Abstract
Background:
Children with attention deficit hyperactivity disorder/developmental coordination disorder (ADHD/DCD) suffer from problems associated with gross and fine motor skills. There is no effective pharmacological therapy for such patients. We aimed to assess the impact of methylphenidate (MPH) on motor performance of children with ADHD/DCD.
Methods:
In this double-blind placebo-controlled, 17 children (12 boys) with ADHD/DCD with a mean age of 7 years 6 months were recruited in Shafa Hospital, Rasht, Iran. The response was defined as ≥25% reduction in the total score of ADHD rating scale-IV from the baseline. Sixteen boys entered phase 2 of the study in which the impact of MPH on motor function was determined through a crossover randomized clinical trial. Eligible individuals were scheduled for baseline and two assessment visits after a one-week period of intervention. We used the short form of Bruininks-Oseretsky test (BOT-2) to identify the disability of motor function. Children were randomly assigned to receive MPH or inert ingredients (placebo). In the second period, medication (MPH/placebo) was crossed over. The effects of MPH were analyzed using χ2 test for related samples to compare the performance during baseline, placebo, and MPH trials. The results were analyzed using the SPSS software version 16.0.
Results:
The mean minimal effective dose of MPH per day was 17.3 mg (0.85 mg/kg). Children with higher ADHD rating scale had a significantly lower standard score in BOT-2 (P=0.03). Following MPH intake, 26.6% of the children showed clinically significant improvement in motor function. However, the improvement was not statistically different between the MPH and placebo.
Conclusion:
Although MPH improved ADHD symptoms, problems with motor performance still remained. Further work is required to determine the probable effects of MPH in a higher dosage or in different subtypes of ADHD.
Trial Registration Number:
IRCT201107071483N2
Keywords: Methylphenidate, Psychomotor performance, Attention deficit disorder with hyperactivity, Motor skills
What’s Known
Fifty percent of ADHD children display developmental coordination disorder.
Methylphenidate improves symptoms in 60%–80% of ADHD children.
What’s New
Minimal effective dose of methylphenidate was intermediate.
Methylphenidate improved motor function in 26.6% of the children.
Introduction
There is some evidence on the association of attention deficit hyperactivity disorder (ADHD) with poor fine motor skills and impaired handwriting.1,2 ADHD is one of the most prevalent neurodevelopmental disorders affecting 3-9% of all school-aged children.3 About 50% of children with ADHD display developmental coordination disorder (DCD).4 According to DSM-IV, the level of motor coordination in DCD is below the expected given child’s chronological age and intelligence and may lead to problems in activities of daily living and/or academic performance.5 These children show poorer outcomes when evaluated in early adulthood in terms of academic achievement and psychosocial adjustment.6
Methylphenidate (MPH) is one of the most common treatment modalities for children with ADHD.7 MPH in 60-80% of children with ADHD improves behavioral problems and inattention. Little is known about the effects of MPH on motor function in children with ADHD. However, some studies have reported that MPH improved fine motor function, handwriting, and postural stability and balance in children with ADHD.8
Only a few studies (Bart et al.8 and Flapper et al.9) have assessed the effects of MPH on motor performances in children fully diagnosed with DCD and ADHD. Bart et al. showed that children with MPH had motor performance superior to children with placebo, but significant clinical improvement was only observed in 33% of the children.8
The present study is the first crossover clinical trial regarding the impact of MPH on ADHD/DCD. The initial goal was to investigate the minimal effective dose of methylphenidate (MED-MPH) that significantly improves ADHD symptoms. MED-MPH was considered due to limited information on drug safety and possible long-term effects of MPH on physical or psychiatric health.10 The second goal was to study the effect of MED-MPH on motor skills in children with ADHD/DCD. It should be noted that the usage of minimal effective dosage instead of a fixed MPH dosage as well as the crossover study design are the novelties of this study compared with previous investigations.
Patients and Methods
Participants
Children were recruited through the Child Psychiatry Department of Shafa Hospital from July 2011 to July 2012. The trial is registered (IRCT201107071483N2) with the Iranian Registry of Clinical Trials. Ethical approval was obtained from Guilan University of Medical Sciences, Iran. The study was described in detail to each child and his/her parents. Written informed consent was obtained from the parents for child’s participation in the study.
ADHD and DCD were diagnosed clinically according to the criteria in the diagnostic and statistical manual of mental disorders, 4th edition, text revision (DSM-IV TR).11 The inclusion criteria were age between 6 and 12 years, diagnosis of ADHD and DCD based on the diagnostic interview according to DSM-IV TR criteria, a recently diagnosed case (less than 3 months), or no prior experience with stimulant medication. The exclusion criteria were the presence of other psychiatric disorders, evidence of a specific learning or neurological disorder, or low intelligence (IQ<70).12
Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL)
The K-SADS-PL is a semi-structured diagnostic interview designed to assess the current and past episodes of psychopathology in children and adolescents according to DSM-IV criteria. The K-SADS-PL is an integrated parent-child interview. Final diagnoses are generated by synthesizing parent and child data, with greater weight typically given to parents’ reports of observable behaviors and children’s reports of subjective experiences.13 Researchers have reported sufficient validity and reliability for the Persian version of the K-SADS-PL. They have shown that the test-retest reliability of ADHD is 0.81.14,15 The K-SADS-PL was administered by an experienced child clinical psychologist (KM).
ADHD Rating Scale-IV
The ADHD rating scale-IV determines parent ratings regarding the frequency of each ADHD symptom based on DSM-IV criteria. Parents are asked to determine the symptomatic frequency that describes child’s home behavior over the previous 6 months. The ADHD rating scale-IV is completed independently by the parent and scored by a clinician. The scale consists of 2 subscales, namely inattention (9 items) and hyperactivity-impulsivity (9 items). Items are rated on a scale of 0 (not at all) to 3 (very much/very often). To obtain the total raw score, we added the inattention and hyperactivity-impulsivity subscale raw scores.
Motor Assessment
Motor function was assessed using the Bruininks-Oseretsky test of motor proficiency, second edition (BOT-2). We used the short form of this test to assess the overall motor proficiency. The BOT-2 comprises 14 items (consisting of eight subtests) in the short form. It uses a composite structure organized around both the muscle groups and limbs involved in the movements. These motor-area composites are fine motor control, manual coordination, body coordination, and strength and agility. After recording child’s raw score for each task, we converted each raw item into a point score. Then, the total point score was obtained by adding the point scores of the individual items. Finally, we ascertained the descriptive category (well above average, above average, average, below average, and well below average). Wuang et al. compared the psychometric properties of several clinical measures for assessing motor function and found sufficient reliability, validity, and responsiveness in preschoolers with intellectual disabilities. This test is most appropriate for children aged 6 years and upwards.16
Procedures
Phase 1 of the study involved identifying MED-MPH on ADHD rating scale in children with ADHD/DCD. We administered the K-SADS-PL to assess other psychopathology in children. Assessments were performed by a resident of psychiatry (THSM) trained to use the questionnaire and checklist. MED-MPH was defined as the minimum level of MPH that improved ADHD symptoms more than 25% on medication. In the present study, 17 children (12 boys) with ADHD/DCD willingly participated. All children had the combined type of ADHD. They were selected from a population of 28 children who could be diagnosed as having ADHD/DCD without other comorbidities between July 2011 and July 2012. Amongst the 28 children, 22 were recently diagnosed cases. The 22 children were asked to enroll in a two-phase trial to assess the effects of MPH on motor skills. In phase 1, the children randomly received three different dosage levels (0.25, 0.5, 1 mg/kg/day, bid) of MPH (Ritalina, Novartis, Switzerland) or placebo) inert ingredient, Sobhandarou, Iran). Each drug was given for a week. These drugs were prepared by the Shafa Hospital Pharmacy and placed into similar gelatinous capsules. The patients were randomly allocated into one of the four groups using a random number generator. Drugs were labeled by study subject number according to the randomization schedule. Parents and the researchers were blinded to child’s drug condition and dosage. The rating of ADHD symptoms was performed weekly to determine changes in ADHD symptoms from the baseline (before medication).
Phase 1 was completed by 16 out of the 17 children. A child withdrew from further assessment due to parental choice (figure 1). All children were MPH-sensitive. The second phase of the study started from week 6 (after one week of washing period). This phase aimed at analyzing the impact of MPH on motor performance of children. Using a crossover trial, we planned a double-blind study and measured the short form of BOT-2 variables. According to the descriptive category of BOT-2, all children were below average or well below average. Using fixed-block randomization, the children were randomized into two groups (A and B). Randomization was done in blocks of four patients in a 1 group A to 1 group B ratio. The consort flow diagram of the study is depicted in figure 2.
Figure 1.
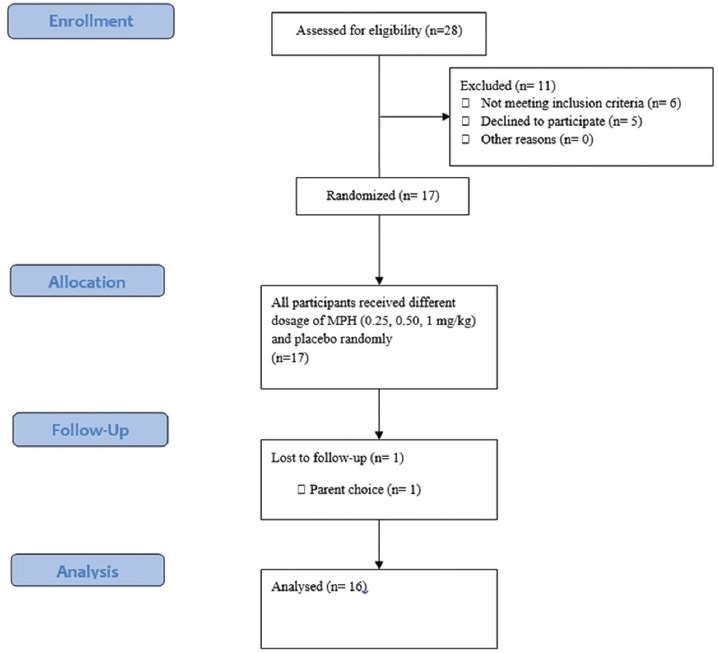
CONSORT flow diagram for the phase 1 of clinical trial methylphenidate versus placebo to identify MED-MPH on ADHD rating scale in children with ADHD/DCD.
Figure 2.
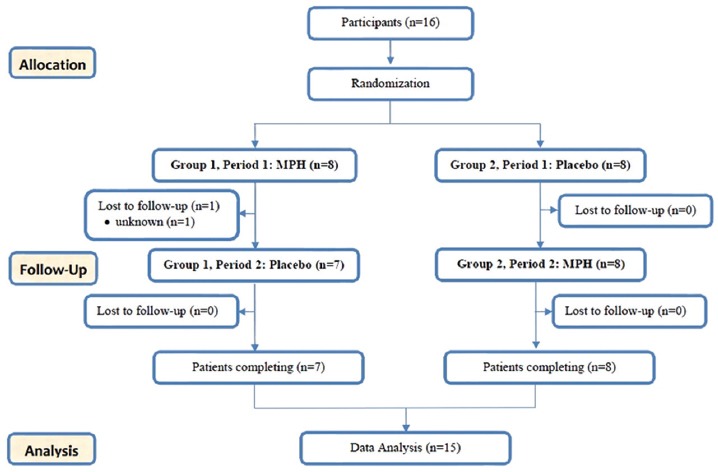
CONSORT flow diagram for the phase 2 of clinical trial methylphenidate versus Placebo. A crossover randomized clinical trial to compare the impact of MPH with placebo on motor performance of children with ADHD/DCD.
Participants received MED-MPH or placebo treatment. The medication was given at one capsule bid for one week. Period 2 covered the seventh week. The primary outcome variable was motor performance as measured on the short form of BOT-2. All children underwent a motor performance assessment by the same resident of psychiatry (THSM). The children were tested with BOT-2 three times. The MPH or placebo was administered after baseline measurement. Other assessments were obtained on days 42 and 49.
The researchers, the participants, and their parents were blinded to group assignment. The key to the randomization was kept by the pharmacy and randomization was broken only in case of potential adverse events. One child belonging to group A dropped out after the fifth day, reducing this group to seven patients.
Statistical Analysis
The sample size was estimated from the previous double-blind study.9 We estimated that the recruitment of 13 cases would provide 90% power to detect a significant difference between the effects of treatment on motor performance at a significance of P<0.05. As we anticipated a dropout rate of 20% in 2 periods, the sample size was increased to a total of 16 patients.
Descriptive statistics were used to describe all relevant variables. The effects of MPH were analyzed using χ2 test for related samples to compare the performance during baseline, placebo, and MPH trials. A significance level of 0.05 was set for all statistical tests (2-tailed). All statistical analyses were performed using the statistical package for social science software version 16.0 (SPSS Inc., Chicago, Illinois).
Results
In phase 1, the mean age and IQ score of the children were 7 years 6 months (±18 months) and 96.6 (±12.5), respectively. Patient’s characteristics are described in table 1. The mean minimal effective dose per day was 17.3 mg (mean 0.85 mg/kg, ±0.16), administered over two doses (at 08:00 and 15:00 hours). As completed by the parents, without medication, the mean total subscale raw score on the ADHD symptom checklist was 27.4 (±4.7). After MPH use, the mean of parental scores was 19.9 (±2.9). Of the 16 children who completed phase 1, four responded favorably to 10 mg MPH per day. MED-MPH of the other children was 20 mg.
Table 1.
The characteristics of children with ADHD/DCD
| Characteristic | Mean (SD) | Range |
|---|---|---|
| Age, mean (SD) | 7y 6mo (18mo) | 6y 5mo11-y 5mo |
| Boy/girl, n | 11/5 | - |
| Height, mean (SD) | 1.27 m (0.06) | 1.16-1.40 |
| Weight, mean (SD) | 27.9 kg (8.9) | 19-47 |
| Systolic blood pressure, mean (SD) | 98.7 mmHg (9.2) | 90-110 |
| Diastolic blood pressure, mean (SD) | 70 mmHg (9.2) | 60-90 |
| IQ score, mean (SD) | 96.6 (12.5) | 80-118 |
| Number of siblings, n | 1.3 (0.5) | 1-2 |
| History of MPH usage, mean (SD) | 22.9 d (22.5) | 0-90 |
Sixteen children entered phase 2 and 15 of them (10 boys and 5 girls) completed this phase. Of the 15 children with ADHD/DCD, all had definite motor problems and fell in the category ‘below average’ (12 cases) or ‘well below average’ (3 cases). Children with higher ADHD rating scale had a significantly lower standard score in BOT-2 (Spearman’s P=-0.55, P=0.03). Inspection of MPH impact on motor performance indicated that 11 out of 15 participants showed improvement on the standard score of BOT-2. The mean percentage of change after MPH was 5.32 (±19.44). However, only 4 children improved descriptive category in BOT-2. Two children showed deterioration of descriptive category in BOT-2. The standard score of BOT-2 of 8 children improved without MPH. The mean percentage of change without MPH was 1.43 (±17.92). Improvements did not always mean improved descriptive category and only 5 of the 15 children showed a better category (table 2). We calculated period, group, and treatment effects and observed no significant difference in the improvement of descriptive category (χ2=0.159, P=0.69).
Table 2.
Response of the children with ADHD/DCD to MPH and placebo
| Child number | Weight (kg) | MED-MPH (mg/day) | ADHD rating scale | Standard score BOT-2 | Descriptive category BOT-2* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Base | MPH | Base | MPH | Placebo | Base | MPH | Placebo | |||
| 1 | 19 | 20 | 23 | 17 | 39 | 42 | 46 | BA | A | A |
| 2 | 20 | 20 | 30 | 22 | 32 | 44 | 43 | BA | BA | WBA |
| 3 | 20 | 10 | 37 | 25 | 20 | 38 | 44 | WBA | WBA | WBA |
| 4 | 20 | 20 | 29 | 21 | 40 | 44 | 43 | BA | A | BA |
| 5 | 21 | 20 | 32 | 24 | 31 | 44 | 28 | BA | A | WBA |
| 6 | 22 | 20 | 24 | 18 | 38 | 38 | 39 | BA | BA | BA |
| 7 | 24 | 20 | 24 | 18 | 36 | 38 | 37 | BA | BA | BA |
| 8 | 26 | 20 | 36 | 24 | 28 | 39 | 41 | WBA | WBA | WBA |
| 9 | 29 | 20 | 29 | 21 | 39 | 29 | 26 | BA | A | A |
| 10 | 30 | 20 | 27 | 20 | 35 | 29 | 26 | BA | BA | A |
| 11 | 30 | 20 | 26 | 19 | 29 | 29 | 26 | WBA | WBA | WBA |
| 12 | 31 | 10 | 24 | 18 | 36 | 37 | 43 | BA | BA | A |
| 13 | 33 | 20 | 27 | 20 | 40 | 40 | 45 | BA | BA | A |
| 14 | 46 | 10 | 21 | 15 | 38 | 38 | 28 | BA | WBA | WBA |
| 15 | 47 | 10 | 23 | 17 | 40 | 33 | 32 | BA | WBA | BA |
A: Average; BA: Below average; WBA: Well below average
We also computed the power of study using the PASS 11.0.4 software. Considering an effect size (W) of 0.13 using two degrees of freedom chi-square test with a significance level (alpha) of 0.7, the power of the study was 81%.
Discussion
It is reasonable to postulate that motor coordination is important and poor motor skills may have a negative impact on a child’s daily living and academic performance. Furthermore, this problem may persist into adulthood with an increase in problems associated with psychosocial adjustment.17 Hence, the management of impairment in motor coordination should be an essential treatment component for children with ADHD/DCD. It is known that MPH improves behavioral symptoms of ADHD. MPH decreases response variability and impulsive response to laboratory cognitive tasks, increases the accuracy of performance, and improves short-term memory, reaction time, math computation, problem-solving in games, and sustained attention.18
We conducted a two-phase study. In phase 1, we showed that the mean minimal effective dose of MPH per day was 17.3 mg. Although the MTA study found that 77 percent of children responded to MPH,19 we had no non-responder in this study. It is possible that this result is due to the small sample size of the study.
The aim of the second phase was to investigate the effectiveness of MED-MPH in improving the ability of children with ADHD/DCD. To prevent confounding factors, we performed a double-blind, crossover randomized clinical trial. By definition, children with ADHD/DCD have problems with motor skills; and as expected, the children in the present study performed poorly in the tasks of BOT-2. The motor performance found in the present study was in line with previous research studies. Flapper et al. found that children with ADHD/DCD performed more poorly on the manual dexterity, had a poorer quality of handwriting, and drew more rapidly and fluently, but less accurately than control on graphomotor task.1 In the present study, although the ADHD rating scale was significantly correlated with the standard score in BOT-2 (Spearman’s P=-0.55, P=0.03), it was not significantly correlated with the descriptive category. It is possible that the small sample size and the structure of BOT-2 contributed to this result. The “below average” and “well below average” categories are considered when there are more one and two standard deviations from the mean, respectively.
Compared with placebo, MPH did not improve the descriptive category in BOT-2 (regardless of the medication response of ADHD). Our results demonstrated that motor performance was unchanged with MPH and the improvement of ADHD symptoms was not related to the improvement of child’s proficiency. In a recent review, it was noted that ADHD and DCD have many similarities, such as prevalence, high rates of comorbidities, and poor psychosocial outcomes. However, they appear to have somewhat different developmental pathways, which could reflect on a combination of genetic and environmental factors that evolve over time. They might be a separate disorder and the combination of ADHD and DCD might be potentially different rather than additive. Therefore, different approaches might be required in the treatment of such patients.20
Our result is consistent with the findings of Brossard-Racine study in which motor difficulties persisted in more than one-half of the treated children.21 As Bart et al. noted, improvement in motor function with MPH should be interpreted with caution. Even in previous studies, it has been demonstrated that despite MPH intake, two-thirds of the children continued to perform poorly on the movement assessment battery for children (MABC) tasks.8 In this study, 4 out of 15 (26.6%) had clinically significant improvement in motor function following MPH intake. However, the improvement was not statistically different between the MPH and placebo. Harvey showed which MPH had no significant effect on the fundamental movement skills of children with ADHD.22 Our finding is in contrast with previous findings on children with ADHD/DCD.1,8,9 Such difference might stem from the difference between the assessments of motor skills since they used manual dexterity items of MABC as a measure of fine motor skills. One possible explanation is that MABC and BOT-2 had different sensitivity in investigating the effectiveness of MPH. Another explanation could be the difference in populations that might have affected the results. In a study by Flapper, 4 out of 12 children had combined type of ADHD. Bart showed that this type of ADHD was observed in 10 out of 18 children.1,8 However, all children in our study had the combined type of ADHD. Therefore, it might be argued that children with this subtype responded differently than the other subtypes of ADHD. This result supports the findings of Pitcher et al. in that the total impairment score of MABC was more severe in predominantly inattentive and combined subtypes of ADHD.23 Finally, although there is some evidence to suggest that methylphenidate might be useful for fine-motor issues like handwriting,12 however, it appears that pharmacological intervention alone should not be given to such children and additional treatment and support should be considered.24
In the present study, the mean minimal effective dose per day was 17.3 mg. Although there is a similarity between the results of Flapper and ours, we could not observe an improvement of motor proficiency in children with ADHD/DCD. The physician’s desk reference (PDR) states that the maximum total daily dose of MPH is 60 mg. In this study, MPH dosage was predominantly 20 mg/day and it is possible that the usage of a higher dosage of MPH improves motor performance. Moreover, Stein et al. revealed that the usage of MPH on three separate doses in each day was associated with a greater improvement on the Conners’ parent rating scale impulsivity-hyperactivity factor than two separate doses.25
There are a couple of limitations in the present study. The participants in this study were children who had been clinically referred; therefore, the results cannot be generalized across other children. The complete form of BOT-2 provides the most reliable measure of the overall motor proficiency, enabling researchers to conduct a comprehensive investigation of child’s proficiency in four motor-area composites, namely fine manual control, manual coordination, body coordination, and strength and agility. Further study is required to identify which composite is prominently impaired and whether there are differences between the responses of children with ADHD/DCD to MPH.
Conclusion
The present study demonstrated that there was a significant association between ADHD symptoms and motor performance. Overall, MPH improves motor performance only in 26.6% of the children. In addition, this study revealed that the change in BOT-2 score between the two periods was not significantly different (period effect) and the improvement to motor performance in our population was not different to with/without MPH. There is the possibility that this finding is due to a relatively short period (one week) of MPH intervention. Therefore, we recommend a replication of this study with a larger sample size and higher dosage of MPH.
Acknowledgment
We would like to thank the children and their parents for their participation in this study.
Conflict of Interest: None declared.
References
- 1.Flapper BC, Houwen S, Schoemaker MM. Fine motor skills and effects of methylphenidate in children with attention-deficit-hyperactivity disorder and developmental coordination disorder. Dev Med Child Neurol. 2006;48:165–9. doi: 10.1017/S0012162206000375. [DOI] [PubMed] [Google Scholar]
- 2.Tseng MH, Henderson A, Chow SM, Yao G. Relationship between motor proficiency, attention, impulse, and activity in children with ADHD. Dev Med Child Neurol. 2004;46:381–8. doi: 10.1017/S0012162204000623. [DOI] [PubMed] [Google Scholar]
- 3.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–48. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 4.Qian Y, Lei G, Castellanos FX, Forssberg H, Heijtz RD. Deficits in fine motor skills in a genetic animal model of ADHD. Behav Brain Funct. 2010;6:51–8. doi: 10.1186/1744-9081-6-51. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niemeijer AS, Smits-Engelsman BC, Reynders K, Schoemaker MM. Verbal actions of physiotherapists to enhance motor learning in children with DCD. Hum Mov Sci. 2003;22:567–81. doi: 10.1016/j.humov.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen P, Gillberg C. Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community-based study. J Am Acad Child Adolesc Psychiatry. 2000;39:1424–31. doi: 10.1097/00004583-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Wilens TE, Spencer TJ. The stimulants revisited. Child Adolesc Psychiatr Clin N Am. 2000;9:573–603. [PubMed] [Google Scholar]
- 8.Bart O, Podoly T, Bar-Haim Y. A preliminary study on the effect of methylphenidate on motor performance in children with comorbid DCD and ADHD. Res Dev Disabil. 2010;31:1443–7. doi: 10.1016/j.ridd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Flapper BC, Schoemaker MM. Effects of methylphenidate on quality of life in children with both developmental coordination disorder and ADHD. Dev Med Child Neurol. 2008;50:294–9. doi: 10.1111/j.1469-8749.2008.02039.x. [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency. Questions and answers on the review of medicines containing methylphenidate [Internet] London: EMEA; 2009. [[cited 2015 Mar 8]]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Methylphenidate_31/WC500011125.pdf . [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. p. 991. [Google Scholar]
- 12.Raven JC, Styles I, Raven M. Coloured Progressive Matrices: mit der Parallelform des Tests und der Puzzle-Form;Manual zu Raven’s Progressive Matrices und Vocabulary Scales. Oxford: Oxford Psychologists Press; 1998. p. 78. [Google Scholar]
- 13.Kim YS, Cheon KA, Kim BN, Chang SA, Yoo HJ, Kim JW, et al. The reliability and validity of Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version- Korean version (K-SADS-PL-K) Yonsei Med J. 2004;45:81–9. doi: 10.3349/ymj.2004.45.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Shahrivar Z, Kousha M, Moallemi S, Tehrani-Doost M, Alaghband-Rad J. The Reliability and Validity of Kiddie-Schedule for Affective Disorders and Schizophrenia - Present and Life-time Version - Persian Version. Child Adolesc Ment Health. 2010;15:97–102. doi: 10.1111/j.1475-3588.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 15.Ghanizadeh A, Mohammadi MR, Yazdanshenas A. Psychometric properties of the Farsi translation of the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version. BMC Psychiatry. 2006;6:10–4. doi: 10.1186/1471-244X-6-10. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wuang YP, Su CY, Huang MH. Psychometric comparisons of three measures for assessing motor functions in preschoolers with intellectual disabilities. J Intellect Disabil Res. 2012;56:567–78. doi: 10.1111/j.1365-2788.2011.01491.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen YW, Tseng MH, Hu FC, Cermak SA. Psychosocial adjustment and attention in children with developmental coordination disorder using different motor tests. Res Dev Disabil. 2009;30:1367–77. doi: 10.1016/j.ridd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41:26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- 19.Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goulardins JB, Rigoli D, Licari M, Piek JP, Hasue RH, Oosterlaan J, et al. Attention deficit hyperactivity disorder and developmental coordination disorder: Two separate disorders or do they share a common etiology. Behav Brain Res. 2015;292:484–92. doi: 10.1016/j.bbr.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Brossard-Racine M, Shevell M, Snider L, Belanger SA, Majnemer A. Motor skills of children newly diagnosed with Attention Deficit Hyperactivity Disorder prior to and following treatment with stimulant medication. Res Dev Disabil. 2012;33:2080–7. doi: 10.1016/j.ridd.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Harvey WJ, Reid G, Grizenko N, Mbekou V, Ter-Stepanian M, Joober R. Fundamental movement skills and children with attention-deficit hyperactivity disorder: peer comparisons and stimulant effects. J Abnorm Child Psychol. 2007;35:871–82. doi: 10.1007/s10802-007-9140-5. [DOI] [PubMed] [Google Scholar]
- 23.Pitcher TM, Piek JP, Hay DA. Fine and gross motor ability in males with ADHD. Dev Med Child Neurol. 2003;45:525–35. doi: 10.1111/j.1469-8749.2003.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 24.Blank R, Smits-Engelsman B, Polatajko H, Wilson P, European Academy for Childhood Disability European Academy for Childhood Disability (EACD): recommendations on the definition, diagnosis and intervention of developmental coordination disorder (long version) Dev Med Child Neurol. 2012;54:54–93. doi: 10.1111/j.1469-8749.2011.04171.x. [DOI] [PubMed] [Google Scholar]
- 25.Stein MA, Blondis TA, Schnitzler ER, O’Brien T, Fishkin J, Blackwell B, et al. Methylphenidate dosing: twice daily versus three times daily. Pediatrics. 1996;98:748–56. [PubMed] [Google Scholar]


