Abstract
In a recent trial, levosimendan therapy failed to ameliorate sepsis-induced organ dysfunction or improve the survival of patients with septic shock. The failure of levosimendan and many other potential therapies for sepsis, together with the findings of histopathologic studies, raise questions regarding the pathophysiologic basis of the disorder.
Sepsis is as difficult a problem today as it was in the sixteenth century when Niccolo Machiavelli wrote “the physicians say it happens in hectic fever, that in the beginning of the malady it is easy to cure but difficult to detect, but in the course of time, not having been either detected or treated in the beginning, it becomes easy to detect but difficult to cure.” Early drainage or excision of the infected site and antimicrobial therapy are key components of effective sepsis therapy. Appropriate fluid resuscitation and maintenance of adequate haemodynamics with vasopressors and inotropes are also indicated and recommended in the Surviving Sepsis Guidelines1.
Gordon et al. recently published the negative findings of their prospective double-blinded, randomized control trial that investigated the efficacy of levosimendan in 516 patients with septic shock2. Levosimendan is a calcium sensitizer with inotropic and vasodilator properties. This drug is approved for the treatment of heart failure in many countries (not including the USA). In the recent trial, patients with septic shock received a 24 h infusion of either levosimendan or placebo in addition to standard care. Additional inotropic support (dobutamine) could also be added at the clinician’s discretion. The effects of levosimendan therapy likely persisted for approximately 1 week owing to the long half-life of its active metabolite.
In this study, levosimendan treatment did not lead to reduced severity of organ dysfunction or lower mortality in comparison to standard care2. Importantly, no significant between-group differences were reported in cardiac index, central venous oxygen saturation, PaO2:FiO2 ratio, serum creatinine or bilirubin levels (a marker of liver function). Patients in the levosimendan group received more noradrenaline and were less likely to be weaned from mechanical ventilation than those in the placebo group. No between-group differences were detected in any measure of renal function.
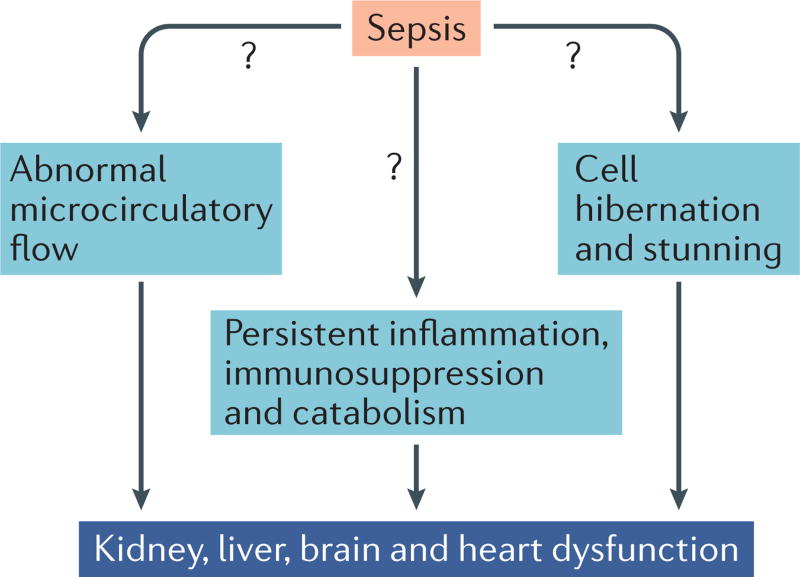
The negative result of this trial underscores a key issue that has long perplexed clinicians and sepsis researchers, namely what is the mechanism of multiple organ failure in sepsis? One of the main theories is that this organ failure results from impaired oxygen delivery at the cellular level owing to altered microcirculatory blood flow3 (FIG. 1). Although global oxygen delivery is typically increased in sepsis (once patients are adequately volume resuscitated), proponents of this theory suggest that many tissue capillary beds do not receive adequate oxygen supplies because of microvascular endothelial injury and the vasodilatory effects of various mediators that are released during sepsis2, 3. The end result is shunting of blood away from selected tissue beds and the development of a relative deficiency in oxygen delivery at the cellular level. Although levosimendan has multiple effects, the most prominent is an increase in oxygen delivery owing to its inotropic and afterload-reducing properties. Thus, if impaired oxygen delivery underlies organ failure in patients with sepsis, levosimendan treatment might improve capillary blood flow and prevent organ failure in these patients.
Figure 1. Current theories of the pathophysiological mechanisms that underlie organ failure in sepsis.
Three main theories exist: abnormal microcirculatory flow leading to cellular oxygen deficiency; inflammation-induced cell injury, protein breakdown and immunosuppression; cell hibernation and cell stunning (that is a switch to fetal gene expression and a low-energy state).
The failure of levosimendan to reduce the severity of organ failure in the recent trial does not disprove the theory that altered cellular microcirculatory flow drives organ dysfunction in sepsis. The fact that no major between-group differences in haemodynamics or oxygen delivery were reported does, however, reduce the likelihood that levosimendan substantially impacts microcirculatory flow. Findings from this study and others suggest that improving global haemodynamics is unlikely to improve cellular oxygen metabolism. Three large randomized controlled trials previously showed no difference in any outcome measure between patients with sepsis who received conventional therapy and those who received therapy that aimed to maximize oxygen delivery (that is, increased fluid loading, blood administration and dobutamine administration, if necessary)4. By contrast, a remarkable study in which patients with septic shock were treated with the β-blocker esmolol to slow heart rate to between 80–94 bpm during the first 96 h of their stay in the intensive care unit (ICU) showed a highly significant mortality benefit in the intervention group (49.4% versus 80.5% in the control group, P < 0.001)5. This therapy reduced oxygen delivery, but apparently not to a level that was detrimental to this population. Microcirculatory impairment with shunting and maldistribution of blood flow, therefore, seems unlikely to be central to the pathophysiology of sepsis-induced organ failure.
If abnormal global circulatory flow is not the driving force for sepsis-induced multiple organ dysfunction, what is responsible? A second evolving theory is that persistent inflammation, immunosuppression, and accelerated protein catabolism leads to loss of lean muscle mass, poor wound healing, and recurrent infections6 (FIG. 1). The end result of this constellation of events, which has been termed persistent inflammation, immunosuppression and catabolism syndrome (PICs), is multiple organ failure and death. Undoubtedly, localized inflammation at sites of infection, muscle wasting, impaired immunity, and delayed healing all occur in patients with sepsis. These adverse processes contribute to the high incidence of secondary hospital-acquired infections and frequent hospital readmissions in this population. It is difficult, however, to directly relate inflammation, immunosuppression and accelerated protein catabolism to organ dysfunction.
The absence of widespread cell injury on histopathologic examination of tissue from septic patients with severe organ dysfunction has led some investigators to propose a third theory — cell hibernation rather than cell death is responsible for organ dysfunction in sepsis (FIG. 1). To investigate the role of cell death, our group performed rapid bedside autopsies in patients who died of sepsis7, 8. We found a remarkable paucity of cell death in heart, kidney and liver tissue. Cardiomyocyte death was rare, occurring in <1–2% of cells. Renal tubular injury was common but focal. Most renal tubular cells appeared normal and the degree of renal tubular injury and tubular cell death did not account for the severity of renal failure and requirement for dialysis. Hepatocyte necrosis and apoptosis was present in seven of 20 patients but was predominantly located in proximity to the hepatic central vein, a region vulnerable to low flow states. The lack of widespread necrotic or apoptotic cell death in these major organs, coupled with laboratory evidence of organ failure, is consistent with cell stunning or hibernation as an aetiology of organ impairment.
If severe cell injury and cell death does not occur in the major organs of septic patients, why do these patients die? Among patients with sepsis in the ICU, most deaths are not the direct result of organ failure. Studies show that cardiac output is maintained until shortly before death. Renal failure occurs but dialysis prevents fatal metabolic derangements, and liver dysfunction rarely progresses to fulminant hepatic failure. Most deaths occur when the patient’s family and physicians decide that the prognosis for meaningful recovery is poor and ongoing efforts are not consistent with patient goals, leading to a shift from aggressive support to comfort measures only.
The take-home message is that clinicians caring for patients with sepsis should follow the fundamentals outlined in the Surviving Sepsis Campaign, including adequate volume resuscitation based upon clinical and laboratory findings, such as urine output, lactate levels and central venous oxygen saturation. Moreover, the sepsis community needs to shift attention to potential new approaches. In oncology, immunotherapy has led to a paradigm shift in patient care, resulting in lengthy remissions and improved durable survival. Given the similarities of the immunologic defects in patients with cancer and those with sepsis, immunotherapy likely offers great promise for the treatment of sepsis9, 10 and multiple clinical trials of immunoadjuvants are currently underway. Effective immunotherapy to restore host immunity might lead to control of the initial infection and prevent new secondary infections. An effective host immune response is the key to reducing organ failure in sepsis, and the lack of substantial cell death in the major organs of patients who have died of the disorder suggests that recovery is possible if infections can be controlled.
Footnotes
Competing interests statement
R.S.H. has received grant support from Medimmune, Bristol-Myers Squibb, and GlaxoSmithKline. T.J.G. declares no competing interests.
Contributor Information
Thomas J. Graetz, Department of Anesthesiology, Washington University, School of Medicine
Richard S. Hotchkiss, Departments of Anesthesiology, Medicine, and Surgery, Washington University School of Medicine, Campus Box 8054, 660 South Euclid, St. Louis, Missouri 63110, USA
References
- 1.Dellinger RP, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2012;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Gordon AC, et al. Levosimendan for the prevention of acute organ dysfunction in sepsis. N. Engl. J. Med. 2016 doi: 10.1056/NEJMoa1609409. http://dx.doi.org/10.1056/NEJMoa1609409. [DOI] [PubMed]
- 3.Hawiger J, Veach RA, Zienkiewicz J. New paradigms in sepsis: from prevention to protection of failing microcirculation. J. Thromb. Haemost. 2015;13:1743–1756. doi: 10.1111/jth.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marik PE. The demise of early goal-directed therapy for severe sepsis and septic shock. Acta Anaesthesiol. Scand. 2015;59:561–567. doi: 10.1111/aas.12479. [DOI] [PubMed] [Google Scholar]
- 5.Morelli A, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–1691. doi: 10.1001/jama.2013.278477. [DOI] [PubMed] [Google Scholar]
- 6.Mira JC, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit. Care Med. 2016 doi: 10.1097/CCM.0000000000002074. http://dx.doi.org/10.1097/ccm.0000000000002074. [DOI] [PMC free article] [PubMed]
- 7.Hotchkiss RS, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Takasu O, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am. J. Respir. Crit. Care Med. 2013;187:509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N. Engl. J. Med. 2014;371:380–383. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Opal S. Immunotherapy for sepsis — a new approach against an ancient foe. N. Engl. J. Med. 2010;363:87–89. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]