Abstract
Background:
Glucose-induced protein glycation has been implicated in the progression of diabetic complications and age-related diseases. The anti-glycation potential of polyphenol-rich plant extracts has been shown previously. Bunium Persicum has been demonstrated to possess a high level of polyphenols. The aim of current in vitro study was to determine the possible inhibitory effect of Bunium Persicum hydroalcoholic extract (BPE) on glucose-induced bovine serum albumin (BSA) glycation, oxidation, and aggregation.
Methods:
Folin-Ciocalteu assay was used to measure the content of total phenolic compounds of BPE. To test the in vitro effect of BPE on the formation of glycated BSA, thiol group oxidation, and protein aggregation of BSA, various concentrations of BPE were incubated with BSA and glucose at 37 °C for 72 hr. Glycation, thiol group oxidation, and aggregation of BSA were then measured using thiobarbituric acid, 2, 4-dinitrophenylhydrazine, and Congo red colorimetric methods, respectively. Data were analyzed using the SPSS software (version 16.0). One-way ANOVA followed by Tukey’s post hoc test was used to compare group means. P<0.05 was accepted as the statistically significant difference between groups.
Results:
The results demonstrated that the content of total phenolics of BPE was 122.41 mg gallic acid equivalents per gram dried extract. BPE (10, 15, and 30 μg/ml) significantly inhibited the formation of GA in a concentration-dependent manner. BPE also significantly decreased the levels of thiol group oxidation and BSA aggregation.
Conclusion:
The results showed that BPE has anti-glycation and antioxidant properties and might have therapeutic potentials in the prevention of glycation-mediated diabetic complications.
Keywords: Glucose, Glycation, Serum albumin, Bovine, Bunium persicum, Apiaceae
What’s Known
Due to numerous pharmacological and therapeutic properties, Bonium Persicum is widely used as a medicinal plant in folk medicine.
The inhibitory effect of Bonium Persicum against hemoglobin glycation has been shown previously.
What’s New
The hydroalcoholic extract of Bonium Persicum caused a significant decrease in albumin glycation, thiol group oxidation, and aggregation.
It seems that the high antioxidant power of Bonium Persicum due to its high level of polyphenolic compound is the main reason for these effects.
Introduction
Protein glycation is defined as a spontaneous and non-enzymatic binding of reducing sugars including glucose, galactose, and fructose to free amino groups of proteins.1 It is a multistep process that is initiated with the formation of Schiff bases between carbonyl group of a sugar and amino group of proteins, followed by Amadori rearrangement and finally several modifications which led to the formation of a variety of compounds known as advanced glycation end products (AGEs).2 AGEs react with the proteins and other important biological molecules and impair their structure and normal functions. The role of protein glycation has been implicated in the pathogenesis of diabetes mellitus associated complication such as retinopathy, neuropathy, and nephropathy.3,4 Protein glycation may also be involved in the pathogenesis of atheroscelerosis, Alzhimer’s disease, rheumatoid arthritis, and chronic heart failure. Thus, naturally occurring chemicals that inhibit protein glycation may be useful for the prevention of these complications.5,6 Phenolic and flavonoid compounds found in spices and medicinal herbs are among the most documented natural products that have been implicated to have anti-glycation properties.7 Black caraway (Bunium persicum; BP) is an important medicinal plant belonging to Apiaceae family.8,9 In herbal medicine, it is recommended for the treatment of some gastro intestinal illnesses, including diarrhea and inflammatory bowel disease.10 Furthermore, its anti-hyperglycemic11 and anti-hyper lipidemic activities12 have been demonstrated. Anti-inflammatory,10 anti-oxidative,13 and anti-nociceptive14 are among other beneficial medicinal properties of BP. Previous studies have reported gamma-terpinene, cuminal, p-cymene, and cumin aldehyde were the main components of BP seed extract (BPE).14,15 The antioxidant properties of BPE have been demonstrated in previous studies. Shahsavari et al.13 evaluated and compared the antioxidant potential of the essential oil of BP (BPEO) with synthetic antioxidants, such as butylated hydroxyanisole (BHA) and showed that BPEO (0.06%) had the same ability as BHA (0.02%) to reduce the oxidation of the soybean oil in the oven test. In addition, Sharififar et al.16 reported that methanolic extract of BP exhibited a high antioxidant activity (IC50=45.7±3.6 µg/mL) in diphenyl-1-picrylhydrazyl (DPPH) assay and also in the inhibition of beta-carotene oxidation and lipid peroxidation. These findings indicate the antioxidant and radical scavenging potential of BP, suggesting the use of this plant in many traditional usages such as an additive in foods and beverages.
Serum albumin is the most abundant protein with a variety of biological function. Albumin is very sensitive to glycation and reacts with glucose more rapidly than hemoglobin, which makes it more reliable test for monitoring blood glucose level than glycated hemoglobin.17,18 Albumin glycation has several clinical implications. Normal structure and function of albumin, including ligand binding and antioxidant properties, alter as a result of glycation. Moreover, glycated albumin has a critical role in the pathogenesis of diabetes mellitus associated complication such as cataract, retinopathy, neuropathy, and nephropathy17,18 Furthermore, an increase in glycated albumin level has been shown in the brain and plasma of Alzheimer’s disease patients, suggesting the role of glycated albumin in the development and progression of neurodegenerative diseases.19
In a recent in vitro study, Naderi et al.20 reported that BPE reduced hemoglobin glycation effectively. To the best of our knowledge, studies regarding the inhibitory effects of BPE on albumin glycation have not been reported. Thus, the first aim of the present study was to determine the possible inhibitory effect of BPE against glucose-mediated bovine serum albumin (BSA) glycation. In addition, glycation has detrimental effects on albumin structure, including oxidation of its free thiols to disulfide linkages or thiol radicals21 and refolding of globular albumin into β-amyloid fibrils structure (β aggregates).22 Because these changes affect the normal functions of albumin and have important clinical implications, we also investigated the effects of BPE on preventing BSA thiol group oxidation and aggregation.
Materials and Methods
BPE Preparation
The seeds of black caraway were obtained from a local market in Shiraz (Fars province, Iran). It was authenticated by a taxonomist at the Department of Botany, Faculty of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran. The voucher specimen with code “PM 977” has been deposited in the Department of Botany, Faculty of Pharmacy, Shiraz University of Medical Sciences. To prepare plant extract, 50 g of dried caraway seed was powdered in a grinder and was soaked in 350 ml ethanol 70% at room temperature. After 72 h, the mixture was filtered through Whatman grade 1 filter paper under the vacuum. The filtered was concentrated using a rotary evaporator and freeze-dried. The obtained powdered extract was stored at -20 °C for further use. A stock solution of BPE was prepared by dissolving the dried extract in dimethyl sulfoxide (DMSO). The stock solution was diluted using phosphate buffer saline (PBS; 0.1 M, PH=7.4) to obtain various concentrations of BPE (5, 10, 15, and 30 µg/ml). The final concentration of DMSO in the incubation medium (both in the control and test tube) was 0.3% that had no significant effect on BSA glycation.
Estimation of Total Polyphenolic Content
Total polyphenolic content of the BPE was measured using Folin-Ciocalteu method.23 Briefly, 0.2 ml of the extract was added to 1 ml of diluted Folin-Ciocalteu reagent and 800 µl of 7% (w/v) sodium carbonate (Na2CO3). The mixture was incubated at room temperature for 30 min and the absorbance of each sample was then measured at 765 nm. Gallic acid was used as a standard (0.8-12.5 mg/ml). The total polyphenolic compounds concentration was reported as mg gallic acid equivalents per gram dried extract.
In Vitro Glycation of BSA
The procedure for the preparation of glycated albumin was according to the method of Monnier et al. with minor modifications.24 Briefly, BSA (0.15 g/ml) was incubated in PBS (0.1 M, PH=7.4, containing 0.01% sodium azide) containing D-glucose (18 mg/ml) as glycated (G+A) group. The control group had the same amount of BSA in PBS (0.15 g/ml) without D-glucose. To investigate the effect of BPE on BSA glycation, different concentrations of BPE (5, 10, 15, and 30 µg/ml) were added to the incubation medium containing BSA (0.15 g/ml) and D-glucose (18 mg/ml) in PBS (0.1 M, PH=7.4, containing 0.01% sodium azide). The solutions incubated at 37 °C for 72 h. The reaction mixtures were then dialyzed against PBS (0.01 M, pH=7.4) for 24 h to remove unbind glucose from the samples.
Measurement of Glycated BSA
The level of glycated BSA was measured by thiobarbituric acid (TBA) colorimetric method. In this method, glycated BSA is hydrolyzed by oxalic acid to produce 5-hydroxymethyl furfural (5-HMF) which reacts with TBA to form a chromophore with a maximum absorbance at 443 nm.13 Briefly, 2 ml of 20% trichloroacetic acid (TCA) was added to 4 ml of glycated sample and centifugated for 10 min at 3000 rpm. 1 ml phosphate buffer and 0.5 ml oxalic acid (0.3 N) were added to the sediment and boiled in the water bath for 1 hr. After cooling, 0.5 ml of 40% TCA was added to each sample and centifugated (10 min, 3000 rpm). 0.5 ml 0.05 M TBA was then added to 1 ml of supernatant and heated in 40 °C for 30 min. The absorbance of each sample was taken at 443 nm. 5-HMF was used as standard and glycated BSA level was calculated and expressed as µmol of HMF per mg protein.
Determination of Protein Thiol Groups
Thiol group of glycated BSA samples was assayed according to the Ellman’s method using 2, 4-dinitrophenylhydrazine (DNPH).25 Briefly, 200 μL of glycated BSA samples were incubated with 1.8 ml of 5, 5’-dithiobis (2-nitrobenzoic acid) (DTNB) (0.5 mM) in 0.1 M PBS (pH 7.4) at room temperature for 15 min and the absorbance of samples was measured at 412 nm. The concentration of thiol groups was calculated using standard curve prepared by L-cysteine as a standard (0.05-0.5 mM). The results were reported as nmol/mg protein.
Determination of Protein Aggregation
Glycation stimulates refolding of globular albumin into β-amyloid structures and leads to the aggregation of albumin. Congo red was used to estimate the effect of plant extract on glycation-induced BSA aggregation. Congo red binds to β-amyloid and forms a complex with maximum absorbance at 530 nm.26 Briefly, 0.5 ml of glycated BSA incubated with 0.5 ml of Congo red (100 μM in PBS with 10% ethanol) at 25°C for 20 min. The absorbance of the sample was then recorded at 530 nm.
Statistical Analysis
The effect BPE on albumin glycation was expressed as % inhibition calculated by the following formula:
Percentage of inhibition=[(G0−G1)/G0]×100
Where Go is the glycated-BSA level of GA group and G1 is the glycated-BSA level of the GA samples co-incubated with BPE.
Data were analyzed using the SPSS software (version 16.0) and one-way ANOVA. All results are reported as mean±SD of at least triplicate values. The Tukey’s post hoc test was used to compare group means. P<0.05 was accepted as the statistically significant difference between groups.
Results
Total Polyphenolic Contents of BPE
The yield of plant extraction (mass of extract/mass of dry matter) was 10.8%. The content of polyphenolic compounds of BPE was 122.41 mg gallic acid equivalents per gram of dried extract.
Effect of BPE on Albumin Glycation
The effects of various concentrations of BPE (5-30 µg/ml) on albumin glycation are shown in table 1. As can be seen, albumin glycation was found to be inhibited by the BPE in a dose-dependent manner (P<0.001).
Table 1.
The Effect of various concentrations of BPE on inhibition of albumin glycation
| Group | Glycation inhibition (%) | P value (compared to G+A group) |
|---|---|---|
| G+A + BPE (5 µg/ml) | 0 | P=0.914 |
| G+A + BPE (10 µg/ml) | 7.38±1.25 | P=0.031 |
| G+A + BPE (15 µg/ml) | 13.62±0.39 | P<0.001 |
| G+A + BPE (30 µg/ml) | 27.80±4.49 | P<0.001 |
The data are mean±SD of at least three independent experiments. The mean of each group presented as percent of glycation inhibition. Percent of glycation inhibition calculated by the following formula: Percentage of inhibition=[(G0−G1)/G0]×100 where G0 is the glycated albumin level of G+A group and G1 is the glycated albumin level of the G+A samples co-incubated with BPE. BPE: Bunium persicum extract; G+A: Glucose+albumin
Effect of BPE on Glycation-Induced Oxidation of Albumin Thiol Groups
The influence of BPE on oxidation of albumin thiol groups is shown in figure 1. The level of thiol groups decreased in G+A sample to about half of the control group (0.41 and 0.86 nmol/mg protein, respectively) (P<0.001). When BPE was added to G+A, the level of thiol group was significantly increased dose-dependently compared to the G+A group. The percentage of the thiol group prevention from oxidation ranged from 9.75 to 51.7 percent by the concentrations of 10, 15, and 30 µg/ml, which showed significant protection against oxidation of thiol groups under these conditions (P<0.001).
Figure 1.
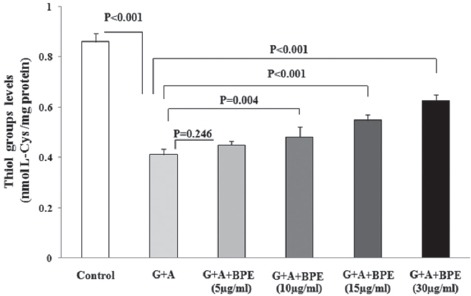
The effect of various concentrations of BPE on albumin thiol groups levels. The level of thiol groups of each group was assayed according to the Ellman’s method using L-cysteine as a standard. The results are reported as nmol L-cysteine per mg protein. The data are mean±SD of at least three independent experiments. G+A: Glucose+albumin; BPE: Bunium persicum extract.
Effect of BPE on Protein Aggregation During Glycation
The protective effect of BPE on protein aggregation is shown in figure 2. The protein aggregation content in glucose-induced glycated albumin was significantly elevated in the G+A group at the end of the experiment time when compared with the control group, whereas BPE at concentrations of 10, 15, and 30 µg/ml reduced the level of amyloid cross-β structure in a concentration-dependent manner (10.5%, 10.8%, and 29.7%) (P<0.001).
Figure 2.
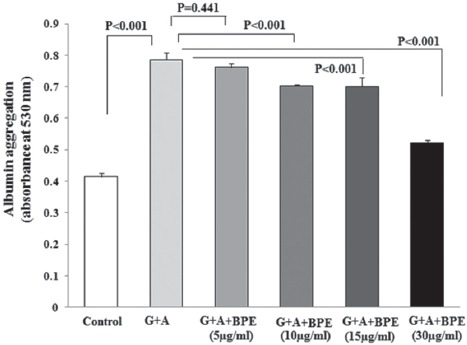
The effect of various concentrations of BPE on the formation of amyloid cross-β structure in BSA incubated with glucose. Formation of amyloid cross-β structure was assayed using Congo red assay. The data are mean±SD of each sample absorbance at 530 nm. G+A: Glucose+albumin; BPE: Bunium persicum extract.
Discussion
The aim of the present study was to evaluate the effects of BPE on glucose-induced glycation, oxidation, and aggregation of BSA. We initially determined the TPC content of BPE extract since a direct association between the level of phenolic compounds in plant extracts and their ability to inhibit mono saccharine-induced glycation and anti-oxidant activity have been demonstrated in previous studies.27-29 The content of TPC in the ethanolic extract of Bunium Persicum in our study was 122.41 mg gallic acid equivalents per gram of dried extract that was very close to 117 mg gallic acid equivalents per gram extract of BPE that has been reported previously.30 The high content of TPC in BPE may be an explanation for its high anti-oxidant and anti-hemoglobin glycation properties observed in previous studies.13
In vitro protein glycation has been studied using several proteins (hemoglobin, collagen, crystalline, and serum albumin) and sugars (glucose, fructose, and ribose).31,32 In the present study, we used glucose-induced BSA glycation because glucose and serum albumin are the main circulating sugar and protein with the highest concentration in the body.32 Our data showed that glycated BSA is increased up to 5-fold of the control group after the incubation of BSA with glucose for 72 hr that is correlated to that reported previously.24 The effect of BPE on glucose-induced BSA glycation was then investigated. The results revealed that BPE dose-dependently and significantly inhibited glycation of BSA. A similar finding has been reported by Naderi et al.20 that demonstrated inhibition of glucose-induced glycation of hemoglobin by BPE. The anti-glycating effects of several phenolic-enriched plant extracts against monosaccharide-induced BSA glycation has been reported previously. For example, Citrus grandis L (Pomelo) extract (0.25-1 mg/ml) decreased the level of fructose-induced formation of glycated BSA approximately by 3.7-30.0%.33 Pomelo extract also prevented thiol groups oxidation of BSA by 20% at the concentration of 2.00 mg/mL.33 The extracts of edible plants (Beijing grass, pennywort, gingko, cat’s whiskers, and grape seed) at the concentration of 1 mg/ml inhibited the fructose-mediated glycation of BSA by 50-30%.34 Incubation of Mesona chinensis Benth extract with BSA inhibited BSA glycation by 39.60-59.42% at the concentrations of 0.25-1.00 mg/mL.35 In the present study, BPE decreased the level of glucose-induced formation of glycated BSA approximately by 7.4-26.7% that showed moderate anti-glycating effect in comparison with the aforementioned phenolic-enriched plant extracts.
Protein glycation is associated with increased level of free radical production. The free radicals cause damage to proteins such as oxidation of thiol group.36,37 Thus, the evaluation of thiol group oxidation in BSA is used to show glycation-induced generation of free radicals. The findings of the present study revealed a significant increase in the oxidation of thiol group of BSA after incubation with glucose. The addition of BPE to incubation medium significantly suppresses oxidation of thiol group dose-dependently. Several studies have shown the antioxidant activity of BPE extract.15,16 Therefore, it can be assumed that the mechanism of BPE for decreasing protein oxidation may be related to its antioxidant properties.
Glycation induced various modifications on albumin function, which is due to alteration in its structure. Glycation is known to induce conformational change in proteins by increasing the level of β-amyloid structure. This structure plays an essential role in the aggregation of proteins. Deposition of aggregated protein is associated with the progression of neurodegenerative disease including Parkinson’s and Alzheimer’s diseases.32 Our data clearly demonstrated that BPE decreased the level of amyloid β-structure of BSA. This beneficial effect of BPE may help to reduce a risk of developing degenerative diseases in diabetic patients.
The molecular mechanisms that are behind the effect of BPE on BSA glycation were not investigated in the present study. GC/MS analysis showed that antioxidant compounds including p-cymene (31.1%), cuminaldehyde (22.2%), and γ-terpinene (11.4%) are the main components of the essential oil of BP. Therefore, the observed BPE effects could be due to antioxidant properties of these components. However other possible mechanisms may also be involved.38 In a recent study, Morshedi et al. reported the inhibitory effects of cuminaldehyde against fibrillation and aggregation of alpha-synuclein, a protein that has a critical role in the pathogenesis of Parkinson’s disease. According to Morshedi et al., cuminaldehyde interacts with amine groups of alpha-synuclein and stalls the formation of insoluble β-fibrils. The same mechanism may be involved in the prevention of BSA aggregation observed in the current study. Separation of BPE components and evaluation of its effects on BSA glycation and aggregation are required to prove this hypothesis. Blocking the formation of Amadori products, blocking carbonyl groups of glucose, and breaking cross-linked structure are among other possible mechanisms. Further investigations are required to clarify the role of these mechanisms in the anti-glycating effects of BPE.
Overall, in the present in vitro study, using common, simple, and validate colorimetric assays, we showed the beneficial inhibitory effects of BPE against BSA glycation, oxidation, and aggregation. These data suggest that BPE could be considered as a useful therapeutic natural product for the prevention of glycation-induced disorders in patients with diabetes mellitus. However, judging the in vivo efficacy of BPE based on the results of an in vitro study is difficult and further comprehensive studies using animal models of diabetes mellitus or diabetic patient are necessary.
Conclusion
In conclusion, our results suggest that BPE can be regarded as a medicinal plant with anti-glycation, oxidation, and aggregation of proteins. More studies are required to establish the mechanisms that are involved and the active constituents which are actually responsible for its beneficial pharmacologic actions of BPE.
Acknowledgment
This manuscript was extracted from the PharmD thesis of Arman Seri and was supported by a grant from the Vice-Chancellor for Research Affairs of Shiraz University of Medical Sciences. We are also grateful to all staff of Diagnostic Laboratory Sciences and Technology Research Center for their technical assistance.
Conflict of Interest: None declared.
References
- 1.Zheng H, Wu J, Jin Z, Yan LJ. Protein Modifications as Manifestations of Hyperglycemic Glucotoxicity in Diabetes and Its Complications. Biochem Insights. 2016;9:1–9. doi: 10.4137/BCI.S36141. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajith TA, Vinodkumar P. Advanced Glycation End Products: Association with the Pathogenesis of Diseases and the Current Therapeutic Advances. Curr Clin Pharmacol. 2016;11:118–27. doi: 10.2174/1574884711666160511150028. [DOI] [PubMed] [Google Scholar]
- 3.Chawla D, Bansal S, Banerjee BD, Madhu SV, Kalra OP, Tripathi AK. Role of advanced glycation end product (AGE)-induced receptor (RAGE) expression in diabetic vascular complications. Microvasc Res. 2014;95:1–6. doi: 10.1016/j.mvr.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr Diabetes Rev. 2017;13:3–10. doi: 10.2174/1573399812666151016101622. [DOI] [PubMed] [Google Scholar]
- 5.Yamagishi S, Nakamura K, Matsui T, Ueda S, Fukami K, Okuda S. Agents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: A novel therapeutic strategy for diabetic vascular complications. Expert Opin Investig Drugs. 2008;17:983–96. doi: 10.1517/13543784.17.7.983. [DOI] [PubMed] [Google Scholar]
- 6.Younus H, Anwar S. Prevention of non-enzymatic glycosylation (glycation): Implication in the treatment of diabetic complication. Int J Health Sci (Qassim) 2016;10:261–77. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui MA, Rasheed S, Saquib Q, Al-Khedhairy AA, Al-Said MS, Musarrat J, et al. In-Vitro dual inhibition of protein glycation, and oxidation by some Arabian plants. BMC Complement Altern Med. 2016;16:276. doi: 10.1186/s12906-016-1225-7. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin GR. Popular medicinal plants of Iran. Tehran: Iranian Research Institute of Medicinal Plants; 1991. [Google Scholar]
- 9.Mozaffarian V. A dictionary of Iranian plant names: Latin, English, Persian. Tehran: Farhang Mo’aser; 1996. [Google Scholar]
- 10.Rahimi R, Shams-Ardekani MR, Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol. 2010;16:4504–14. doi: 10.3748/wjg.v16.i36.4504. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giancarlo S, Rosa LM, Nadjafi F, Francesco M. Hypoglycaemic activity of two spices extracts: Rhus coriaria L and Bunium persicum Boiss. Nat Prod Res. 2006;20:882–6. doi: 10.1080/14786410500520186. [DOI] [PubMed] [Google Scholar]
- 12.Khaksari M, Ahmadi M, Najafipour H, Shahrokhi N. Effect of Bunium persicum aqueous extract plus endurance exercise on cardiorespiratory capacity and serum lipid profile. Avicenna J Phytomed. 2014;4:118–26. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahsavari N, Barzegar M, Sahari MA, Naghdibadi H. Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant Foods Hum Nutr. 2008;63:183–8. doi: 10.1007/s11130-008-0091-y. [DOI] [PubMed] [Google Scholar]
- 14.Hajhashemi V, Sajjadi SE, Zomorodkia M. Antinociceptive and anti-inflammatory activities of Bunium persicum essential oil, hydroalcoholic and polyphenolic extracts in animal models. Pharm Biol. 2011;49:146–51. doi: 10.3109/13880209.2010.504966. [DOI] [PubMed] [Google Scholar]
- 15.Nickavar B, Adeli A, Nickavar A. Analyses of the essential oil from Bunium persicum fruit and its antioxidant constituents. J Oleo Sci. 2014;63:741–6. doi: 10.5650/jos.ess13168. [DOI] [PubMed] [Google Scholar]
- 16.Sharififar F, Yassa N, Mozaffarian V. Bioactivity of major components from the seeds of Bunium persicum (Boiss.) Fedtch. Pak J Pharm Sci. 2010;23:300–4. [PubMed] [Google Scholar]
- 17.Vetter SW. Glycated Serum Albumin and AGE Receptors. Adv Clin Chem. 2015;72:205–75. doi: 10.1016/bs.acc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, et al. Review: Glycation of human serum albumin. Clin Chim Acta. 2013;425:64–76. doi: 10.1016/j.cca.2013.07.013. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos-Fernandez E, Tajes M, Palomer E, Ill-Raga G, Bosch-Morato M, Guivernau B, et al. Posttranslational nitro-glycative modifications of albumin in Alzheimer’s disease: Implications in cytotoxicity and amyloid-beta peptide aggregation. J Alzheimers Dis. 2014;40:643–57. doi: 10.3233/JAD-130914. [DOI] [PubMed] [Google Scholar]
- 20.Naderi GH, Dinani NJ, Asgary S, Taher M, Nikkhoo N, Boshtam M. Effect of some high consumption spices on hemoglobin glycation. Indian J Pharm Sci. 2014;76:553–7. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 21.Rondeau P, Bourdon E. The glycation of albumin: Structural and functional impacts. Biochimie. 2011;93:645–58. doi: 10.1016/j.biochi.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Khan MS, Rabbani N, Tabrez S, Islam BU, Malik A, Ahmed A, et al. Glycation Induced Generation of Amyloid Fibril Structures by Glucose Metabolites. Protein Pept Lett. 2016;23:892–7. doi: 10.2174/0929866523666160831153858. [DOI] [PubMed] [Google Scholar]
- 23.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2:875–7. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 24.Safari M, Sheikh N, Mani Kashani K. Study on the effect of vitamin C on the in vitro albumin glycation reaction. Iran J Pharm Res. 2010:275–9. [Google Scholar]
- 25.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Klunk WE, Jacob RF, Mason RP. Quantifying amyloid by congo red spectral shift assay. Methods Enzymol. 1999;309:285–305. doi: 10.1016/s0076-6879(99)09021-7. [DOI] [PubMed] [Google Scholar]
- 27.Peng X, Cheng KW, Ma J, Chen B, Ho CT, Lo C, et al. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J Agric Food Chem. 2008;56:1907–11. doi: 10.1021/jf073065v. [DOI] [PubMed] [Google Scholar]
- 28.Muanda FN, Bouayed J, Djilani A, Yao C, Soulimani R, Dicko A. Chemical Composition and, Cellular Evaluation of the Antioxidant Activity of Desmodium adscendens Leaves. Evid Based Complement Alternat Med. 2011;2011:620862. doi: 10.1155/2011/620862. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorsey PG, Greenspan P. Inhibition of nonenzymatic protein glycation by pomegranate and other fruit juices. J Med Food. 2014;17:447–54. doi: 10.1089/jmf.2013.0075. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haghirossadat F, Bernard F, Kalantar M, Sheikhha M, Hokmollahi F, Azimzadeh M, et al. Bunium persicum (Black Caraway) of Yazd province: Chemical assessment and Evaluation of its antioxidant effects. Journal of Shahid Sadoughi University of Medical Sciences. 2010;18:284–91. Persian. [Google Scholar]
- 31.Kumar PA, Reddy PY, Srinivas PN, Reddy GB. Delay of diabetic cataract in rats by the antiglycating potential of cumin through modulation of alpha-crystallin chaperone activity. J Nutr Biochem. 2009;20:553–62. doi: 10.1016/j.jnutbio.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Arasteh A, Farahi S, Habibi-Rezaei M, Moosavi-Movahedi AA. Glycated albumin: An overview of the In Vitro models of an In Vivo potential disease marker. J Diabetes Metab Disord. 2014;13:49. doi: 10.1186/2251-6581-13-49. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caengprasath N, Ngamukote S, Makynen K, Adisakwattana S. The protective effects of pomelo extract (Citrus grandis L. Osbeck) against fructose-mediated protein oxidation and glycation. EXCLI J. 2013;12:491–502. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 34.Adisakwattana S, Jiphimai P, Prutanopajai P, Chanathong B, Sapwarobol S, Ariyapitipan T. Evaluation of alpha-glucosidase, alpha-amylase and protein glycation inhibitory activities of edible plants. Int J Food Sci Nutr. 2010;61:295–305. doi: 10.3109/09637480903455963. [DOI] [PubMed] [Google Scholar]
- 35.Adisakwattana S, Thilavech T, Chusak C. Mesona Chinensis Benth extract prevents AGE formation and protein oxidation against fructose-induced protein glycation in vitro. BMC Complement Altern Med. 2014;14:130. doi: 10.1186/1472-6882-14-130. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesne S, Rondeau P, Armenta S, Bourdon E. Effects of oxidative modifications induced by the glycation of bovine serum albumin on its structure and on cultured adipose cells. Biochimie. 2006;88:1467–77. doi: 10.1016/j.biochi.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–7. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 38.Sadowska-Bartosz I, Bartosz G. Effect of glycation inhibitors on aging and age-related diseases. Mech Ageing Dev. 2016;160:1–18. doi: 10.1016/j.mad.2016.09.006. [DOI] [PubMed] [Google Scholar]


