Abstract
In breast cancer the use of small molecule inhibitors of tyrosine kinase activity of the ERBB family members improves survival thus represents a valuable therapeutic strategy. The addition of calcitriol, the most active metabolite of vitamin D, or some of its analogs, to conventional anticancer drugs, including tyrosine kinase inhibitors (TKIs), has shown an increased effect on the inhibition of cancer cell growth. In this work, we have evaluated the effects and the mechanism of action of the combination of calcitriol or its analog EB1089 with lapatinib or neratinib on EGFR and/or HER2 positive breast cancer cell lines. Lapatinib, neratinib, calcitriol and EB1089 inhibited breast cancer cell proliferation in a concentration-dependent manner. Addition of calcitriol or EB1089 to TKIs treatment induced more effective inhibiting effect on cell growth and AKT and MAPK phosphorylation than all compounds alone. The combined treatments incremented also the expression of active caspase 3 and induced cell death in two and three-dimensional cell culture and significantly inhibited anchorage-independent colony formation. Our results suggest that the addition of calcitriol or its analog EB1089 to conventional targeted therapies, including lapatinib or neratinib might be of benefit to patients with breast cancer, particularly those with an EGFR and/or HER2 positive phenotype.
Keywords: Lapatinib, neratinib, calcitriol, EB1089, HER2/EGFR positive breast cancer
Introduction
Breast cancer, a life-threatening disease, is one of the most common and the second leading cause of death in women, and its worldwide incidence continues to increase [1]. The mammary carcinomas are mainly classified into three molecular subtypes; luminal A/B, human epidermal growth factor receptor 2 (HER2) positive and triple-negative [2]. The latter two subtypes are aggressive, highly proliferative and metastatic. Both comprise approximately 20-30% and 15-20%, respectively, of all breast tumours [3,4]. In breast cancer, the overexpression and deregulation of some of the epidermal growth factor receptor (ERBB) family members (EGFR/HER1, HER2, HER3, and HER4) have been found to importantly contribute to the genesis and development of the tumorigenic process. In addition, coexpression of EGFR and HER2 have been associated with poorly differentiated tumours, metastases and worse prognosis than those tumours only expressing a single receptor [5,6]. In fact, a subset of triple negative breast cancer tumours contains elevated EGFR expression [7]. Activation and autophosphorylation of ERBB members, in specific tyrosine kinase residues, triggers the activation of tumour-promoting effects such as those mediated by the PI3K/AKT and Ras/Raf/MEK/MAPK signaling pathways that promotes proliferation, tumour cell growth and migration in breast cancer [8]. Hence, ERBB receptor family members have been intensely studied as therapeutic targets and several ERBB inhibitors are now been developed and of use in the clinic. In fact, HER2 positive breast cancers are treated with anti-HER2 therapies such as monoclonal antibodies and TKIs. On the other hand, triple negative breast cancer does not have conventional targeted therapies due to the lack of the receptors commonly found on other breast tumours, such as HER2, progesterone or estrogen receptors (PR, ER). Therefore; the treatment options for these tumours are chemotherapy and radiation therapy.
Lapatinib, a small molecule, inhibits HER2 and EGFR activation and subsequent down regulation of PI3K/AKT and MAPK pathways. Currently, this TKI is used for metastatic HER2 positive breast cancer treatment [9,10]. Interestingly, lapatinib showed an anti-proliferative effect in triple negative breast cancer cells through inhibition of the signaling pathway cancerous inhibitor of protein phosphatase 2A (CIP2A)/protein phosphatase 2A (PP2A)/AKT and induction of apoptosis [11,12].
Neratinib is another TKI of several ERBB family members (EGFR, HER2 and HER4) that blocks their downstream signaling pathways [13,14]. Currently, this inhibitor is being evaluated in a phase I/II clinical trials in patients with HER2 positive [13,15,16] and may also serve to treat metastatic breast cancer that overexpresses HER2 [16,17].
Several factors contribute to the progression and high incidence of HER2 positive and triple negative breast cancer, including vitamin D deficiency. This deficiency is typically defined as serum levels less than 80 nmol/L of calcidiol, a vitamin D metabolite that delimitates nutritional vitamin D status [18]. In fact, a low serum calcidiol concentration is associated with increased breast cancer risk, incidence and metastasis [19]. Calcitriol, the hormonally active form of vitamin D, exerts pleiotropic effects as growth arrest, cell differentiation, migration, invasion and apoptosis, through its binding with the vitamin D receptor (VDR) [20]. In fact, VDR knockout mice showed to be more prone to develop ER and PR negative breast tumours compared with their wild type counterparts after treatment with a carcinogen [21]. These findings suggest that vitamin D deficiency may play a critical role in the origin and development of breast cancer.
Calcitriol and several of its analogs increase tumour-cell sensitivity to diverse chemotherapeutic agents, antihormonal compounds and ionizing radiation [22]. In addition, recent evidences from our laboratory have shown that calcitriol or its analog EB1089, which has lower calcemic effects, enhance the antiproliferative activity of others antineoplastic agents, in EGFR and/or HER2 positive breast cancer cells [23,24]. Considering this, herein we study in breast cancer cell lines, overexpressing EGFR or HER2, if calcitriol or EB1089 affect the antiproliferative and pro-apoptotic activity of lapatinib and neratinib.
Material and methods
Reagents
Cell culture media were obtained from Life Technologies (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Hyclone Laboratories Inc (Logan, UT, USA). Lapatinib and neratinib were acquired from Sequoia Research Products (United Kingdom). Calcitriol (1α, 25-dihydroxyvitamin D3) and propidium iodide were purchased from Sigma (St. Louis, MO, USA). EB1089 (seocalcitol) was obtained from Tocris Bioscience (Bristol, United Kingdom). RNase A solution was acquired from Promega (Madison, WI, USA). Matrigel was obtained from Corning (NY, USA), F(ab) solution (1 mg/mL) was purchased from Jackson Immuno Research (PA, USA), Prolong solution was obtained from Molecular Probes (Thermo Fisher Scientific, MA, USA).
Cell culture
SUM-229PE cell line was acquired from Asterand (San Francisco, CA), SK-BR-3, HCC1937 and MDA-MB-231 cell lines were obtained from ATCC (Manassas, VA. USA). The cells were seeded in their specific medium following indications from the supplier. The media were supplemented with 5% heat-inactivated-FBS, 100 units/mL penicillin plus 100 µg/mL streptomycin and maintained at 37°C with a 5% atmosphere of CO2 and 95% humidity.
Proliferation and drug combination treatment
The cells were seeded in 96-well culture plates at a density of 1000-2000 cells per well depending on each line, then, the cells were incubated in the presence of increasing concentrations of calcitriol, EB1089, lapatinib and neratinib or vehicle alone (0.1% v/v ethanol or dimethyl sulfoxide) for 7 days. Subsequently, the DNA concentration was quantified by the CyQuant proliferation kit (Invitrogen) according to manufacturer’s instructions. The values of the inhibitory concentrations (IC) were calculated by non-linear regression sigmoidal fitting with a dose-response curve by means of a scientific graphing software (OriginLab Corporation, Northampton, MA, version 5.0). Experiments were performed in sextuplicate on at least 3 different occasions. Combinations of TKIs with calcitriol or its synthetic analog were performed using the IC20 and IC50 values. Pharmacological effect of combination studies was calculated with the combination index (CI) multi-drug equation of Chou Talalay [25]. For this analysis, the parameters are as follows: IC < 1 synergistic effect, IC > 1 antagonistic effect and CI = 1 additive effect.
Cell cycle distribution
Cells were incubated with the IC50 values of lapatinib or neratinib alone or in combination for 96 hours. After treatment, the cells were collected and washed with phosphate buffer (PBS) pH 7.2, fixed in 70% v/v ethanol and stored at -20°C. For cell cycle analysis, the samples were washed and incubated in RNAse (10 μg/ml), 0.1% v/v triton X-100 and propidium iodide (1 μg/ml) solution in the dark at room temperature for 20 min. The DNA content was determined using a FACSCanto II flow cytometer (Becton Dickinson, San Jose, CA, USA). For cell cycle analysis and detection of SubG0 peak a total of 35,000 events from PI-area vs. PI-wide gate were acquired. The results were analyzed using FlowJo Software (LLC, Ashland, OR, USA).
Detection of active form of caspase 3
In order to evaluate the effect of combinations on caspase 3 activation, monolayer and 3D cultures of breast cancer cells were used.
For monolayer cell culture, the cells were incubated with the antineoplastics independently or in combination for 72 h. Positive cells for the active form of caspase 3 were detected with a commercial apoptosis kit (BD PharMingen, CA, USA). Cells were collected, washed and resuspended in BD Cytofix/Cytoperm buffer and incubated for 20 minutes at 4°C. The cell suspension was centrifuged and washed twice with the BD Perm/Wash buffer. Subsequently, the cells were incubated with the fluorescein isothiocyanate (FITC) anti-active caspase 3 antibody for 30 min. Samples were washed, and resuspended with BD Perm/Wash buffer and analyzed by flow cytometry. A total of 20,000 events were acquired. Then, percentage of active caspase 3 positive cells was obtained from FSC-A vs. FITC active caspase 3 contour-plot.
3D culture assays were performed for the evaluation of the active form of caspase 3, as previously described [26]. SUM-229PE cells were seeded in 8 wells plates previously covered with Matrigel. After 7 days of culture, cells were exposed to different treatments alone or in combination for 48 h. Subsequently, the cells were fixed with 4% formalin, washed with PBS pH 7.2/glycine, and exposed to a primary block buffer and 10% goat serum for 1 h at room temperature. After this period, the blocking solution was aspirated and the cells were incubated with a second blocking buffer (immunofluorescence buffer, 10% goat serum, F (ab) 1 mg/mL) and the primary antibody (active form of caspase 3, Cell Signaling, dilution 1:100) overnight at 4°C. After this period, the samples were washed 3 times for 20 min with immunofluorescence buffer at room temperature. Subsequently, the cells were incubated for 1 h at room temperature with a solution containing the first blocking buffer with the secondary antibody. Finally, the samples were washed 3 times with the first blocking buffer and incubated with 4’,6-diamidino-2-phenylindole (DAPI) for 20 min at room temperature. After this period, the samples were washed with the first blocking buffer, aspirated and the Prolong solution was added. Finally, the slides were dried at room temperature and stored at -20°C until further analysis by confocal microscopy.
Western blot
After 72 h of treatment, the cells were washed with PBS pH 7.3 and lysed in a buffer containing HEPES 50 mM pH 7.4, NaCl 250 mM, EDTA 5 mM, Nonidet P-40 0.1%, NaF 10 mM, β-glycerophosphate 50 mM, Na3VO4 1 mM and complete EDTA-free protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Subsequently, 20 micrograms of protein were separated on SDS-PAGE and transferred to a polyvinylidene fluoride membrane, which was blocked with 5% milk for 30 min. Membranes were incubated overnight at 4°C with different antibodies, anti-EGFR (1:1000 Cell Signaling, Boston, Massachusetts), anti-VDR (1:1000, Santa Cruz Biotechnology Inc., CA, USA), anti-HER2 (1:1000 Cell Signaling), anti-phospho-p44/42 MAPK ERK1/2 (1:1000 Cell Signaling) or anti-AKT (serine 453, 1:1000 Cell Signaling). After incubation with the primary antibody, the membranes were washed and incubated with their respective peroxidase coupled secondary antibody (1:1000) for 1 h at room temperature. For loading control, the membranes were incubated with an anti-GAPDH antibody (1:1000 Santa Cruz Biotechnology Inc.). Proteins were detected with ECLPlus western blotting detection system (GE Healthcare, UK) and visualized using a Kodak developer. Densitometry was performed using Image J software (NIH, USA). The normalization of the values of each treatment was performed with respect to the total protein of ERK or AKT.
Soft-agar assays
The determination of the ability of the cells to form colonies after being exposed to the treatments was performed by the soft agar technique. Cells were seeded at a concentration of 5000 cells per well in 6 well plates with an agarose mixture consisting of 0.65% top agar and 0.35% bottom agar and their respective specific medium. The cells were treated with the antineoplastics alone or in combinations and allowed to grow for 30 days. The cells were incubated in a humidified chamber at 37°C with 5% CO2 and the medium was replaced every 7 days. After 30 days, the colonies were fixed and stained with a solution of methanol and violet crystal. Representative images were taken with the EVOS® FL Cell Imaging System (Life Technologies, San Francisco, USA) in clear field.
Statistical analyses
Data are expressed as the mean ± standard deviation (S.D.). Statistical analyses were determined by one-way ANOVA followed by the Holm-Sidak method, using a specialized software package (SigmaStat 3.5 version, Jandel Scientific).
Results
Effect of the antineoplastic agents on breast cancer cells proliferation
Protein expression of VDR, EGFR and HER2 was confirmed in established human breast cancer cell lines SUM-229PE, SK-BR-3, HCC1-937 and MDA-MB-231 by Western blots. All cell lines were VDR and EGFR positive, but only SK-BR-3 cells were HER2 positive (Table 1).
Table 1.
Cellular characterization of human breast cancer lines
| Cell line | VDR | EGFR | HER2 |
|---|---|---|---|
| SUM-229PE | + | + | - |
| SK-BR-3 | + | + | + |
| HCC1937 | + | + | - |
| MDA-MB-231 | + | + | - |
Vitamin D receptor (VDR), human epidermal growth factor receptor type I (EGFR), and type II (HER2).
The effects of lapatinib and neratinib on cell proliferation were evaluated using a DNA quantification assay. Both TKIs inhibited cell proliferation in a dose dependent manner; however, the compounds showed different potencies among the cell lines tested (Figure 1). Based on the calculated IC50 values (Table 2), the SK-BR-3 cell line, that overexpresses HER2, was the most sensitive, while HCC1937 and MDA-MB-231 (data not shown) were those significantly less sensitive to the TKIs. These results indicated and confirmed that HER2 overexpression is associated with an increased sensitivity to TKIs [27]. Both SUM-229PE and SK-BR-3 cells responded to calcitriol or its analog in terms of cell growth inhibition, as previously described (Table 2) [23]; however, HCC1937 responded significantly less and no response was observed in the case of MDA-MB-231 cells (data not shown).
Figure 1.
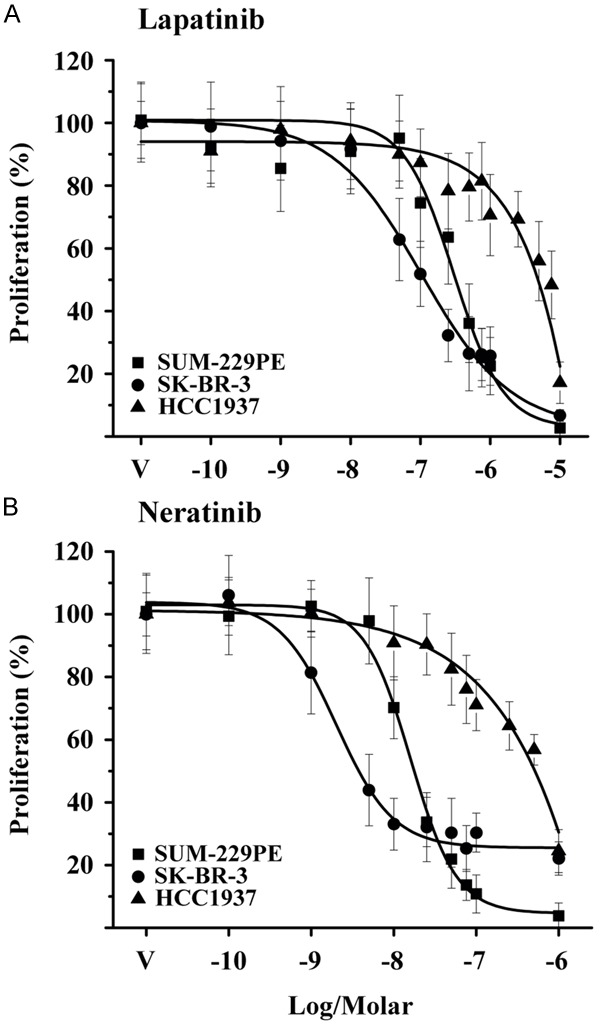
Antiproliferative effect of lapatinib and neratinib on breast cancer cells. The cell lines were incubated in the presence of different concentrations of lapatinib (A) or neratinib (B) during 7 days. Cell proliferation was evaluated by quantification of DNA. Results are shown as the mean ± S.D. of sextuplicate determinations of three independent experiments. Data from vehicle-treated cells (V) were normalized to 100%.
Table 2.
Inhibitory concentration (IC)20 and 50 values of lapatinib and neratinib on breast cancer cells proliferation
| Compound | IC | SUM-229PE (mol/L) | SK-BR-3 (mol/L) | HCC1937 (mol/L) |
|---|---|---|---|---|
| Lapatinib | 20 | 1.3 × 10-7 | 1.6 × 10-8 | 4.5 × 10-8 |
| 50 | 3.5 × 10-7 | 8.9 × 10-8 | 6.0 × 10-6 | |
| Neratinib | 20 | 6.7 × 10-9 | 8.1 × 10-10 | 8.5 × 10-8 |
| 50 | 1.4 × 10-8 | 2.1 × 10-9 | 7.5 × 10-7 | |
| Calcitriol (23) | 20 | 4.6 × 10-9 | 4.6 × 10-10 | - |
| 50 | 1.4 × 10-8 | 2.4 × 10-9 | - | |
| EB1089 (23) | 20 | 2.0 × 10-10 | 4.3 × 10-10 | - |
| 50 | 2.7 × 10-9 | 7.5 × 10-10 | - |
Effects of calcitriol or its analog on lapatinib or neratinib activities on cell growth
We next analyzed the combination of calcitriol or EB1089 with TKIs on breast cancer cells proliferation using IC20 and IC50 values (Table 2). For the particular cases where IC values were not obtained, only significant results upon cell proliferation are used. The results obtained from co-incubations are shown in Table 3. As depicted, the treatment of calcitriol or its analog with TKIs resulted in a significant and more notable inhibition of cell growth than that obtained with either drug alone, in most of the combinations used (Table 3). Except all combinations with neratinib using its IC50 values in SK-BR-3 and HCC1937 cell lines, although inhibitory, did not result in higher effect on cell growth. Interestingly, in cells that were insensitive to the effects of antineoplastics, such as MDA-MB-231 cells, drug resistance was reversed when cells were treated in combination with calcitriol or EB1089 (Table 3).
Table 3.
Growth inhibitory effects (I%) exerted by lapatinib, neratinib, calcitriol or EB1089 alone or in combination in breast cancer cells
| SUM-229PE | L | N | ||||
|
| ||||||
| IC20 | IC50 | IC20 | IC50 | |||
|
| ||||||
| Lg | IC | I% | 23.0±12.9 | 48.0±10.0 | 12.6±11.7 | 35.6±11.6 |
| Combinations | ||||||
| C | 20 | 15.4±8.7 | 26.3±14.2 | 59.2±12.5 | 48.7±8.4* | 58.0±11.7* |
| 50 | 45.7±7.8 | 56.9±14.2 | 72.5±7.4* | 64.7±10.3* | 73.8±8.5* | |
| E | 20 | 10.8±6.0 | 40.0±10.0* | 55.7±11.5 | 37.3±12.6* | 47.9±8.8 |
| 50 | 41.6±8.3 | 65.2±7.1* | 76.5±7.6* | 65.5±9.5* | 73.2±8.2* | |
|
| ||||||
| SK-BR-3 | L | N | ||||
|
| ||||||
| IC20 | IC50 | IC20 | IC50 | |||
|
| ||||||
| Lg | IC | I% | 24.4±10.2 | 60.4±10.8 | 52.3±9.9 | 77.7±4.6 |
| Combinations | ||||||
| C | 20 | 10.6±7.7 | 33.4±7.1 | 71.9±4.5* | 78.0±5.4* | 58.1±7.1 |
| 50 | 22.0±9.4 | 36.8±10.8 | 70.3±5.0* | 78.5±4.5* | 63.1±11.7 | |
| E | 20 | 14.6±7.7 | 28.6±5.9 | 70.6±6.7* | 79.5±3.7* | 59.5±7.0 |
| 50 | 31.6±11.2 | 43.0±11.1 | 71.1±4.6* | 78.4±4.8* | 63.9±7.9* | |
|
| ||||||
| HCC1937 | L | N | ||||
|
| ||||||
| IC20 | IC50 | IC20 | IC50 | |||
|
| ||||||
| Lg | IC mol/L | I% | 11.6±9.4 | 39.7±9.8 | 22.5±8.3 | 64.9±6.1 |
| Combinations | ||||||
| C | 1 × 10-8 | 7.3±1.5 | 67.1±3.1* | 39.8±6.8 | 42.9±13.1* | 63.3±8.4 |
| E | 1 × 10-8 | 8.9±6.9 | 53.3±6.9* | 57.1±8.8* | 33.9±5.6* | 61.1±5.9 |
|
| ||||||
| MDA-MB-231 | L | N | ||||
|
| ||||||
| 1 × 10-5 mol/L | 1 × 10-6 M mol/L | |||||
|
| ||||||
| Lg | IC mol/L | I% | 18.5±2.2 | 24.2±3.5 | ||
| Combinations | ||||||
| C | 1 × 10-7 | 4.0±9.2 | 31.7±10.9* | 21.2±3.8 | ||
| E | 1 × 10-7 | 1.7±9.7 | 42.5±8.7* | 34.2±8.8* | ||
Ligand (Lg), Inhibitory concentration (IC), lapatinib (L), neratinib (N), calcitriol (C), EB1089 (E). Results are expressed as the mean ± S.D. percent growth inhibition of sextuplicate determinations and represent at least three different experiments.
P < 0.001 vs. each drug alone.
The nature of the interactions between calcitriol or EB1089 and both of the TKIs studied was performed by the determination of the combination index (CI), as described under Material and Methods section. In general, combinations of lapatinib (Figure 2A) and neratinib (Figure 2B) with any of the vitamin D compounds in SUM-22PE and SK-BR-3 cells resulted in a combination index < 1, which denoted a synergistic interaction mechanism between calcitriol or EB1089 with TKIs. Of note, mostly synergic combinations were obtained with neratinib.
Figure 2.
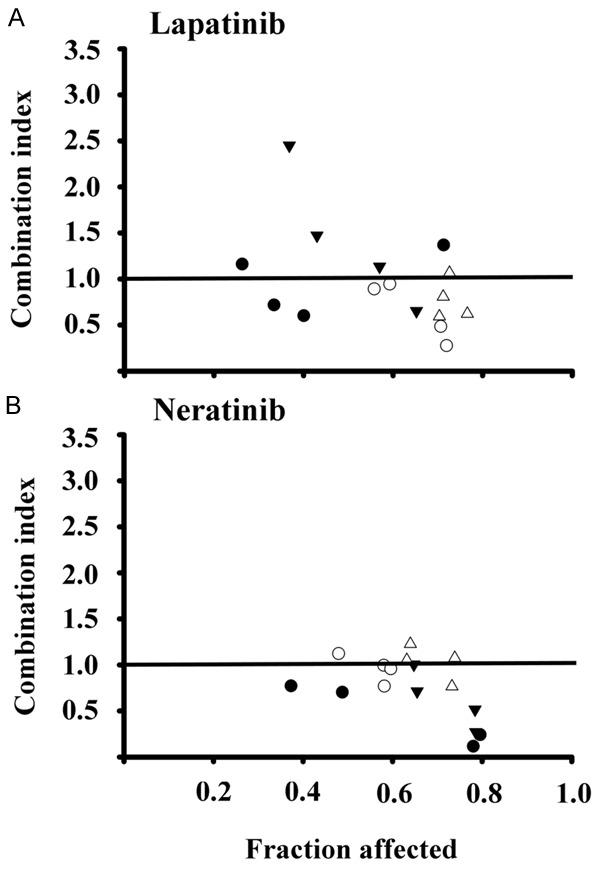
Combination index values obtained in breast cancer cells exposed to different drug combinations schemes. SUM-229PE and SK-BR-3 cells were incubated with drug combinations of vitamin D derivative: lapatinib (A) or neratinib (B); 20:20 (●); 20:50 (○); 50:20 (▼); and 50:50 (Δ). The combination index was calculated with the equation of Chou and Talalay. The data points below combination index values of 1 denoted by a horizontal line on each plot are indicative of synergistic interactions.
Treatment of breast cancer cells with calcitriol or EB1089 in combination with TKIs inhibited MAPK and AKT signaling pathways
Since SUM-229PE cells were the most sensitive to the growth inhibitory effects of drug combinations, we decided to investigate the effects of the combined treatment on ERK and AKT phosporylation, two signaling pathways activated by ERBB family members. Combined treatments of TKIs with calcitriol or its analog inhibited ERK and AKT phosphorylation more notably than either drug alone (Figure 3A and 3B). In addition, total ERK protein levels were also diminished in the presence of all combinations, however, total AKT levels were decreased only when calcitriol or EB1089 were added to neratinib.
Figure 3.
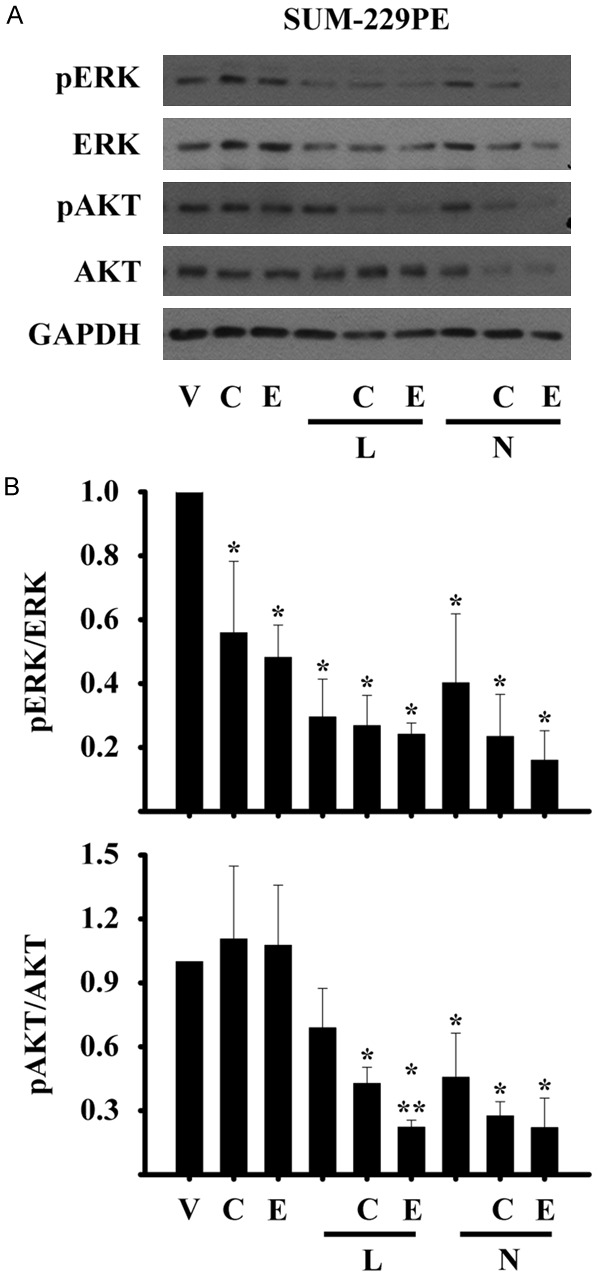
Inhibition of ERK1/2 and AKT phosphorylation by the co-treatments in breast cancer cells. SUM-229PE cells were incubated in the absence (V) or presence of its corresponding IC50 values of lapatinib (L), neratinib (N), calcitriol (C) or EB1089 (E) alone or in combination during 72 h. The phosphorylated form of the ERK or AKT proteins was determined by Western blots. Glyceraldehyde 3-phosphate dehydrogenase protein (GAPDH) was used as the loading control. Normalization of values was done against total ERK or AKT protein in SUM-229PE cells. The representative image (A) and the densitometry of three (SUM-229PE) different experiments (B) are shown.
Calcitriol or EB1089 combined with TKIs induced apoptosis and inhibited cell cycle progression
To confirm the results obtained with the cell growth assays and in order to elucidate the mechanisms of action of combination treatments, we then analyzed the cell cycle distribution by flow cytometry in SUM-229PE cells. Figure 4 shows the histograms of the cell cycle profile of the cells treated with the IC50 values of only one drug or in combination during 96 hours. As depicted, incubations in the presence of lapatinib or neratinib reduced the percentages of cells in S and G2/M phases compared to those observed with the vehicle alone and induced cell death, as detected by the increase in the percentage of cells in subG0 phase (Table 4).
Figure 4.
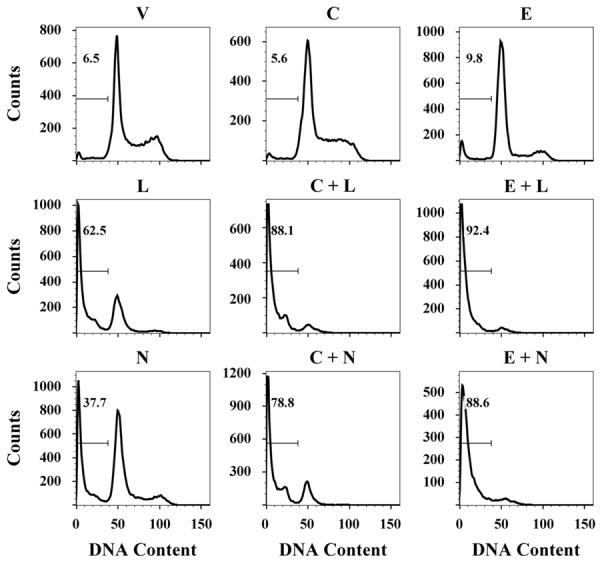
Effects of the addition of calcitriol or EB1089 to lapatinib or neratinib on the cell cycle of SUM-229PE cell line. Cells were incubated in the absence (V) or presence of their corresponding IC50 value of lapatinib (L), neratinib (N), calcitriol (C) or EB1089 (E) alone or in combination for 96 h. The DNA content was analyzed. Histograms show representative DNA profiles of cells treated with the agents. SubG0 peak is indicated with a marker. Images are representative from three independent experiments.
Table 4.
Percentage of SUM-229PE cells exposed to calcitriol, EB1089, lapatinib, neratinib alone or their combination in the cell cycle phases
| Treatment | SubG0 | G1 | S | G2 |
|---|---|---|---|---|
| V | 10.7±6.1 | 34.0±2.6 | 39.7±1.9 | 15.0±1.6 |
| C | 9.7±4.4 | 42.7±5.5 | 35.1±2.9 | 13.2±3.0 |
| E | 14.6±7.2 | 59.5±10.8* | 18.5±3.8* | 7.9±1.2* |
| L | 62.5±0.0* | 29.4±2.3 | 10.6±0.3* | 2.0±2.8* |
| C+L | 86.2±3.3*,** | 13.4±3.9*,** | 0.2±0.0*,** | 0.0±0.0* |
| E+L | 98.5±0.2*,** | 1.4±0.1*,** | 0.0±0.0*,** | 0.0±0.0* |
| N | 31.8±9.8* | 36.8±0.1 | 23.6±5.0* | 7.1±4.7* |
| C+N | 98.7±0.2*,** | 11.3±3.8*,** | 3.9±5.0*,** | 0.3±0.0*,** |
| E+N | 95.3±5.0*,** | 0.6±0.5*,** | 3.9±5.0*,** | 0.0±0.0*,** |
V, vehicle; C, calcitriol; E, EB1089; L, lapatinib; N, neratinib. Results are the mean ± S.D. from three independent assays.
P < 0.001 vs. V;
P < 0.001 vs. each drug alone.
Cells incubated only with calcitriol or EB1089 did not increase the percentage of cells in the subG0 phase but in the case of the analog, cells increased significantly the G1 fraction and decreased S and G2/M phases. On the other hand, the addition of calcitriol or its analog to TKIs increased even more the percentage of cells in the SubG0 region, while the percentage of cells in G1 phase was reduced and the percentages of cells in the S and G2/M phase were totally abolished as compared with each treatment alone or with the control (Figure 4 and Table 4). Thus our data suggest that the combination of calcitriol or EB1089 with lapatinib or neratinib induced cell cycle arrest and then cell death in higher proportions compared with the drugs alone.
To determine whether the high percentage of cells in the subG0 peak induced by combinations was due to the induction of apoptosis, the presence of active form of caspase 3 was evaluated. Figure 5A shows the presence of caspase activated in the gate. The combined treatments of calcitriol or EB1089 with TKIs significantly increased the percentages of active caspase 3-positive cells when compared to those incubations with all compounds alone (Figure 5B).
Figure 5.
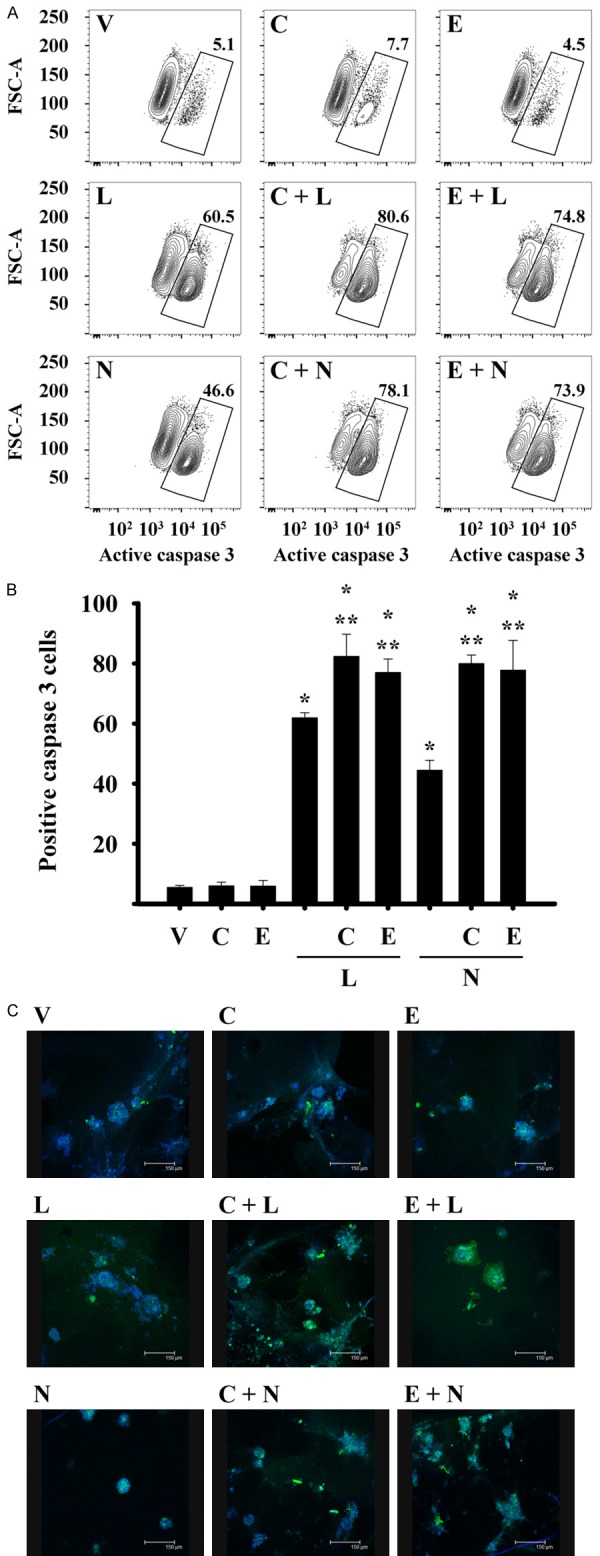
The addition of calcitriol or EB1089 to TKIs treatment increased the caspase 3 active form in breast cancer cells. SUM-229PE cells were incubated in the absence (V) or presence of lapatinib (L), neratinib (N), calcitriol (C) or EB1089 (E) alone or in combination during 72 h (A and B) and 48 h (C) with their corresponding IC50 value. (A) SUM-229PE cells were permeated, fixed and stained with an anti-caspase 3 active antibody. Subsequently, the cells were analyzed by flow cytometry and cells positive to caspase 3 are shown in the gate. (B) Quantification of percentage of cells positive to caspase 3 obtained of three experiments independently. (C) SUM-229PE cells were grown in the presence of matrigel, after 7 days of culture the cells were treated with the compounds for 48 h. The cells were fixed, permeated and stained with DAPI and anti-caspase 3 antibody (Alexa 488 fluorophore). Subsequently, images were acquired with a confocal microscope. Data images are representative of two to three experiments independently.
Because three-dimensional (3D) epithelial cell culture models maintain the structural organization and multicellular complexity of the mammary epithelium and are useful for evaluating experimental therapies, it was decided to assess the presence of caspase-3-induced by compounds in a 3D culture. The combination of calcitriol or its analog with lapatinib or neratinib resulted in activation of caspase 3 activity, as judged by the increase in fluorescence intensity, compared to the compounds alone, denoting increased abundance of this protein in its active form in SUM-229PE cells treated with the combined drugs (Figure 5C). Taken together these findings indicate that the combination of compounds inhibits proliferation and induces apoptotic pathways.
Combined treatment of calcitriol or EB1089 with lapatinib and neratinib inhibited the anchorage-independent growth of breast cancer cells
To assess the ability of the SUM-229PE cells to divide and form anchorage-independent colonies following treatment with the antineoplastics, an in vitro soft agar assay was performed. Figure 6A shows the results in SUM-229PE cells incubated in the presence or absence of the compounds. The formation of colonies was not affected in the cells treated with the vehicle; in contrast, the colony formation and size were considerably inhibited in cells treated with the individual compounds. Of note, the development of colonies was completely abolished by the combined treatments (Figure 6B). Considering that the soft agar assay of cells in vitro is a valuable pre-clinical technique to test the anti-tumour potential of new cancer therapies [28], these results highlight the effectiveness of calcitriol or EB1089 as adjuvant in the treatment of breast cancer.
Figure 6.
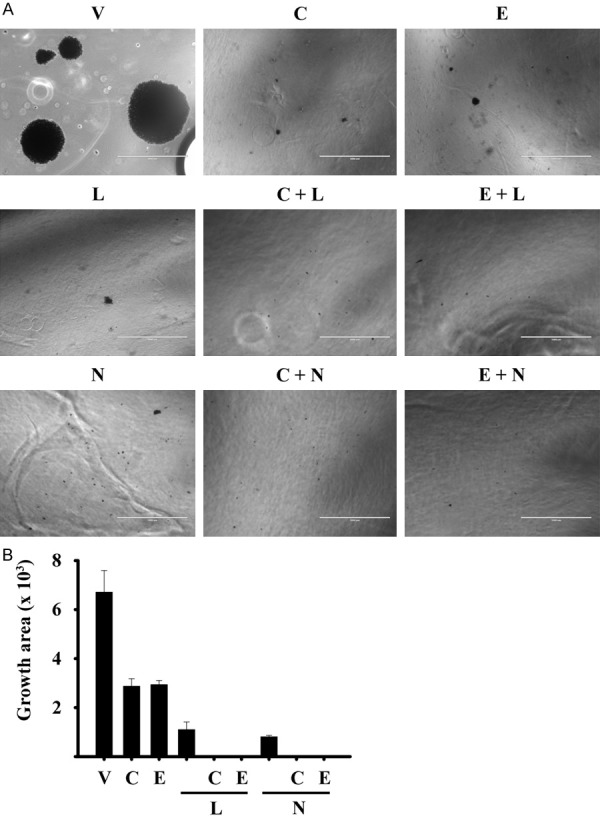
The combination of calcitriol or EB1080 with lapatinib or neratinib inhibited the clonogenic capacity of breast cancer cells. SUM-229PE cells were seeded on soft agar, and then they were incubated in the presence of each compound at their corresponding IC50, alone or in combination with calcitriol for 30 days. Every 7 days, the medium was changed and treatments refreshed. The colonies were stained with violet crystal. A: Representative images of two experiments are shown. B: The growth area size was quantified using Image J. Each bar represents the mean ± SD of two experiments.
Discussion
Patients with malignant breast tumours that overexpress EGFR and/or HER2 generally have poor prognosis, aggressive clinical behavior and resistance to conventional treatments [5,29]. Thus, inhibition of EGFR family members activation results in an important therapeutic strategy in breast cancer tumours [30,31]. An example of this is the use of lapatinib which is currently approved as second-line therapy and the phase I studies of neratinib in patients with HER2 positive breast cancer [16,32]. In this regard, lapatinib and neratinib have also been shown to reduce the proliferation of breast cancer cells expressing different levels of EGFR or HER2, by inhibiting the kinase activity of these receptors [10,13,33,34]. In addition, calcitriol is a potent cell growth inhibitor of breast cancer cells regardless of their molecular phenotype on in vitro and in vivo models. The antiproliferative effects of this compound have been evaluated in several treatments combined with multiple antineoplastic agents in preclinical and clinical phases, resulting in an increase in their antitumour activity [22,35]. The main objective of this study was to evaluate the effects derived from the addition of calcitriol or EB1089 to lapatinib or neratinib on cultured ERBB receptor positive breast cancer cells.
Both lapatinib and neratinib had important antiproliferative effects, particularly on those cells with an EGFR and HER2 positive phenotype. In this study, neratinib was more potent than lapatinib to inhibit cell proliferation. Interestingly, the SK-BR-3 cell line, that overexpresses HER2, was the most sensitive to these TKIs, finding that was consistent with previous publications [27]. In addition, the IC50 values of lapatinib and neratinib obtained in the different cell lines evaluated are similar to those reported in other studies [27].
Regarding the effects of calcitriol or its analog on cell proliferation, the breast cancer lines studied showed differences in sensitivity to both compounds. The most sensitive lines to calcitriol and EB1089 were SUM-229PE and SK-BR-3. It is also important to mention that the IC50 values for calcitriol or EB1089 obtained in these cells were similar to those reported in previous studies using hormone receptor positive breast cancer cells [36], suggesting that the effect of the mentioned compounds is independent of the presence of the hormonal receptors and the ERBB family members.
In this work, we demonstrated that addition of calcitriol or its analog to lapatinib or neratinib treatment resulted in significantly better antiproliferative effects compared to those with the compounds alone in various breast cancer tumour phenotypes; thus, supporting previous studies in which addition of calcitriol to diverse therapies, including the TKI gefitinib, potentiates the antineoplastic activity [22,23].
Of note, the cell lines that resulted less sensitive to calcitriol and its analog were the cells with triple negative phenotype HCC1937 and MDA-MB-231. This phenomenon might be attributed to the fact that these cell lines are poorly differentiated [37,38]. High expression of the enzyme CYP24A1, involved in calcitriol degradation, has been associated with calcitriol resistance and more malignant and metastatic tumours. In fact, CYP24A1 basal expression is highly expressed in MDA-MB-231 cells [39]. Our data agree with Peng, et al., who demonstrated that the MDA-MB-231 line is not sensitive to the antiproliferative effects of calcitriol [40].
On the other hand, and as previously shown, administration of calcitriol or EB1089 or various of the TKIs alone affects proliferation and induces apoptosis of breast cancer cells [10,13,41], however, when they are co-administered with other antineoplastic agents, largely inhibit cancer cell proliferation [23,42-44], indicating that combination therapy has better results than monotherapy. In this study, addition of calcitriol or its analog to lapatinib or neratinib, had greater efficacy in inhibiting breast cancer cell proliferation and caused a significant downregulation of phosphorylated and total form of ERK and AKT than that observed with either treatment alone. This fact suggests that these combination treatments not only act by inhibiting the kinases activity, but also their protein expression, what eventually may result in inhibition of cell proliferation, tumour cell growth and migration in breast cancer [45].
This finding suggests that other mechanisms, in addition to those concerning the inhibition of tyrosine kinase enzyme activity, are taking place when calcitriol is being added to cultured cells. As previously shown by this laboratory, calcitriol was able to inhibit cell growth through the downregulation of gene transcription of a unique ether á go-go 1 potassium channel (EAG1) in breast and cervical cancer primary and established human cell lines [39,46]. In addition, using microarray assays, it was demonstrated that RXR-VDR heterodimer, known as a transcription factor, was differentially modulated in response to lapatinib in breast cancer cell lines [47], suggesting that the addition of calcitriol or EB1089 could favor the antineoplastic effects of TKIs. Interestingly enough was the finding, in MDA-MB-231 cells, that TKIs in the presence of calcitriol or EB1089 reversed the drug resistance phenotype of these cells, indicating the ability of this hormone to improve drug sensitivity, probably through a mechanisms involving cell death or a cell differentiation promoting effect, as previously shown in the case of estrogen receptor negative breast cancer cells [24].
Most of the combinations tested in this work showed a synergistic effect to inhibit cell proliferation. Notably, the SUM-229PE cell line was the most sensitive to the combination of TKIs with calcitriol or EB1089, compared to the other cell lines used. Moreover, the mostly synergic combinations were obtained with neratinib possibly for its pan-HER inhibitor activity, highlighting its potential use in the treatment of breast cancer. Of note, the concentrations of all tested compounds are therapeutically achievable at serum levels in human blood, except in MDA-MB-231 cells, as mentioned in previous studies [17,48-50]. Our results raise the possibility of using calcitriol or its analog in the therapy schemes of lapatinib or neratinib in order to reduce the side effects of these inhibitors and increase their antineoplastic activity.
TKIs activate apoptosis by increasing the subG0 cell population and decreasing the percentage of cells undergoing synthesis and mitosis [11,13]. Accordingly, treatment with lapatinib resulted in activation of caspase 3, as previously reported [11]. Similar to lapatinib, neratinib increased the active form of caspase 3. It is noteworthy to mention that, to our knowledge, this study is the first to demonstrate the ability of neratinib to induce the active form of caspase 3 in breast cancer cells. The addition of calcitriol or its analog to lapatinib and neratinib treatment, favored the accumulation of cells in G1 phase, markedly reducing the percentage of cells in S and G2/M phases, and leading to induction of apoptosis. It should be noted that in the case of EB1089, its addition to lapatinib increased even more the percentage of cell death. This effect could be attributed to the higher activity of the analog when compared to calcitriol [51]. This result supports the use of analogs of calcitriol as adjuvants in the treatment of breast cancer due to its antineoplastic effects and low calcemic effects. Further in vivo studies should be performed with the purpose to ascertain these assumptions.
The ability of a cell to form colonies has been associated with the potential of cancer cells to cause post-treatment relapse on in vivo models [52]. Herein under in vitro conditions, we demonstrated that the presence of calcitriol or EB1089 in the co-incubations with TKIs decreased the formation of colonies and anchorage-independence was completely inhibited in breast cancer cells. These findings, may suggest that the antitumor effects of calcitriol added to conventional treatments with TKIs may involve the modulation of oncogenes and cell cycle genes.
Simultaneous treatment of lapatinib or neratinib with vitamin D metabolite calcitriol and analog EB1089 resulted in a greater inhibition of cell proliferation, abolished anchorage-independent growth and induced apoptosis in breast cancer cells. These effects were mediated by the modulation of the PI3K/AKT and MAPK pathways, as well as the increase of subG0 phase, which resulted in induction of apoptosis.
These findings, may suggest that combination therapy of lapatinib or neratinib with calcitriol or its analog may have utility and could optimize the clinical applications of these compounds in the treatment of patients with breast cancer. Therefore, further research in in vivo models and clinical investigations would be of great relevance to corroborate this effect.
Conclusion
The overall results may provide, with scientific evidence, the bases for the potential use of calcitriol or its analogs as antiproliferative, antitumoral adjuvant agent for the treatment of patients affected with EGFR and HER2 positive breast cancer. We showed that the anticancer effects of calcitriol or EB1089 when added to lapatinib or neratinib are mediated via inhibition of the expression and activity of important intracellular signaling pathways involved in cell cycle regulation, cell survival, and cell death. Our results correlated well with those of similar studies supporting the role of calcitriol as adjuvant for the treatment of cancer.
Acknowledgements
This work was supported by grants 256994 from the Consejo Nacional de Ciencia y Tecnología (CONACyT), México and INCMN/110/08/PI/86/15 from Instituto Científico Pfizer, to RGB. MSM has a post-doctoral fellowship from the “Fundación para la salud y la Educación Dr. Salvador Zubirán” (grant number P-318). The authors would like to thank Biol. Salvador Ramirez Jiménez, who is responsible of the repository of cell lines from “Programa de Investigación en Cáncer de Mama” Universidad Nacional Autónoma de México, for providing the HCC1937 cell line.
Disclosure of conflict of interest
None.
Authors’ contribution
RGB was involved in the conception, design and coordination of the study as well as in data analysis, interpretation of results, actively participated in experimental procedures, and was involved in drafting the manuscript. MSM was in charge of all experimental procedures, participated in coordination, analysis and interpretation of data, as well as in drafting of manuscript. LD and MJR participated in interpretation of results and made a significant contribution to the study and drafting the manuscript. HPG participated in the experimental procedures and revised critically the content of the manuscript. FL participated in the interpretation of data, made substantive intellectual contribution to the study and drafting the manuscript. All authors read and approved the final manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Haibe-Kains B, Desmedt C, Piette F, Buyse M, Cardoso F, Van’t Veer L, Piccart M, Bontempi G, Sotiriou C. Comparison of prognostic gene expression signatures for breast cancer. BMC Genomics. 2008;9:394. doi: 10.1186/1471-2164-9-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Saha P, Nanda R. Concepts and targets in triple-negative breast cancer: recent results and clinical implications. Ther Adv Med Oncol. 2016;8:351–359. doi: 10.1177/1758834016657071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osaki A, Toi M, Yamada H, Kawami H, Kuroi K, Toge T. Prognostic significance of co-expression of c-erbB-2 oncoprotein and epidermal growth factor receptor in breast cancer patients. Am J Surg. 1992;164:323–326. doi: 10.1016/s0002-9610(05)80897-9. [DOI] [PubMed] [Google Scholar]
- 6.Harlozinska A, Bar JK, Wenderski R, Bebenek M. Relationship between c-erbB-2 oncoprotein, epidermal growth factor receptor, and estrogen receptor expression in patients with ductal breast carcinoma. Association with tumor phenotypes. In Vivo. 1996;10:217–222. [PubMed] [Google Scholar]
- 7.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 8.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 9.Segovia-Mendoza M, Gonzalez-Gonzalez ME, Barrera D, Diaz L, Garcia-Becerra R. Efficacy and mechanism of action of the tyrosine kinase inhibitors gefitinib, lapatinib and neratinib in the treatment of HER2-positive breast cancer: preclinical and clinical evidence. Am J Cancer Res. 2015;5:2531–2561. [PMC free article] [PubMed] [Google Scholar]
- 10.Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 11.Liu CY, Hu MH, Hsu CJ, Huang CT, Wang DS, Tsai WC, Chen YT, Lee CH, Chu PY, Hsu CC, Chen MH, Shiau CW, Tseng LM, Chen KF. Lapatinib inhibits CIP2A/PP2A/p-Akt signaling and induces apoptosis in triple negative breast cancer cells. Oncotarget. 2016;7:9135–9149. doi: 10.18632/oncotarget.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T, Yacoub R, Taliaferro-Smith LD, Sun SY, Graham TR, Dolan R, Lobo C, Tighiouart M, Yang L, Adams A, O’Regan RM. Combinatorial effects of lapatinib and rapamycin in triplenegative breast cancer cells. Mol Cancer Ther. 2011;10:1460–1469. doi: 10.1158/1535-7163.MCT-10-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, Hallett WA, Johnson BD, Nilakantan R, Overbeek E, Reich MF, Shen R, Shi X, Tsou HR, Wang YF, Wissner A. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 14.Wissner A, Overbeek E, Reich MF, Floyd MB, Johnson BD, Mamuya N, Rosfjord EC, Discafani C, Davis R, Shi X, Rabindran SK, Gruber BC, Ye F, Hallett WA, Nilakantan R, Shen R, Wang YF, Greenberger LM, Tsou HR. Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2) J Med Chem. 2003;46:49–63. doi: 10.1021/jm020241c. [DOI] [PubMed] [Google Scholar]
- 15.Crown J, O’Shaughnessy J, Gullo G. Emerging targeted therapies in triple-negative breast cancer. Ann Oncol. 2012;23(Suppl 6):vi56–65. doi: 10.1093/annonc/mds196. [DOI] [PubMed] [Google Scholar]
- 16.Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, Awada A, Ranade A, Jiao S, Schwartz G, Abbas R, Powell C, Turnbull K, Vermette J, Zacharchuk C, Badwe R. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J. Clin. Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 17.Chow LW, Xu B, Gupta S, Freyman A, Zhao Y, Abbas R, Vo Van ML, Bondarenko I. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br J Cancer. 2013;108:1985–1993. doi: 10.1038/bjc.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rainville C, Khan Y, Tisman G. Triple negative breast cancer patients presenting with low serum vitamin D levels: a case series. Cases J. 2009;2:8390. doi: 10.4076/1757-1626-2-8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janowsky EC, Lester GE, Weinberg CR, Millikan RC, Schildkraut JM, Garrett PA, Hulka BS. Association between low levels of 1,25-dihydroxyvitamin D and breast cancer risk. Public Health Nutr. 1999;2:283–291. doi: 10.1017/s1368980099000385. [DOI] [PubMed] [Google Scholar]
- 20.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005;97:153–164. doi: 10.1016/j.jsbmb.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Trump DL, Johnson CS. Vitamin D in combination cancer treatment. J Cancer. 2010;1:101–107. doi: 10.7150/jca.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segovia-Mendoza M, Diaz L, Gonzalez-Gonzalez ME, Martinez-Reza I, Garcia-Quiroz J, Prado-Garcia H, Ibarra-Sanchez MJ, Esparza-Lopez J, Larrea F, Garcia-Becerra R. Calcitriol and its analogues enhance the antiproliferative activity of gefitinib in breast cancer cells. J Steroid Biochem Mol Biol. 2015;148:122–131. doi: 10.1016/j.jsbmb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Santos-Martinez N, Diaz L, Ordaz-Rosado D, Garcia-Quiroz J, Barrera D, Avila E, Halhali A, Medina-Franco H, Ibarra-Sanchez MJ, Esparza-Lopez J, Camacho J, Larrea F, Garcia-Becerra R. Calcitriol restores antiestrogen responsiveness in estrogen receptor negative breast cancer cells: a potential new therapeutic approach. BMC Cancer. 2014;14:230. doi: 10.1186/1471-2407-14-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 26.Kambach DM, Sodi VL, Lelkes PI, Azizkhan-Clifford J, Reginato MJ. ErbB2, FoxM1 and 14-3-3zeta prime breast cancer cells for invasion in response to ionizing radiation. Oncogene. 2014;33:589–598. doi: 10.1038/onc.2012.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Neill F, Madden SF, Clynes M, Crown J, Doolan P, Aherne ST, O’Connor R. A gene expression profile indicative of early stage HER2 targeted therapy response. Mol Cancer. 2013;12:69. doi: 10.1186/1476-4598-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horibata S, Vo TV, Subramanian V, Thompson PR, Coonrod SA. Utilization of the soft agar colony formation assay to identify inhibitors of tumorigenicity in breast cancer cells. J Vis Exp. 2015:e52727. doi: 10.3791/52727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris AL, Nicholson S, Sainsbury JR, Farndon J, Wright C. Epidermal growth factor receptors in breast cancer: association with early relapse and death, poor response to hormones and interactions with neu. J Steroid Biochem. 1989;34:123–131. doi: 10.1016/0022-4731(89)90072-1. [DOI] [PubMed] [Google Scholar]
- 30.Singh JC, Jhaveri K, Esteva FJ. HER2-positive advanced breast cancer: optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111:1888–1898. doi: 10.1038/bjc.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li SG, Li L. Targeted therapy in HER2-positive breast cancer. Biomed Rep. 2013;1:499–505. doi: 10.3892/br.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H. Lapatinib for the treatment of breast cancer in the People’s Republic of China. Onco Targets Ther. 2014;7:1367–1373. doi: 10.2147/OTT.S60586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spector NL, Xia W, Burris H 3rd, Hurwitz H, Dees EC, Dowlati A, O’Neil B, Overmoyer B, Marcom PK, Blackwell KL, Smith DA, Koch KM, Stead A, Mangum S, Ellis MJ, Liu L, Man AK, Bremer TM, Harris J, Bacus S. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J. Clin. Oncol. 2005;23:2502–2512. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 34.Canonici A, Gijsen M, Mullooly M, Bennett R, Bouguern N, Pedersen K, O’Brien NA, Roxanis I, Li JL, Bridge E, Finn R, Siamon D, McGowan P, Duffy MJ, O’Donovan N, Crown J, Kong A. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget. 2013;4:1592–1605. doi: 10.18632/oncotarget.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trump DL, Deeb KK, Johnson CS. Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J. 2010;16:1–9. doi: 10.1097/PPO.0b013e3181c51ee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swami S, Krishnan AV, Feldman D. 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000;6:3371–3379. [PubMed] [Google Scholar]
- 37.Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S. Triple negative breast cancerprognostic factors and survival. Radiol Oncol. 2011;45:46–52. doi: 10.2478/v10019-010-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehman A. Triple-negative phenotype of poorly-differentiated metaplastic breast carcinoma in a male: an oncological rarity. J Coll Physicians Surg Pak. 2013;23:370–372. [PubMed] [Google Scholar]
- 39.Garcia-Becerra R, Diaz L, Camacho J, Barrera D, Ordaz-Rosado D, Morales A, Ortiz CS, Avila E, Bargallo E, Arrecillas M, Halhali A, Larrea F. Calcitriol inhibits Ether-a go-go potassium channel expression and cell proliferation in human breast cancer cells. Exp Cell Res. 2010;316:433–442. doi: 10.1016/j.yexcr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Peng X, Jhaveri P, Hussain-Hakimjee EA, Mehta RG. Overexpression of ER and VDR is not sufficient to make ER-negative MDAMB231 breast cancer cells responsive to 1alphahydroxyvitamin D5. Carcinogenesis. 2007;28:1000–1007. doi: 10.1093/carcin/bgl230. [DOI] [PubMed] [Google Scholar]
- 41.Fife RS, Sledge GW Jr, Proctor C. Effects of vitamin D3 on proliferation of cancer cells in vitro. Cancer Lett. 1997;120:65–69. doi: 10.1016/s0304-3835(97)00298-x. [DOI] [PubMed] [Google Scholar]
- 42.Clavarezza M, Puntoni M, Gennari A, Paleari L, Provinciali N, D’Amico M, DeCensi A. Dual block with lapatinib and trastuzumab versus single-agent trastuzumab combined with chemotherapy as neoadjuvant treatment of HER2-positive breast cancer: a meta-analysis of randomized trials. Clin Cancer Res. 2016;22:4594–4603. doi: 10.1158/1078-0432.CCR-15-1881. [DOI] [PubMed] [Google Scholar]
- 43.Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, Lee SC, Mehta AO, Kim SB, Bachelot T, Goswami C, Deo S, Bose R, Wong A, Xu F, Yao B, Bryce R, Carey LA. Neratinib plus paclitaxel vs. trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2:1557–1564. doi: 10.1001/jamaoncol.2016.0237. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Quiroz J, Garcia-Becerra R, Barrera D, Santos N, Avila E, Ordaz-Rosado D, Rivas-Suarez M, Halhali A, Rodriguez P, Gamboa-Dominguez A, Medina-Franco H, Camacho J, Larrea F, Diaz L. Astemizole synergizes calcitriol antiproliferative activity by inhibiting CYP24A1 and upregulating VDR: a novel approach for breast cancer therapy. PLoS One. 2012;7:e45063. doi: 10.1371/journal.pone.0045063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stivarou T, Patsavoudi E. Extracellular molecules involved in cancer cell invasion. Cancers (Basel) 2015;7:238–265. doi: 10.3390/cancers7010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avila E, Garcia-Becerra R, Rodriguez-Rasgado JA, Diaz L, Ordaz-Rosado D, Zugel U, Steinmeyer A, Barrera D, Halhali A, Larrea F, Camacho J. Calcitriol down-regulates human ether a go-go 1 potassium channel expression in cervical cancer cells. Anticancer Res. 2010;30:2667–2672. [PubMed] [Google Scholar]
- 47.O’Neill F, Madden SF, Aherne ST, Clynes M, Crown J, Doolan P, O’Connor R. Gene expression changes as markers of early lapatinib response in a panel of breast cancer cell lines. Mol Cancer. 2012;11:41. doi: 10.1186/1476-4598-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beer TM. Development of weekly high-dose calcitriol based therapy for prostate cancer. Urol Oncol. 2003;21:399–405. doi: 10.1016/s1078-1439(03)00170-4. [DOI] [PubMed] [Google Scholar]
- 49.Burris HA 3rd, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O’Neil B, Marcom PK, Ellis MJ, Overmoyer B, Jones SF, Harris JL, Smith DA, Koch KM, Stead A, Mangum S, Spector NL. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J. Clin. Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 50.Gulliford T, English J, Colston KW, Menday P, Moller S, Coombes RC. A phase I study of the vitamin D analogue EB 1089 in patients with advanced breast and colorectal cancer. Br J Cancer. 1998;78:6–13. doi: 10.1038/bjc.1998.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathiasen IS, Lademann U, Jaattela M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;59:4848–4856. [PubMed] [Google Scholar]
- 52.Crowley LC, Waterhouse NJ. Measuring survival of hematopoietic cancer cells with the colony-forming assay in soft agar. Cold Spring Harb Protoc. 2016;2016 doi: 10.1101/pdb.prot087189. pdb.prot087189. [DOI] [PubMed] [Google Scholar]


