were increased in patients with CHB.
(A) CD14+ monocyte-derived IP-10 levels were significantly increased in patients with CHB compared with patients with LC and HCs. (B) Patients with CHB exhibited a significantly higher percentage of CD14+IP-10+ cells (n = 10) than HCs (n = 10) and patients with CHB complicated with LC (n=10). Each circle represents an individual. **p < 0.01.
Recombinant IP-10 promoted hepatocyte apoptosis
We analysed the effect of IP-10 on HepG2 cells in vitro to directly investigate the role of IP-10 in liver fibrosis and inflammation. As shown in Fig. 6, 1 ng/ml IP-10 markedly induced apoptosis in HepG2 cells after a 6 h treatment. Approximately 12.99% of the treated cells were positive for Annexin V, whereas only 8.48% of the control cells were positive for Annexin V. Higher concentrations of IP-10 did not have the same effect.
Fig. 6.
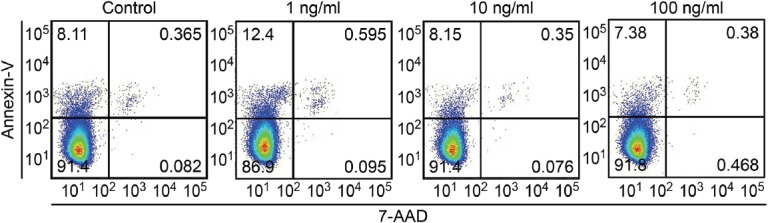
IP-10 promoted hepatocyte apoptosis.
Representative dot plots showing a significant increase in the rate of HepG2 cell apoptosis after a 6 h stimulation with 1 ng/ml IP-10.
DISCUSSION
In patients with HBV infection, antiviral therapy is a long-term and effective treatment. Unfortunately, many patients with CHB have a drug-resistant disease, and antiviral treatment is ineffective in these patients after a long duration of therapy. Thus, the identification of a baseline factor that predicts the outcome of antiviral treatment is urgently needed. IP-10 and its non-cognate receptor Toll-like receptor 4 (TLR4) are involved in the pro-apoptotic signalling cascade during liver injury; thus, antagonism of the chemokine C-X-C motif ligand (CXCL) 10/TLR4 pathway may be a therapeutic option for patients with liver diseases associated with increased apoptosis (Sahin et al., 2013). Although the use of IP-10 as a prognostic marker to predict the outcomes of antiviral therapy still faces significant opposition, lower serum IP-10 levels at year 0 were the only factor associated with HBsAg seroclearance in a previous study (Wong et al., 2016). Moreover, baseline IP-10 levels were associated with the response of HBeAg-positive patients treated with a combination of Peg-IFN and adefovir, and higher pretreatment IP-10 levels were associated with an increased probability of HBeAg loss after Peg-IFN therapy. In another study, higher IP-10 levels, particularly levels >350 pg/ml, were associated with a decrease in the HBsAg levels ≥ 0.5 log10 (Willemse et al., 2016).
IP-10 has recently been shown to be a useful biomarker for identifying patients with significant fibrosis (Sevgi et al., 2016), and IP-10 levels are associated with a decrease in ALT levels (Yoshio et al., 2016). In this study, we first measured IP-10 levels in different patient groups using peripheral blood collected before and after antiviral therapy. Baseline serum IP-10 levels were associated with the severity of liver fibrosis and inflammation. The baseline serum IP-10 level was positively correlated with clinical markers, such as ALT, AST, HBV DNA, and HBsAg levels. Moreover, serum IP-10 levels were positively correlated with the decrease in HBsAg levels during the year-long course of antiviral therapy. However, no relationship was observed between serum IP-10 levels and ALT levels, AST levels, HBV DNA load or the decrease in HBeAg levels after entecavir therapy.
Intrahepatic expression of IP-10 mRNA is correlated with ALT levels and HAI scores in patients with CHB and with the baseline HBV DNA load in patients with HBeAg-negative CHB (Deng et al., 2008). In this study, we examined the intrahepatic baseline IP-10 mRNA levels in patients with HBeAg-positive CHB and analysed the relationship between intrahepatic IP-10 expression and the outcomes of antiviral treatment. First, the serum and intrahepatic IP-10 levels were consistent. Second, higher levels of intrahepatic expression of the IP-10 mRNA were observed in patients with CHB than in patients with LC and HCs, and the intrahepatic expression of IP-10 mRNA was increased in patients with CHB who presented higher degrees of liver inflammation and fibrosis. The group of patients with a high degree of fibrosis (S≥3) did not include patients rated as S≥4 because most of the hepatic cells were necrotic in patients with LC. As IP-10 is mainly produced by hepatic cells, IP-10 expression was reduced in patients with LC. Third, intrahepatic IP-10 mRNA levels were positively correlated with the decrease in the HBsAg levels after antiviral treatment. Most importantly, patients with CHB who had good prognoses had higher baseline IP-10 levels in the liver and exhibited a marked decrease in IP-10 levels after antiviral therapy.
Furthermore, monocyte-derived IP-10 levels were increased in patients with CHB compared with patients with LC and HCs. In the in vitro study, 1 ng/ml IP-10 promoted hepatocyte apoptosis, but higher concentrations of IP-10 did not exert the same effect, which might be associated with the time of incubation. Further studies are needed to assess this dose-dependent effect. We explored the role of IP-10 in liver damage among patients with CHB and confirmed that IP-10 directly promotes hepatocyte apoptosis through the CXCR3 receptor, but we did not ascertain the timing of apoptosis induction. Our research did not assess the colocalization of CD14 and IP-10 in the liver, and our in vitro analyses were conducted with a small number of samples; therefore, studies with larger sample sizes are still needed to confirm whether IP-10 is a predictive marker of the outcomes of patients with CHB who are receiving entecavir therapy.
In conclusion, baseline serum and intrahepatic IP-10 levels are positively correlated with liver inflammation and fibrosis, and baseline IP-10 levels may predict the decrease in the HBsAg levels after entecavir therapy. Thus, IP-10 may be an important clinical marker in predicting the outcomes of entecavir therapy.
ACKNOWLEDGEMENTS
This work was supported by the Research Centre for Biological Therapy, Institute of Translational Hepatology, Beijing 302 Hospital and Department of Liver Disease, the 88th Hospital of PLA and grants from the National Natural Science Foundation of China (81273212) and the Science and Technology Development Project of Shandong Province, China (2014GSF118044).
REFERENCES
- Deng G., Zhou G., Zhang R., Zhai Y., Zhao W., Yan Z., Deng C., Yuan X., Xu B., Dong X., et al. Regulatory polymorphisms in the promoter of CXCL10 gene and disease progression in male hepatitis B virus carriers. Gastroenterology. 2008;134:716–726. doi: 10.1053/j.gastro.2007.12.044. [DOI] [PubMed] [Google Scholar]
- Gong L.-L., Zhao B.-B., Fan W.-F., Gong L.-Y., Chen C.-F., Liu J.-J., Lu X. Correlations of IFN-γ-inducible protein-10 with the risk of chronic hepatitis B and the efficacy of interferon therapy in Asians. Int J Clin Exp Pathol. 2015;8:8367–8375. [PMC free article] [PubMed] [Google Scholar]
- Jaroszewicz J., Ho H., Markova A., Deterding K., Wursthorn K., Schulz S., Bock C.T., Tillmann H.L., Manns M.P., Wedemeyer H., et al. Hepatitis B surface antigen (HBsAg) decrease and serum interferon-inducible protein-10 levels as predictive markers for HBsAg loss during treatment with nucleoside/nucleotide analogues. Antiviral Ther. 2011;16:915–924. doi: 10.3851/IMP1866. [DOI] [PubMed] [Google Scholar]
- Sahin H., Borkham-Kamphorst E., do O N.T., Berres M.L., Kaldenbach M., Schmitz P., Weiskirchen R., Liedtke C., Streetz K.L., Maedler K., et al. Proapoptotic effects of the chemokine, CXCL 10 are mediated by the noncognate receptor TLR4 in hepatocytes. Hepatology. 2013;57:797–805. doi: 10.1002/hep.26069. [DOI] [PubMed] [Google Scholar]
- Scheuer P.J. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- Sevgi D.Y., Bayraktar B., Gunduz A., Ozguven B.Y., Togay A., Bulut E., Uzun N., Dokmetas I. Serum soluble urokinase-type plasminogen activator receptor and interferon-gamma-induced protein 10 levels correlate with significant fibrosis in chronic hepatitis B. Wien Klin Wochenschr. 2016;128:28–33. doi: 10.1007/s00508-015-0886-4. [DOI] [PubMed] [Google Scholar]
- Sonneveld M.J., Arends P., Boonstra A., Hansen B.E., Janssen H.L. Serum levels of interferon-gamma-inducible protein 10 and response to peginterferon therapy in HBeAg-positive chronic hepatitis B. J Hepatol. 2013;58:898–903. doi: 10.1016/j.jhep.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao C., Zhang L., Yu W., Shen C., Wang W., Zhen Z., Zhou J. Predictive value of interferon-gamma inducible protein 10 kD for hepatitis B e antigen clearance and hepatitis B surface antigen decline during pegylated interferon alpha therapy in chronic hepatitis B patients. Antiviral Res. 2014;103:51–59. doi: 10.1016/j.antiviral.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Willemse S.B., Jansen L., de Niet A., Sinnige M.J., Takkenberg R.B., Verheij J., Kootstra N.A., Reesink H.W. Intrahepatic IP-10 mRNA and plasma IP-10 levels as response marker for HBeAg-positive chronic hepatitis B patients treated with peginterferon and adefovir. Antiviral Res. 2016;131:148–155. doi: 10.1016/j.antiviral.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Wong G.L., Chan H.L., Chan H.Y., Tse C.H., Chim A.M., Lo A.O., Wong V.W. Serum interferon-inducible protein 10 levels predict hepatitis B s antigen seroclearance in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2016;43:145–153. doi: 10.1111/apt.13447. [DOI] [PubMed] [Google Scholar]
- Xu R., Lin F., He J., Jin L., Zhang J.Y., Fu J., Liu H., Wang S., Zhang Z., Wang F.S. Complement 5a stimulates hepatic stellate cells in vitro, and is increased in the plasma of patients with chronic hepatitis B. Immunology. 2013;138:228–234. doi: 10.1111/imm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshio S., Sugiyama M., Shoji H., Mano Y., Mita E., Okamoto T., Matsuura Y., Okuno A., Takikawa O., Mizokami M., et al. Indoleamine-2,3-dioxygenase as an effector and an indicator of protective immune responses in patients with acute hepatitis B. Hepatology. 2016;63:83–94. doi: 10.1002/hep.28282. [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Zhang Z., Lin F., Zou Z.S., Xu R.N., Jin L., Fu J.L., Shi F., Shi M., Wang H.F., et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]
- Zhang G., Chen Y., BilalWaqar A., Han L., Jia M., Xu C., Yu Q. Quantitative analysis of rabbit coronary atherosclerosis. Practical techniques utilizing open-source software. Anal Quant Cytol Histol. 2015;37:115–122. [PubMed] [Google Scholar]


