Abstract
Gestational diabetes mellitus (GDM), one of the common metabolic disorders of pregnancy, leads to functional alterations in various cells including stem cells as well as some abnormalities in fetal development. Perivascular stem cells (PVCs) have gained more attention in recent years, for the treatment of various diseases. However, the effect of GDM on PVC function has not been investigated. In our study, we isolated PVCs from umbilical cord of normal pregnant women and GDM patients and compared their phenotypes and function. There is no significant difference in phenotypic expression, response to bFGF exposure and adipogenic differentiation capacity between normal (N)-PVCs and GDM-PVCs. However, when compared with N-PVCs, early passage GDM-PVCs displayed decreased initial rates of cell yield and proliferation as well as a reduced ability to promote wound closure. These results suggest that maternal metabolic dysregulation during gestation can alter the function of endogenous multipotent stem cells, which may impact their therapeutic effectiveness.
Keywords: differentiation, gestational diabetes mellitus, peri-vascular stem cells, proliferation
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder caused by deficiency (type 1) or ineffectiveness (type 2) of endogenous insulin and associated with an increased risk of vascular diseases in various tissues (Eriksson et al., 1989; Huysman and Mathieu, 2009; Kolluru et al., 2012). The third main form of DM is gestational DM (GDM), which is one of the most common metabolic derangements and characterized by glucose intolerance during pregnancy due to decreased insulin sensitivity (Buchanan et al., 2012). Children whose mothers had GDM during pregnancy are prone to obesity, malformation and diabetes (Leddy et al., 2008). Recent studies reported that GDM affects the growth and functions of endogenous stem or progenitor cells by inducing oxidative stress, senescence and mitochondrial dysfunctions. For examples, women with GDM have reduced concentration of circulating endothelial progenitor cells in late stages of pregnancy when compared with women without GDM (Acosta et al., 2011; Buemi et al., 2007; Penno et al., 2011). Two recent studies have demonstrated the adverse effect of GDM on proliferation, viability, differentiation and mitochondrial functions in mesenchymal stem cells (MSCs) derived from human umbilical cord (UC) (Kim et al., 2015; Wajid et al., 2015). More recently, Hadarits et al. reported that the proportion of hematopoietic stem cells (CD34+ CD45dim) is significantly higher in the cord blood of newborn from women with GDM compared to non-GDM women (Hadarits et al., 2016). These results suggest that changes in glucose tolerance during pregnancy may have adverse effects on the growth and function of endogenous stem or progenitor cells in various tissues.
In recent years, perivascular stem cells (PVCs) have been considered as an ancestor of MSCs with greater proliferative activity and multi-lineage differentiation potential when compared to bone marrow (BM)-derived MSCs. Human UC (HUC) is an ideal source to obtain PVCs because it represents discarded tissue after birth, is less invasive to mothers and babies and HUCPVCs are well-tolerated by the immune system. Furthermore, increasing evidence has demonstrated the broader effectiveness of HUCPVCs as an alternative therapeutic agent for various diseases and injuries such as pulmonary disease, cutaneous wound, infertility, diabetes and bone/cartilage defects (Kim et al., 2016; Maghen et al., 2016; Montemurro et al., 2011; Tsang et al., 2013; Zebardast et al., 2010). Thus, it seems plausible that the growth and regenerative potential of HUCPVCs can be influenced by maternal GDM. In this study, we non-enzymatically isolated HUCPVCs from umbilical cord collected at birth from healthy and GDM pregnancies and compared their functional properties to determine if GDM affects the growth and functional properties of HUCPVCs.
MATERIALS AND METHODS
Isolation and expansion of HUCPVCs
UCs were collected after caesarian section from 15 healthy women and 15 GDM mothers. The study was performed with IRB approval (Kangwon National University Hospital) and participants provided written informed consent. For all 30 patients, we collected maternal age, gestational period, newborn birth weight and Apgar score (Table 1). A previously described non-enzymatic HUCPVC isolation method was used (An et al., 2015). Briefly, dissected vessels from UC were incubated for 10 days in α-MEM (Gibco) supplemented with 10% FBS (Hyclone), 1% penicillin/streptomycin (Sigma) and 1 mM L-glutamine at 37°C in a humidified 5% CO2 incubator. Fibroblast-like colonies that migrated from the explanted vessels were subcultured by dissociation with 0.05% trypsin-EDTA (Sigma).
Table 1.
Donor demographics
| Parameters | Normal (n = 15) | GDM (n = 15) | P value |
|---|---|---|---|
| Donor ages (years) | 33 ± 0.91 | 34.33 ± 0.74 | 0.302 |
| Gestational period (weeks) | 38.75 ± 0.2 | 37.93 ± 0.45 | 0.097 |
| 1 min Apgar score | 7.33 ± 0.15 | 7.25 ± 0.13 | 0.699 |
| 5 min Apgar score | 8.9 ± 0.06 | 9 | 0.381 |
| Birth weight (kg) | 3.15 ± 0.1 | 3.13 ± 0.14 | 0.942 |
| Maternal medical problems | n.d. | n.d. | |
| Congenital fetal anomaly | n.d. | n.d. |
n.d. not detected
Flow cytometry assay
Single cell suspensions were prepared from normal pregnant women and GDM patient-derived HUCPVC (hereafter called N-PVC and GDM-PVC) cultures by dissociation with 0.05% trypsin-EDTA. The cells were resuspended in 1% FBS-PBS and were stained for 1 hr with the following fluorochrome-conjugated anti-human antibodies: CD31-phycoerythrin (PE), CD34-luorescein-isothiocyanate (FITC), CD45-allophycoerythrin (APC), SSEA-4-FITC, CD146-FTIC, CD44-APC and CD90-APC (all BD Biosciences) or their corresponding isotype controls. After immunostaining, the cells were incubated with the viability dye 7-aminoactinomycin D (7AAD) to exclude dead cells. Data was analyzed using FACSCanto II (BD Biosciences) and FlowJo software.
Cell proliferation assay
To compare the population doubling time (PDT) of the N-PVCs and GDM-PVCs, 6 × 104 cells (passage 1) were plated into 6-well tissue culture plate and continuously cultured from passage 1 to 8. The cells were passaged every 5 days and the total number of viable cells was counted at each passage. PDT was calculated as previously described (Hong et al., 2013). To evaluate the effects of bFGF (Invitrogen) on the proliferation of N-PVCs and GDM-PVCs, 4 × 104 cells were plated into 6-well plate and cultured for 4 days in the absence or presence of basic fibroblast growth factor (bFGF) (20 ng/ml). Viable cells were counted on a hemocytometer using trypan blue dye exclusion.
Cell cycle assessment
Cell cycle was analyzed with bromodeoxyuridine (BrdU) assay kit (BD Pharmingen) according to manufacturer’s instruction. Briefly, N-PVCs and GDM-PVCs were treated with 10 μM of BrdU for 4 h before cells were harvested. Single cell suspensions were stained with APC-conjugated anti-BrdU antibody for 1 h. The cells were washed and analyzed by FACSCanto II (BD Biosciences).
Adipogenic and osteogenic differentiation
The in vitro adipogenic and osteogenic differentiation potentials of HUCPVCs were assesed as previously described (An et al., 2015). Briefly, HUCPVCs were plated at 4.5 × 103 cells per cm2 in 12-well tissue culture plates and cultured in the presence of adipogenic and osteogenic supplements (Gibco). The medium was changed every 3 days for 21 days. The lipid vacuoles and mineralization, respectively, were visualized by Oil Red O or Alizarin Red S (Lifeline Cell Technology) and assessed quantitatively using a spectrophotometer (BioTek Instruments).
Harvest of conditioned medium and in vitro wound healing assay
N-PVCs and GDM-PVCs (passage 2) were plated at a density of 4 × 104 cells per well of 6-well plate. At 90% confluence, the cells were washed with PBS and replenished with serum-free α-MEM. After being incubated for 24 h at 37°C in a humidified 5% CO2, conditioned medium (CM) was collected and centrifuged at 2,000 rpm for 10 min to remove cell debris. To compare the wound healing potential of N-PVC and GDM-PVC, human bronchial epithelial cells (BEAS-2B, 17 × 104 cells/well) were plated in 6-well tissue culture plate and allowed to attach for 24 h. When BEAS-2B cells reach 90% confluency, an artificial wound was created by scratching the cell monolayer using P1000 pipette tip. The cultures were then rinsed with PBS and replenished with CM harvested from N-PVC and GDM-PVC cultures. Wound images were captured with a digitial camera at 12 h and the average rates of wound closure were analyzed using ImageJ version 1.47.
Data analysis
All results were expressed as mean ± standard deviation (SD). Comparisons for all experiments were performed with Student’s t-test. Significance levels were set at p < 0.05.
RESULTS
GDM does not alter the morphology and immunophenotype of PVCs
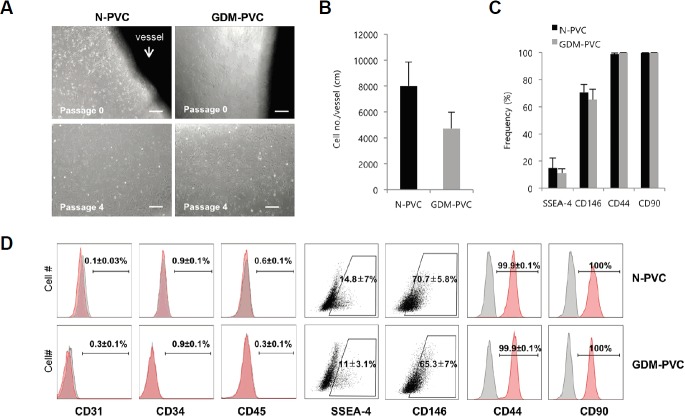
To investigate the effect of maternal hyperglycemia on PVCs, we isolated PVCs from dissected UCs of normal pregnant women and GDM patients using our previous non-enzymatic method. PVCs migrated from the explanted vessels of both normal and GDM UCs and were successfully subcultured up to passage 9. While both N-PVCs and GDM-PVCs exhibited similar spindle-like fibroblast morphology (Fig. 1A), less cells were harvested per centimeter of GDM versus normal (4,730 ± 1253 vs. 7,999 ± 1,855) (Fig. 1B). Flow cytometric analysis at passage 2 showed no significant difference in immunophenotype between N-PVCs and GDM-PVCs. Both N-PVCs and GDM-PVCs were negative for endothelial- (CD31 and CD34) and hematopoietic-associated (CD45) markers (Figs. 1C and 1D). The major proportion of both N-PVCs and GDM-PVCs were positive for MSC/PVC-associated markers CD44 (99.9 ± 0.1% vs. 99.9 ± 0.1%), CD90 (100% vs. 100%) and CD146 (70.7 ± 5.8% vs. 65.3 ± 7.0%). They also showed comparable frequencies of SSEA-4(+) cells (14.8 ± 7.0% vs. 11 ± 3.1%) (Fig. 1D). Taken together, these results suggest that a high concentration of glucose resulted in reduced harvested PVC numbers, but did not impact PVC morphology and immunophenotype.
Fig. 1.
Isolation and phenotypic characterization of HUCPVCs.
(A) Bright field images of HUCPVCs obtained from healthy and GDM patients. Arrow indicates dissected vessel from HUC. Scale bars. 500 μm. (B) Average number of harvested PVCs per centimeter of vessel length. (C, D) Representative flow cytometry dot plots and histograms for comparing phenotypes between N-PVCs and GDM-PVCs. Frequencies indicate average and SD. Error bars indicate SD.
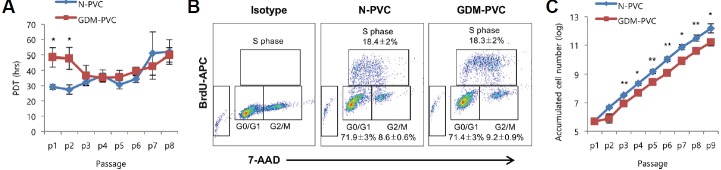
The proliferative capacity of N-PVCs and GDM-PVCs became homogeneous over passages
We next compared the proliferative potential of GDM-PVCs to N-PVCs. The proliferative capacity of GDM-PVCs was significantly lower than that of N-PVCs until passage 2 (p < 0.05). However, after passage 3, the proliferative capacity of both cell types became relatively homogeneous (Fig. 2A), which was confirmed using the BrdU incorporation assay. Both N-PVCs and GDM-PVCs (passage 4) showed similar S phase accumulation (18.4 ± 2.0% vs. 18.3 ± 2.0%) (Fig. 2B). The difference in the initial proliferation rate resulted in approximately 4-fold increase in cumulative cell number after 9 passages in comparison to GDM-PVCs (Fig. 2C). We previously reported that supplementation of bFGF profoundly promotes proliferation of HUCPVCs. Thus, we further examined whether the treatment of bFGF enhances the proliferation of GDM-PVCs. Equal number of N-PVCs and GDM-PVCs (passage 3) were plated and cultured for 4 days in the absence or presence of bFGF (20 ng/mL). Both cell types showed a decrease in cell cycle length following treatment with bFGF (Fig. 3A). In addition, supplementation of bFGF resulted in a similar degree of cell number increase in both cell types (Fig. 3B). BrdU incorporation assay revealed that both N-PVCs and GDM-PVCs exhibited a significant increased proportion of S phase (32.6 ± 1.9% vs. 28.2 ± 2.4%) by treatment of bFGF (Fig. 3B). These findings suggest that GDM leads to adverse effects on cell yield and proliferation of HUCPVCs during early passages. Interestingly, we found that slowed proliferative capacity of GDM-PVCs during early passages was recovered at later passages under low glucose conditions.
Fig. 2.
Comparison of proliferative capacity between N-PVCs and GDM-PVCs.
(A) Population doubling time (PDT) of HUCPVCs (n = 5) from passage 1 (p1) to p8. (B) Comparison of cell cycle compartments between N-PVCs and GDM-PVCs (passage 4) using BrdU incorporation assay (n = 5). (C) Accumulated cell numbers of HUCPVCs (n = 5) from p1 to p9. *p < 0.05, **p < 0.01. Error bars indicate SD.
Fig. 3.
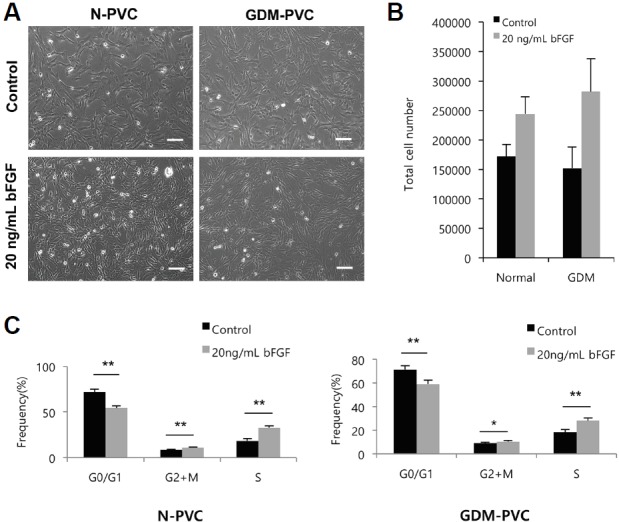
Supplementation of bFGF promotes the proliferation of N-PVCs and GDM-PVCs.
(A) Representative images of N-PVC and GDM-PVC cultured in the presence or absence of bFGF for 4 days. Scale bars. 500 μm. (B) N-PVCs (black bars) and GDM-PVCs (gray bars) at passage 4 were plated at a density of 4 × 104 cells and treated with 20 ng/ml of bFGF for 4 days. (C) Changes of cell cycle compartments by bFGF treatment were estimated by BrdU incorporation assay. *p < 0.05. **p < 0.01. Error bars indicate SD.
Comparison of regenerative potential between GDM-PVCs and N-PVCs
We next asked if maternal hyperglycemic condition affects the regenerative potential of HUCPVCs. We first compared the abilities of N-PVCs and GDM-PVCs differentiate into adipocytes and osteocytes. We detected lipid vacuoles by Oil Red O staining and calcium deposition by Alizarin Red S staining in both groups (Fig. 4A). Spectrophotometric quantification revealed a significant increase in osteogenic induction of GDM-PVC (1.8 fold) compared with N-PVC, while no difference in adipogenic induction (p < 0.01, Fig. 4B). PVCs and MSCs secret various trophic factor that modulate the microenvironment to evoke responses from resident stem or progenitor cells in tissues. Thus, many studies make efforts to elucidate their paracrine mechanisms for regenerative medicine. Here, we collected CM from GDM-PVC cultures and further assessed its paracrine effect using a wound closure model. We found that GDM-PVC-CM significantly reduced wound closure in BEAS-2B bronchial epithelial cell monolayers compared to N-PVCs (59.8 ± 5.8% vs. 76.3 ± 2.9%, p < 0.01) (Figs. 4C and 4D). These results indicate that maternal metabolic derangement during gestation can alter the regenerative potential of harvested PVCs.
Fig. 4.
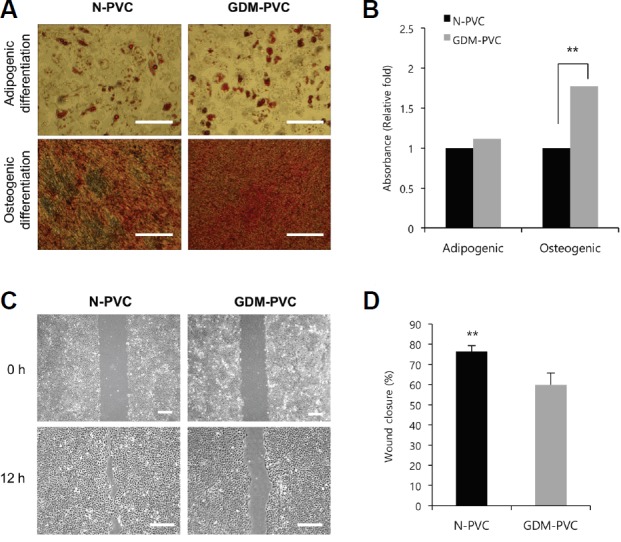
Comparison of in vitro regeneration potential between N-PVCs and GDM-PVCs.
(A) Representative images of Oil Red O and Alizarin Red S staining for adipocytes and osteocytes. Scale bars. 500 μm. (B) Measurements of Oil Red O and Alizarin Red S contents using spectrophotometry. (C) Representative images were captured by microscope and analyzed using ImageJ software at 0 and 12 h. Scale bars. 500 μm. (D) Percentage of uncovered wound area from BEAS-2B cell monolayers treated with N-PVC-CM and GDM-PVC-CM for 12 h. **p < 0.01. Error bars indicate SD.
DISCUSSION
In the present study, we clearly showed that the proliferation and function of HUCPVCs are adversely affected by hyperglycemic condition. Notably, women who had experienced GDM during pregnancy had a low yield of HUCPVCs compared to healthy pregnant women. The major causes of diabetic complications are the persistent production of reactive oxygen species and inflammation caused by exposure to hyperglycemia (Manea et al., 2015). This induces capillaries collapse by accelerating the loss of pericytes, which destroys the homeostasis of cells or tissues. Thus, our result showing lower HUCPVC yield from the GDM patients was somewhat predictable, indicating that we may not be able to obtain sufficient number of PVCs from donors with metabolic abnormalities.
Two independent studies reported the lower proliferation rate of early passage GDM-MSCs isolated from Wharton’s jelly compared with normal MSCs (Kim et al., 2015; Wajid et al., 2012). It was attributable to premature senescence, which is driven by depression of cyclin-dependent kinase inhibitors. Consistent with these findings, we also found the longer PDT in GDM-PVCs compared to N-PVCs during early passage even though no significant morphological and phenotypic differences between N-PVCs and GDM-PVCs were not seen. Furthermore, early passage GDM-PVCs showed significantly reduced ability to promote wound closure in BEAS-2B cell monolayers compared to N-PVCs. These results suggest that the biological properties or regenerative potentials of PVCs may be influenced by the pathological conditions of donors. This possibility can be inferred from previous findings showing functional deficits of MSCs derived from diseases or older donors. For example, BM-MSCs derived from rats with chronic kidney disease showed reduced proliferative capacity and no beneficial effect on anti-Thy1.1 nephritis due to premature cellular senescence (Klinkhammer et al., 2014). Aging and Parkinson’s disease lead to functional changes in mitochondria of BM-MSCs, which may be associated with their limited regenerative potential (Geissler et al., 2012; Mantovani et al., 2012; Moon et al., 2013). In addition, BM-MSCs obtained from multiple myeloid patients have an impaired osteogenic differentiation potential (Xu et al., 2012). All these findings strongly indicate that pathological conditions of donors are determinant factors when applying PVCs to clinical practice.
In summary, our results suggest that maternal metabolic abnormalities such as GDM may affect the proliferation and function of PVCs in the body, which should be considered in their use for regenerative medicine. Understanding the detailed molecular mechanism of functional abnormalities in GDM-PVCs and effects of other pathological conditions on PVC functions need to be further elucidated to develop their therapeutic strategies.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Ministry of Science, ICT and Future Planning (2015R1A4A1038666 and 2016R1A2B4014890). The authors thank Andrée Gauthier-Fisher (CReATe Fertility Center, Toronto, Ontario, Canada) for intellectual input to the drafts of the manuscript.
REFERENCES
- Acosta J.C., Haas D.M., Saha C.K., Dimeglio L.A., Ingram D.A., Haneline L.S. Gestational diabetes mellitus alters maternal and neonatal circulating endothelial progenitor cell subsets. Am J Obstet Gynecol. 2011;204:254.e8–254 e15. doi: 10.1016/j.ajog.2010.10.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An B., Heo H.R., Lee S., Park J.A., Kim K.S., Yang J., Hong S.H. Supplementation of growth differentiation factor-5 increases proliferation and size of chondrogenic pellets of human umbilical cord-derived perivascular stem cells. Tissue Eng Regen Med. 2015;12:181–187. [Google Scholar]
- An B., Na S., Lee S., Kim W.J., Yang S.R., Woo H.M., Kook S., Hong Y., Song H., Hong S.H. Non-enzymatic isolation followed by supplementation of basic fibroblast growth factor improves proliferation, clonogenic capacity and SSEA-4 expression of perivascular cells from human umbilical cord. Cell Tissue Res. 2015;359:767–777. doi: 10.1007/s00441-014-2066-7. [DOI] [PubMed] [Google Scholar]
- Buchanan T.A., Xiang A.H., Page K.A. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8:639–649. doi: 10.1038/nrendo.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buemi M., Allegra A., D’Anna R., Coppolino G., Crasci E., Giordano D., Loddo S., Cucinotta M., Musolino C., Teti D. Concentration of circulating endothelial progenitor cells (EPC) in normal pregnancy and in pregnant women with diabetes and hypertension. Am J Obstet Gynecol. 2007;196:68 e61–66. doi: 10.1016/j.ajog.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Eriksson J., Franssila-Kallunki A., Ekstrand A., Saloranta C., Widen E., Schalin C., Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Eng J Med. 1989;321:337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- Geissler S., Textor M., Kuhnisch J., Konnig D., Klein O., Ode A., Pfitzner T., Adjaye J., Kasper G., Duda G.N. Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PloS one. 2012;7:e52700. doi: 10.1371/journal.pone.0052700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadarits O., Zoka A., Barna G., Al-Aissa Z., Rosta K., Rigo J., Jr, Kautzky-Willer A., Somogyi A., Firneisz G. Increased proportion of hematopoietic stem and progenitor cell population in cord blood of neonates born to mothers with gestational diabetes mellitus. Stem Cells Deve. 2016;25:13–17. doi: 10.1089/scd.2015.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.H., Maghen L., Kenigsberg S., Teichert A.M., Rammeloo A.W., Shlush E., Szaraz P., Pereira S., Lulat A., Xiao R., et al. Ontogeny of human umbilical cord perivascular cells: molecular and fate potential changes during gestation. Stem Cells Dev. 2013;22:2425–2439. doi: 10.1089/scd.2012.0552. [DOI] [PubMed] [Google Scholar]
- Huysman E., Mathieu C. Diabetes and peripheral vascular disease. Acta Chir Belg. 2009;109:587–594. doi: 10.1080/00015458.2009.11680493. [DOI] [PubMed] [Google Scholar]
- Kim J., Piao Y., Pak Y.K., Chung D., Han Y.M., Hong J.S., Jun E.J., Shim J.Y., Choi J., Kim C.J. Umbilical cord mesenchymal stromal cells affected by gestational diabetes mellitus display premature aging and mitochondrial dysfunction. Stem Cells Dev. 2015;24:575–586. doi: 10.1089/scd.2014.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Hong K.S., Song W.K., Bae D., Hwang I.K., Kim J.S., Chung H.M. Perivascular progenitor cells derived from human embryonic stem cells exhibit functional characteristics of pericytes and improve the retinal vasculature in a rodent model of diabetic retinopathy. Stem Cells Transl Med. 2016;5:1268–1276. doi: 10.5966/sctm.2015-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkhammer B.M., Kramann R., Mallau M., Makowska A., van Roeyen C.R., Rong S., Buecher E.B., Boor P., Kovacova K., Zok S., et al. Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential. PLoS One. 2014;9:e92115. doi: 10.1371/journal.pone.0092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluru G.K., Bir S.C., Kevil C.G. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy M.A., Power M.L., Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- Maghen L., Shlush E., Gat I., Filice M., Barretto T., Jarvi K., Lo K., Gauthier-Fisher A.S., Librach C.L. Human umbilical perivascular cells: a novel source of MSCs to support testicular niche regeneration. Reproduction. 2016 doi: 10.1530/REP-16-0220. pii: REP-16–0220. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Manea A., Manea S.A., Todirita A., Albulescu I.C., Raicu M., Sasson S., Simionescu M. High-glucose-increased expression and activation of NADPH oxidase in human vascular smooth muscle cells is mediated by 4-hydroxynonenal-activated PPARα and PPARβ/δ. Cell Tissue Res. 2015;361:593–604. doi: 10.1007/s00441-015-2120-0. [DOI] [PubMed] [Google Scholar]
- Mantovani C., Raimondo S., Haneef M.S., Geuna S., Terenghi G., Shawcross S.G., Wiberg M. Morphological, molecular and functional differences of adult bone marrow- and adipose-derived stem cells isolated from rats of different ages. Exp Cell Res. 2012;318:2034–2048. doi: 10.1016/j.yexcr.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Montemurro T., Andriolo G., Montelatici E., Weissmann G., Crisan M., Colnaghi M.R., Rebulla P., Mosca F., Peault B., Lazzari L. Differentiation and migration properties of human foetal umbilical cord perivascular cells: potential for lung repair. J Cell Mol Med. 2011;15:796–808. doi: 10.1111/j.1582-4934.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H.E., Yoon S.H., Hur Y.S., Park H.W., Ha J.Y., Kim K.H., Shim J.H., Yoo S.H., Son J.H., Paek S.L., et al. Mitochondrial dysfunction of immortalized human adipose tissue-derived mesenchymal stromal cells from patients with Parkinson’s disease. Exp Neurobiol. 2013;22:283–300. doi: 10.5607/en.2013.22.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penno G., Pucci L., Lucchesi D., Lencioni C., Iorio M.C., Vanacore R., Storti E., Resi V., Di Cianni G., Del Prato S. Circulating endothelial progenitor cells in women with gestational alterations of glucose tolerance. Diab Vasc Dis Res. 2011;8:202–210. doi: 10.1177/1479164111408938. [DOI] [PubMed] [Google Scholar]
- Tsang W.P., Shu Y., Kwok P.L., Zhang F., Lee K.K., Tang M.K., Li G., Chan K.M., Chan W.Y., Wan C. CD146+ human umbilical cord perivascular cells maintain stemness under hypoxia and as a cell source for skeletal regeneration. PLoS One. 2013;8:e76153. doi: 10.1371/journal.pone.0076153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajid N., Naseem R., Anwar S.S., Awan S.J., Ali M., Javed S., Ali F. The effect of gestational diabetes on proliferation capacity and viability of human umbilical cord-derived stromal cells. Cell Tissue Bank. 2015;16:389–397. doi: 10.1007/s10561-014-9483-4. [DOI] [PubMed] [Google Scholar]
- Xu S., Evans H., Buckle C., De Veirman K., Hu J., Xu D., Menu E., De Becker A., Vande Broek I., Leleu X., et al. Impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients is associated with a blockade in the deactivation of the Notch signaling pathway. Leukemia. 2012;26:2546–2549. doi: 10.1038/leu.2012.126. [DOI] [PubMed] [Google Scholar]
- Zebardast N., Lickorish D., Davies J.E. Human umbilical cord perivascular cells (HUCPVC): A mesenchymal cell source for dermal wound healing. Organogenesis. 2010;6:197–203. doi: 10.4161/org.6.4.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]