Abstract
Current guidelines for chronic kidney disease (CKD) recommend using albuminuria as well as estimated glomerular filtration rate (eGFR) to stage CKD. However, CKD progression is solely defined by change in eGFR with little regard to the risk implications of change in albuminuria. This is an observational study from the Stockholm CREAtinine Measurements (SCREAM) project, a health care utilization cohort from Stockholm, Sweden, with laboratory measures from 2006–2011 and follow-up through December 2012. Included were 31,732 individuals with two or more ambulatory urine albumin to creatinine ratio (ACR) tests. We assessed the association between change in ACR during a baseline period of 1, 2, or 3 years and end-stage renal disease (ESRD) or death. Using a 2-year baseline period, there were 378 ESRD events and 1712 deaths during a median of 3 years of follow-up. Compared to stable ACR, a 4-fold increase in ACR was associated with a 3.08-times (95% confidence interval 2.59 to 3.67) higher risk of ESRD while a 4-fold decrease in ACR was associated with a 0.34-times (0.26 to 0.45) lower risk of ESRD. Similar associations were found in people with and without diabetes mellitus, with and without hypertension, and also when adjusted for the change in eGFR during the same period. The association between change in ACR and mortality was weaker: ACR increase was associated with mortality, but the relationship was largely flat for ACR decline. Results were consistent for 1-, 2-, and 3-year ACR changes. Thus, changes in albuminuria are strongly and consistently associated with the risk of ESRD and death.
Keywords: albuminuria, changes in albuminuria, estimated glomerular filtration rate, end-stage renal disease, death
INTRODUCTION
Chronic kidney disease (CKD) is a significant global public health problem with poor prognosis and elevated healthcare costs1. Recent clinical guidelines on CKD incorporate albuminuria as well as estimated glomerular filtration rate (eGFR) to define and stage CKD2–4. However, guidelines define CKD progression solely by eGFR changes, and disagree regarding the usefulness and/or frequency of albuminuria monitoring 5–7. The progression of CKD is often slow and there are few specific symptoms until very advanced disease. Changes in albuminuria may serve as early indicators of CKD progression and complications beyond eGFR, but the risk implications of these changes are not well documented, with existing studies predominantly limited to subjects with diabetic kidney disease 8, 9.
There is increased interest in surrogate endpoints of CKD progression for the testing of new treatments in patients with CKD, given that the clinically meaningful event of ESRD requires large and lengthy trials to assess drug efficacy. A 30% decline in eGFR has been proposed as an acceptable surrogate of CKD progression in some circumstances10, 11. Changes in albuminuria may also be considered a potential surrogate endpoint, with the advantage that they often occur earlier in the disease course than eGFR decline. Recent meta-analyses of randomized controlled trials attempted to validate the usefulness of short-term albuminuria changes to predict ESRD incidence12–14. Collectively, they concluded that placebo-adjusted treatment effects on albuminuria correlate well with the treatment effect on ESRD endpoints. However, they also acknowledge as limitations that the duration of those trials was relatively short (maximum 24-month intervention) and that they included a selected population, composed mainly of diabetic and hypertensive patients. Definitive conclusions have not been reached and recent debate has highlighted the significant controversy15–18.
Observational studies in large representative healthcare-utilization cohorts can fill some of these knowledge gaps, with the possibility of modeling longer time-frames for albuminuria change, testing its predictive accuracy in real-life heterogeneous populations, and overall contributing to provide clinical guidance as to how to interpret albuminuria changes at the bedside in the face of substantial biologic variability. Against this background, the objective of this study was to analyze the prognostic nature of albuminuria changes with regards to the subsequent risk of ESRD and mortality in a real-life healthcare setting.
RESULTS
Participant selection and baseline characteristics
There were 88 055 individuals ≥18 years of age who underwent urine albuminuria testing in the region of Stockholm during 2006–2011, with 202 598 albumin to creatinine ratio (ACR) tests performed. Of those individuals, there were 39 864 individuals with ≥2 ACR tests. We then imposed the requirement that ≥2 ACR tests must have been performed in the outpatient setting, excluding 1541 individuals. Of the 38 323 individuals with ≥2 outpatient ACR tests, 6533 individuals had urine ACR changes not fitting the pre-specified baseline periods; thus, our analyses were finally based on 31 732 individuals. Depending on the baseline period considered, different numbers of individuals were eligible (Supplemental Figure 1).
Table 1 describes baseline characteristics of 19 897 participants with data on 2-year ACR changes. Mean age was 59 (minimum 18, maximum 96) years, 41% were women, 61% had diabetes mellitus, and 69% had a history of hypertension. History of cardiovascular disease (CVD) was present in 16%. Baseline average eGFR was 81 (standard deviation, SD 30) ml/min/1.73m2 and baseline median ACR was 1.9 (IQR 0.8, 8.5) mg/mmoL (16 [7–75] mg/g). When stratified by change in ACR, individuals with increases in ACR tended to be older, with more comorbid conditions and a somewhat lower baseline eGFR than individuals with decreases in ACR. Similar patient characteristics were observed for the 1-year and 3-year baseline periods (Supplemental Tables 1 and 2).
Table 1.
Baseline characteristics of individuals eligible for 2-year ACR changes, further stratified by pre-specified ACR-fold changes.
| Overall | 4+ fold decrease | 2–4 fold decrease | Stable | 2–4 fold increase | 4+ fold increase | |
|---|---|---|---|---|---|---|
| N | 19897 | 1977 | 2511 | 10568 | 2817 | 2024 |
| Age (years), mean (SD) | 59 (18) | 56 (20) | 59 (18) | 59 (18) | 60 (18) | 60 (19) |
| Women, n(%) | 8105 (41%) | 912 (46%) | 1044 (42%) | 4063 (38%) | 1160 (41%) | 926 (46%) |
| Diabetes Mellitus, n(%) | 12090 (61%) | 1015 (51%) | 1512 (60%) | 6511 (62%) | 1791 (64%) | 1261 (62%) |
| Hypertension, n(%) | 13671 (69%) | 1363 (69%) | 1699 (68%) | 7146 (68%) | 1992 (71%) | 1471 (73%) |
| Cardiovascular disease, n(%) | 3191 (16%) | 307 (16%) | 375 (15%) | 1623 (15%) | 481 (17%) | 405 (20%) |
| Total cholesterol (mmol/L), mean (SD) | 4.9 (1.1) | 5.2 (1.3) | 5.0 (1.2) | 4.9 (1.1) | 4.9 (1.1) | 4.9 (1.1) |
| eGFR (ml/min/1.73m2), mean (SD) | 81 (30) | 82 (35) | 82 (29) | 82 (28) | 80 (29) | 77 (35) |
| ACR (mg/mmoL), median (IQR) | 1.9 (0.8–8.5) | 11 (3–45) | 3 (1–14) | 2 (1, 6) | 1 (1–5) | 1 (1–4) |
| ACR (mg/g), median (IQR) | 16 (7–75) | 97 (27–398) | 27 (9–124) | 13 (6, 57) | 9 (9–44) | 9 (9–35) |
| Number of ACR tests, median (IQR) | 3.0 (2.0–4.0) | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) |
| ACR <30 mg/g, n(%) | 12171 (61%) | 495 (25%) | 1279 (51%) | 6949 (66%) | 1977 (70%) | 1471 (73%) |
| ACR 30–299 mg/g, n(%) | 5495 (28%) | 916 (46%) | 879 (35%) | 2571 (24%) | 667 (24%) | 462 (23%) |
| ACR 300+ mg/g, n(%) | 2231 (11%) | 566 (29%) | 353 (14%) | 1048 (10%) | 173 (6%) | 91 (4%) |
| ACR fold-change, median (IQR) | 1.0 (0.5–2.0) | 0.14 (0.08–0.20) | 0.38 (0.32–0.44) | 1.01 (0.75, 1.41) | 2.63 (2.28–3.14) | 6.95 (5.00–12.62) |
| Median ACR (IQR) across ACR categories | ||||||
| ACR < 30 mg/g | 8 (4, 14) | 16 (10, 22) | 12 (8, 18) | 8 (4, 13) | 7 (4, 12) | 7 (4, 12) |
| ACR 30–299 mg/g | 74 (45, 132) | 90 (51, 147) | 75 (43, 141) | 72 (45, 128) | 69 (43, 119) | 69 (42, 122) |
| ACR 300+ mg/g | 806 (482, 1639) | 880 (492, 2092) | 911 (518, 1872) | 839 (491, 1627) | 613 (412, 1008) | 558 (404, 832) |
ACR, albumin to creatinine ratio; SD, standard deviation; IQR, interquartile range; ESRD, end-stage renal disease
When stratified by the presence of diabetes mellitus, diabetic patients were older, more often men, and more often with a history of hypertension or CVD as compared to non-diabetic patients. Baseline eGFR was slightly higher and median ACR slightly lower among diabetic compared to non-diabetic patients (Supplemental Table 3).
During a median follow up of 3.0 years, 378 (2%) individuals developed ESRD, and 1712 (9%) died (Table 2). Of the 1712 deaths, 672 were attributed to cardiovascular causes. Death and ESRD events were more common in patient groups with increases in ACR, and more common in individuals with higher baseline ACR. The same pattern was observed for the 1-year and 3-year baseline periods (Supplemental Tables 4 and 5).
Table 2.
Follow up and outcomes of individuals eligible for 2-year ACR changes, further stratified by pre-specified ACR-fold changes.
| Overall | 4+ fold decrease | 2–4 fold decrease | stable | 2–4 fold increase | 4+ fold increase | |
|---|---|---|---|---|---|---|
| N | 19897 | 1977 | 2511 | 10568 | 2817 | 2024 |
| Follow-up (years), median (IQR) | 3.0 (1.9–4.0) | 3.1 (1.9–4.1) | 3.0 (1.9–3.9) | 3.1 (1.9, 4.0) | 2.9 (1.9–3.9) | 2.9 (1.8–3.9) |
| ESRD events, n(%) | 378 (2%) | 22 (1%) | 29 (1%) | 205 (2%) | 49 (2%) | 73 (4%) |
| Incidence rate (1000 person-years) | 6,4 | 3,7 | 3,9 | 6.5 | 5,9 | 12,7 |
| Deaths, n(%) | 1712 (9%) | 172 (9%) | 214 (9%) | 778 (7%) | 273 (10%) | 275 (14%) |
| Incidence rate (1000 person-years) | 28,8 | 28,7 | 28,6 | 24.5 | 32,5 | 46,6 |
| Cardiovascular disease deaths, n | 672 | 63 | 89 | 295 | 104 | 121 |
| Non-cardiovascular disease deaths, n | 1040 | 109 | 125 | 483 | 169 | 154 |
| Kidney disease deaths, n | 31 | 2 | 6 | 15 | 4 | 4 |
| ESRD events and total number of patients stratified by baseline ACR | ||||||
| ACR < 30 mg/g | 19/12171 | 0/495 | 1/1279 | 4/6949 | 2/1977 | 12/1471 |
| ACR 30–299 mg/g | 86/5495 | 7/916 | 3/879 | 30/2571 | 10/667 | 36/462 |
| ACR 300+ mg/g | 273/2231 | 15/566 | 25/353 | 171/1048 | 37/173 | 25/91 |
| Deaths and total number stratified by baseline ACR | ||||||
| ACR < 30 mg/g | 662/12171 | 26/495 | 52/1279 | 307/6949 | 131/1977 | 146/1471 |
| ACR 30–299 mg/g | 678/5495 | 80/916 | 99/879 | 297/2571 | 100/667 | 102/462 |
| ACR 300+ mg/g | 372/2231 | 66/566 | 63/353 | 174/1048 | 42/173 | 27/91 |
ACR, albumin to creatinine ratio; SD, standard deviation; IQR, interquartile range; ESRD, end-stage renal disease
ESRD risk according to fold-change in ACR
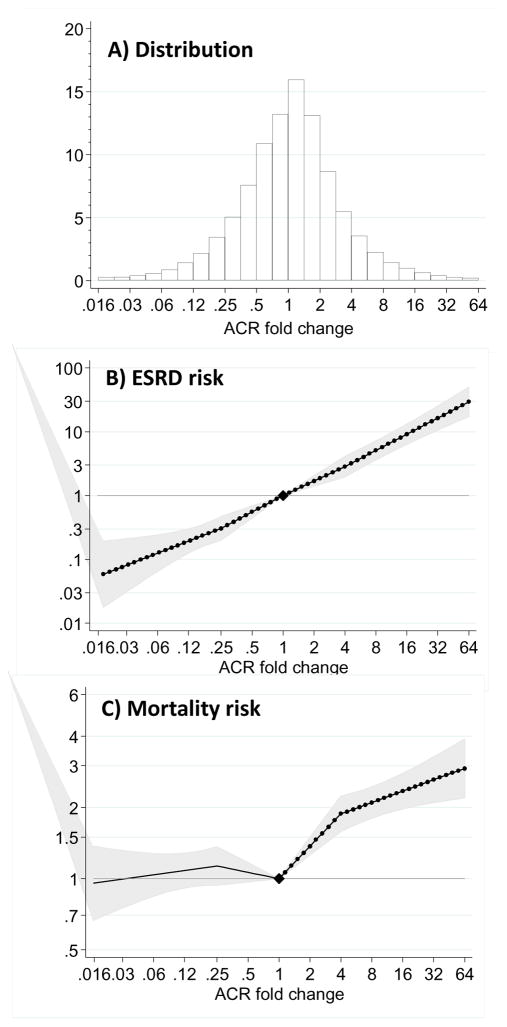
ACR fold-change followed a normal distribution for each baseline period, with increases in ACR being slightly more common than decreases in ACR (panel A: Figure 1, Supplemental Figures 2 and 3). The association between ACR change and ESRD was strong and showed a nearly linear relationship (Figure 1, Panel B). There was a 3.08-times (95% confident interval, CI 2.59 to 3.67) higher risk of ESRD associated with a 4-fold increase in ACR over a 2-year period, and 0.34-times (95% CI 0.26 to 0.45) lower risk of ESRD associated with a 4-fold decrease in ACR (0.25-fold increase in ACR).
Figure 1.
Distribution of 2-year ACR fold changes (Panel A) and adjusted hazard ratio of end-stage renal disease (ESRD, Panel B) and mortality (Panel C) associated with a 2-year fold change in ACR. Adjusted for baseline (log) ACR, baseline eGFR (knot at 60 ml/min/1.73 m2), age, gender, total cholesterol, diabetes mellitus, hypertension and history of cardiovascular disease. Filled circles denote statistical significance (P<.05) compared with the reference (diamond) at stable ACR (1-fold change). Grey area represents 95% confidence intervals.
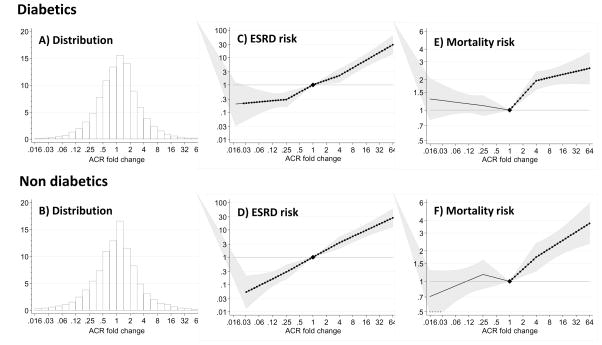
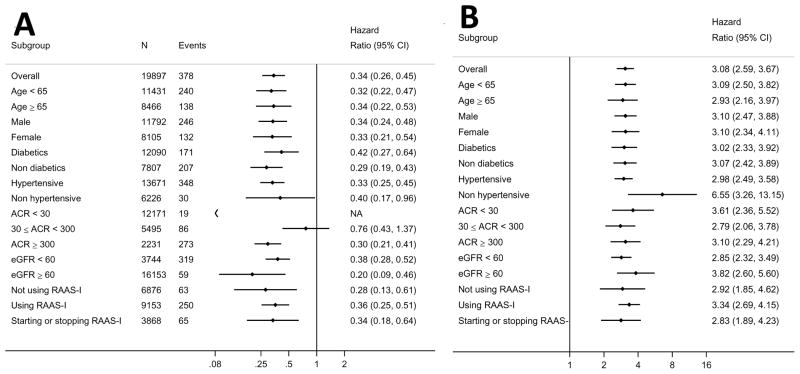
The shape of the association between change in ACR and ESRD remained essentially unchanged for both men and women (P for interaction>0.1; Supplemental Figure 4, Panel A) or according to the presence/absence of diabetes (Figure 2, Supplemental Figures 5 and 6). In fact, the risk associated with a 4-fold increase in ACR over a 2-year period was similar for both diabetics (3.07 [2.42, 3.89]) and non-diabetics (3.02 [2.33, 3.92]). Results were confirmed across other pre-specified subpopulations, including different age strata, hypertensive patients, or individuals with different baseline levels of ACR and eGFR (Figure 3, additional details on number of participants and events in Supplemental Table 6). Although the distribution of ACR change was shifted slightly to the left (favoring decrease in ACR) among users of RAAS inhibitors, there were similar associations between ACR change and the risk of ESRD among both users and non-users of RAAS inhibitors (Supplemental Figure 7, Figure 3).
Figure 2.
Distribution of 2-year ACR fold changes (Panels A and B) and adjusted hazard ratio of end-stage renal disease (ESRD, Panels C and D) and mortality (Panels E and F) associated with a 2-year fold change in ACR stratified by the presence of diabetes mellitus. Adjusted for baseline (log) ACR, baseline eGFR (knot at 60 ml/min/1.73 m2), age, gender, total cholesterol, hypertension and history of cardiovascular disease. Median ACR (IQR) was 19 (7, 106) mg/g in non-diabetics and 16 (7, 60) mg/g in diabetics. Filled circles denote statistical significance (P<.05) compared with the reference (diamond) at stable ACR (1-fold change). Grey area represents 95% confidence intervals. P value for the product diabetes x ACR fold change >0.1.
Figure 3.
Forest plots of 4-fold decrease (A) and 4-fold increase (B) in ACR during a 2-year period and ESRD risk, overall and in subgroups.
NA indicates that there were no ESRD events in persons with ACR <30 mg/g and a ≥4-fold decrease in ACR during the 2-year period. “Not using RAAS-I” indicates participants were not taking a RAAS-I at the start or end of the 2-year baseline period. “Using RAAS-I” indicates participants were taking a RAAS-I at the start and end of the 2-year baseline period. “Starting or stopping RAAS-I” indicates that participants had a change in RAAS-I use from the start to the end of the 2-year baseline period.
The results appeared robust through several sensitivity analyses, which included adjusting for the concomitant change in eGFR, adjusting for the number of available tests per individual, considering death as a competing risk, considering the composite endpoint of ESRD plus renal death, excluding individuals with concomitant diagnosis of urinary tract infection, and studying the selected population of individuals free of diabetes and hypertension (Supplemental Figure 8, Supplemental Tables 7, 8). Analyses assessing the fold-change in ACR over shorter (1 year) and longer (3 year) periods yielded comparable associations to the main analysis (panel B: Supplemental Figures 2 and 3).
Mortality risk according to fold-change in ACR
Compared to those with stable ACR, the adjusted HR of all-cause mortality was higher with greater ACR increases, but was largely flat in the range of ACR decline (Figure 1, Panel C). This mortality risk pattern was similar for both men and women (P for interaction>0.1; Supplemental Figure 4, Panel B), diabetics and non-diabetics (Figure 2, Panels E and F), and through all baseline periods (Supplemental Figures 2, 3, 5 and 6). In general, the association between ACR increases and the risk of death was lower in magnitude than that observed for ESRD risk. Adjusted HRs for mortality associated with fold-increases and decreases in ACR are shown in Supplemental Figure 9: A 4-fold increase in ACR during a 2-year period was associated with a 1.5-times higher risk of mortality (95% CI 1.39 to 1.62), and a 4-fold decrease in ACR did not show any association with this outcome (adjusted HR 0.98 [95% CI 0.89, 1.08]). We observed consistent results in subgroup analyses (Supplemental Figure 9 and additional detail on number of participants and events in Supplemental Table 6) and for both non-cardiovascular and cardiovascular-specific mortality (Supplemental Figure 10–12).
DISCUSSION
The purpose of this study was to assess the risk implications of a change in ACR for the development of ESRD, particularly as a potential early marker of ESRD risk. Previous studies have shown the strong prediction of albuminuria measures at a single time point for ESRD and mortality19–23, but little is known outside the RCT setting about whether changes in ACR over time translate into changes in these outcomes. In this analysis of 31,732 individuals with repeated ACR tests from the same region and healthcare system, we documented that changes in ACR over a 1, 2 or 3-year time interval were linearly associated with subsequent risk of ESRD. The HR of ESRD adjusted for baseline ACR, eGFR and other covariates was substantially higher with greater ACR increases, and substantially lower with greater ACR reductions. Our study also reported associations with mortality that were quantitatively smaller than associations with ESRD risk but still important for ACR increases. Strengths of this study are its healthcare setting as well as its large size and population heterogeneity, reflecting real-life associations from the clinical arena. This allowed us to model larger time intervals than previous RCTs, and provides overall robust results across relevant subgroup populations scarcely investigated before (including non-diabetics and non-hypertensives, or individuals with normo/moderately/severely increased ACR). Altogether, these novel data provide a basis to better interpret ACR monitoring at the bedside and to foster consideration of change in albuminuria as a surrogate endpoint for clinical trials.
In the interpretation of our results, the healthcare utilization nature of our study should be duly noted. ACR testing is not universal in healthcare, and we have no direct information on the indications for or the reasons behind such testing. This results in a cohort with more comorbid conditions than the general Swedish population and generalizability to other settings should not be directly assumed. Additional limitations of our study is that we lack information on smoking, blood pressure or body weight, and that as in all observational studies, we can neither infer causality in the associations, nor ascertain the etiologies or therapeutic strategies that explained the variation in our exposure (for instance, whether treatment or discontinuation of RAAS inhibitors were responsible for the observed changes in ACR). Nevertheless, we were reassured in the robustness of our findings by the fact that the association remained strikingly similar among a number of stratified analyses, allowing us to speculate that these associations may be independent of etiology.
In contrast to current recommendations4–7, which rely only on eGFR changes to define CKD progression, our study clearly shows how a change in ACR adds important prognostic information to initial ACR as well as eGFR changes. In our study, the prognostic association between ACR changes and ESRD outcomes was strong and robust in our real-life healthcare setting. Notably, the association was not materially modified by baseline albuminuria, suggesting that reducing albuminuria both in the moderately and severely increased range may slow kidney disease progression24. An interesting finding, both from a prognostic and from an etiological point of view, is that the association between ACR changes and ESRD risk remained strong beyond adjustment for baseline eGFR and the eGFR change occurring over the same time period. This provides additional support for the notion that albuminuria and eGFR represent two interconnected but distinct measures of pathways leading to kidney failure19, 20. Our observations may have clinical implications for considering albuminuria surveillance in clinical practice to monitor CKD progression. An increase in ACR monitoring in clinical practice could lead to better prognostication and intensified interventions in high-risk individuals with worsening albuminuria before a decline in eGFR. This may prove to be of particular importance given the ongoing global increase in CKD burden. In this sense, our data are useful for defining what level of albuminuria change in the future may be considered clinically important and what its consequences might be in order to motivate how frequently people should be monitored.
Our results also contribute to the current debate concerning the use of surrogate outcomes to facilitate the conduct of clinical trials in nephrology. Because number and type of RCTs targeting albuminuria in CKD are limited12–14, particularly with respect to length of follow-up, number of ESRD events reached and the selection of mainly diabetic patients, observational studies such as ours are necessary to assess a range of potential scenarios and thus evaluate the utility, robustness and power of ACR changes. In evaluating future trials it will be important to consider the mechanism of action for lowering albuminuria and reducing CKD progression since no surrogate is perfect and hence the challenge is to define the range of circumstances under which using albuminuria as a surrogate is useful. In this regard, consistency of effects over time and across all predefined strata in our study may suggest applicability for shorter as well as for longer trials, which is relevant for diseases that are progressing more rapidly or slowly, respectively.
Evaluation of the outcome of death is important because it is a common outcome in CKD and may precede ESRD. In our study, ACR increases were linearly associated with the risk of death, which was attributed to excess of both cardiovascular-related and non-cardiovascular-related deaths. Again, we are not aware of previous community-based studies evaluating the association between ACR changes with mortality, but our data is in line with previous evidence documenting the powerful predictive power of single albuminuria tests for hard endpoints21–23. Conversely, no association was observed for ACR decreases; in our healthcare setting, decreases in ACR were less common, and occurred in a healthier subset with few mortality events. Our data expand to the greater community the post hoc analysis from an RCT 25, where a ≥ twofold increase in ACR over 2 years was associated with nearly 50% higher mortality (HR 1.48; 1.32–1.66), and a ≥ twofold decrease in ACR was associated with 15% lower mortality (HR 0.85; 95% CI 0.74 to 0.98) compared with those with lesser ACR changes and after adjustment for baseline albuminuria.
We recognize that fold-change in ACR based on a single first and single last ACR is less precise than alternative designs in which multiple measures are available at each time point. However, the two-measure approach is simpler and easier to implement in clinical trials than an endpoint defined on the average rate of decline in ACR. The inter- and intra-laboratory as well as intra-individual variability of ACR causes concern about the ability to monitor this variable over time26. In our study, the performance of all ACR analyses by three centralized laboratories with frequent audits for harmonization should therefore be seen as an advantage. Nonetheless, we acknowledge the intra-individual variability inherent in urine albumin excretion and that we cannot distinguish first morning voids from spot urine samples in the lab records. Finally, there is some controversy regarding the standardization of urine albumin with urine creatinine. We note that, as we assess ACR fold-change over time, the method of standardization should have less impact, and that our results were robust by sex and age and also after additional adjustment for change in eGFR.
To conclude, changes in ACR in a healthcare setting were strongly and consistently associated with the risk of ESRD and mortality, documenting the value of albuminuria monitoring in estimating patients’ risk and providing real world evidence for the notion of ACR change as a relevant surrogate endpoint.
METHODS
Patient selection
The Stockholm CREAtinine Measurements (SCREAM) project is a healthcare utilization cohort from the sole healthcare provider of the region of Stockholm, Sweden (Stockholm County Council), described elsewhere 27. Briefly, all Stockholm residents over the age of 18 years and who had a measurement of serum creatinine in either inpatient or outpatient care during 2006–2011 were included. For these, all standard laboratory tests performed during the period were collected; the dataset was then linked to regional and national administrative databases with complete information on demographic data, healthcare utilization, diagnoses, validated ESRD outcomes, vital status and pharmacy-dispensed medicines 27. The institutional review board at Karolinska Institutet, Stockholm, Sweden and the Swedish National Board of Welfare approved the study.
Biochemical assessments and study covariates
Blood and urine laboratory tests were performed as part of a healthcare encounter. In this analysis, we considered only those tests performed in the outpatient setting, which may better reflect stable medical conditions. Biochemical assessments were performed by three different laboratories that provide services to the region 27. Inter- as well as intra-laboratory variation is considered minimal, with the three laboratories frequently being audited for quality and harmonization by the national Government-funded organisation EQUALIS (www.equalis.se). Serum and urine creatinine measurements were standardized to isotope dilution mass spectrometry. ACR was calculated by taking the ratio between urinary albumin and urinary creatinine, and expressed in mg/mmol (to convert to mg/g multiply by 8.84). Serum creatinine was used to calculated eGFR using the CKD-EPI 2009 creatinine equation 28. Information on race was not available by law, and all individuals were assumed to be Caucasian.
Other study covariates are considered as follows: Age, defined as of time of first ACR test and analyzed continuously. Presence of diabetes mellitus, defined by relevant ICD-10 codes before the first ACR test; or pharmacy dispensation of oral antidiabetic medication (ATC codes A10A, A10B) within 3 months before first ACR test. Presence of hypertension was defined by relevant ICD-10 codes (ICD-10 I10–I15) or pharmacy dispensation of antihypertensive medication (ATC codes C03, C09, C03DA, C07 and C08). Participants with a history of myocardial infarction (ICD-10 – I21, I22), coronary revascularization (surgical codes FNA, FNB, FNC, FND, FNE, FNF, FNG), heart failure (ICD-10 I50), or stroke (ICD-10 I61, I63, I64) were considered to have a history of cardiovascular disease (CVD). Information on drug-dispensations was derived from linkage with the Swedish Prescribed Drug Registry, which collects information on all prescription drugs dispensed at Swedish pharmacies29. Data on blood pressure, weight and smoking were not available
Study exposure
The study exposure was the fold-change in ACR during a baseline period. The implications of the magnitude of change in ACR may vary depending on the time over which change is assessed. For that reason, we evaluated three distinct pre-specified baseline periods (1, 2, and 3 years). For each baseline period, a 0.5 year of margin before and after the end of the period was allowed for determining the last available ACR to calculate the change (e.g., ACR between 0.5 and 1.5 years after the first available ACR could be used for the 1 year baseline period analysis), but the ACR closest to the end of the period of interest was selected for each participant11.
Study outcomes
The primary outcome of interest was ESRD after the baseline period. We defined ESRD as initiation of renal replacement therapy (dialysis or renal transplantation). ESRD events were ascertained by linkage with the Swedish Renal Registry30, with follow up until December 31st, 2012 and censoring for death. As sensitivity analysis we considered the composite endpoint of ESRD and renal death (death attributed to renal causes, ICD-10 codes N18, N19). Secondary outcomes were all-cause mortality as well as cause-specific mortality, grouped as cardiovascular and non-cardiovascular deaths. Cardiovascular mortality was defined as death due to myocardial infarction, heart failure, stroke, or sudden cardiac death reported as 1st position cause of death (any ICD-10 code from the I chapter). The remainder of deaths were considered non-cardiovascular. Information on vital status and causes of death was obtained via linkage with the Swedish Population Registry, with end of observation on December 31st, 2012 and no loss to follow up.
Statistical analysis
Analyses were performed using Stata/SE software version 14 (StataCorp). We considered 2-sided P values of less than 0.05 as statistically significant. We imputed missing values of serum cholesterol using the cohort-specific mean values. We did not impute change in ACR or change in eGFR, as this data was available in all cases. We modeled the adjusted hazard ratios (HRs) of ESRD and mortality after the end of the baseline period as a spline function of log-transformed fold change of ACR. We fit piecewise linear splines for log-transformed fold change of ACR (knots were placed at 4-fold increase, 4-fold decrease and stable ACR). We used stable ACR (−1.25 to +1.25 fold increase) as a reference in categorical analysis. Cox models were adjusted for age, sex, total cholesterol, hypertension, diabetes, history of cardiovascular disease, first eGFR (knot at 60 ml/min/1.73m2) and first log-transformed ACR. Interaction terms assessed the potential effect modification of the presence of diabetes mellitus with change in ACR, and analyses were performed separately within each diabetes strata. Spline curves were also constructed as above for the association between fold change in ACR and cause-specific mortality.
Several sensitivity analyses were pre-specified for the primary study outcome of ESRD risk. First, we adjusted for change in eGFR during the same baseline period10; second, given that this is a healthcare extraction with sicker individuals more often accessing healthcare, we adjusted for the number of tests available for each individual during the period; Third, we considered death as a competing risk; Fourth, we considered the composite endpoint of ESRD and death before ESRD attributed to CKD ((kidney disease death); Fifth, we excluded patients in which baseline ACR measurements occurred concomitantly with a diagnostic code for urinary tract infection. Finally, analyses were stratified by a) sex, b) presence of diabetes mellitus, c) presence of hypertension, d) initial ACR category (<30 mg/g, ≥30–299 mg/g, ≥300 mg/g), level of eGFR (<60 ml/min/1.73 m2, ≥60 ml/min/1.73 m2), and RAAS inhibitor use. For simplicity of presentation, the main analysis here presented refers to the 2-year ACR change. Corresponding results for other baseline periods are presented as supplementary materials.
Supplementary Material
Acknowledgments
Funding: Stockholm County, the Swedish Heart and Lung Foundation, the US National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases.
The SCREAM project has obtained financial support from Stockholm County. We also acknowledge grant support from the Swedish Heart and Lung Foundation, The Swedish Research Council, the Marianne and Marcus Wallenberg Foundation, Dalarna University, the County Council of Dalarna, Martin Rind’s and Westman’s foundations. Baxter Novum is the result of a grant from Baxter Healthcare to Karolinska Institutet. Analysis and writing were partially supported by grants from the US National Kidney Foundation (NKF funding sources include AbbVie, Amgen and Relypsa) and the NIDDK (R01DK100446-01). MG receives support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; K08DK092287).
Footnotes
Declaration of Interests: Bengt Lindholm declares being employed by Baxter Healthcare Inc. All other authors declare no competing interests.
Contributors: Study concept and design: JJC, MEG, JA, SHB, KM, RTG, ASL, JC; Analysis and interpretation of data: all authors; Drafting of the manuscript: JJC, MEG, SHB, YS, KM, JC; Critical revision of the manuscript for important intellectual content: all authors; Statistical analysis: YS; Study supervision: JC. Drs. Carrero and Coresh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Online-Only Material: eTables and e-Figures are available online
References
- 1.Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 2.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 3.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of internal medicine. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 4.KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Hopkins RH, Jr, Sweet DE, et al. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: A clinical practice guideline from the American College of Physicians. Annals of internal medicine. 2013;159:835–847. doi: 10.7326/0003-4819-159-12-201312170-00726. [DOI] [PubMed] [Google Scholar]
- 6.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 7.European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur J Prev Cardiol. 2012;19:585–667. doi: 10.1177/2047487312450228. [DOI] [PubMed] [Google Scholar]
- 8.Parving HH, Andersen AR, Smidt UM, et al. Reduced albuminuria during early and aggressive antihypertensive treatment of insulin-dependent diabetic patients with diabetic nephropathy. Diabetes care. 1981;4:459–463. doi: 10.2337/diacare.4.4.459. [DOI] [PubMed] [Google Scholar]
- 9.Viberti GC, Hill RD, Jarrett RJ, et al. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–1432. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–835. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. Jama. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inker LA, Levey AS, Pandya K, et al. Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient meta-analysis. Am J Kidney Dis. 2014;64:74–85. doi: 10.1053/j.ajkd.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jun M, Turin TC, Woodward M, et al. Assessing the Validity of Surrogate Outcomes for ESRD: A Meta-Analysis. J Am Soc Nephrol. 2015;26:2289–2302. doi: 10.1681/ASN.2014040396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heerspink HJ, Kropelin TF, Hoekman J, et al. Drug-Induced Reduction in Albuminuria Is Associated with Subsequent Renoprotection: A Meta-Analysis. J Am Soc Nephrol. 2015;26:2055–2064. doi: 10.1681/ASN.2014070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambers Heerspink HJ, Gansevoort RT. Albuminuria Is an Appropriate Therapeutic Target in Patients with CKD: The Pro View. Clin J Am Soc Nephrol. 2015;10:1079–1088. doi: 10.2215/CJN.11511114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LF, Lewis J. Albuminuria is Not an Appropriate Therapeutic Target in Patients with CKD: The Con View. Clin J Am Soc Nephrol. 2015;10:1089–1093. doi: 10.2215/CJN.10681014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cravedi P, Ruggenenti P, Remuzzi G. Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol. 2012;8:301–306. doi: 10.1038/nrneph.2012.42. [DOI] [PubMed] [Google Scholar]
- 18.Thompson A. Proteinuria as a surrogate end point--more data are needed. Nat Rev Nephrol. 2012;8:306–309. doi: 10.1038/nrneph.2012.43. [DOI] [PubMed] [Google Scholar]
- 19.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal R, Duffin KL, Laska DA, et al. A prospective study of multiple protein biomarkers to predict progression in diabetic chronic kidney disease. Nephrol Dial Transplant. 2014;29:2293–2302. doi: 10.1093/ndt/gfu255. [DOI] [PubMed] [Google Scholar]
- 22.Hallan SI, Ritz E, Lydersen S, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 25.Schmieder RE, Mann JF, Schumacher H, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011;22:1353–1364. doi: 10.1681/ASN.2010091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witte EC, Lambers Heerspink HJ, de Zeeuw D, et al. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20:436–443. doi: 10.1681/ASN.2008030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Runesson B, Gasparini A, Qureshi AR, et al. The Stockholm CREAtinine Measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J. 2016;9:119–127. doi: 10.1093/ckj/sfv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–735. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 30.Evans M, Suttorp MM, Bellocco R, et al. Trends in haemoglobin, erythropoietin-stimulating agents and iron use in Swedish chronic kidney disease patients between 2008 and 2013. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.