Abstract
Background
Atrial fibrillation (AF) occurs in 30% of patients with mitral regurgitation referred for surgery. However, the underlying mechanisms in this population are poorly understood. The purpose of this study was to examine the effects of acute left atrial volume overload on atrial electrophysiology and the inducibility of AF.
Methods
Ten canines underwent insertion of an atrioventricular shunt between the left ventricle and left atrium. Shunt and aortic flows were calculated and the shunt was titrated to a shunt fraction to 40–50% of cardiac output. An epicardial plaque with 250 bipolar electrodes was used to determine activation and refractory periods. Biatrial pressures and volumes, conduction times, and atrial fibrillation inducibility were recorded. Data were collected at baseline and 20 minutes following shunt opening and closure.
Results
Mean shunt flow was 1.3±0.5L/min with a shunt fraction of 43±6% simulating moderate to severe mitral regurgitation. Compared to baseline, left atrial volumes and maximum pressures increased by 27% and 29% respectively following shunt opening. Biatrial effective refractory periods did not change significantly following shunt opening or closure. Conduction times increased by 9% with shunt opening and returned to baseline after closure. There were no changes in atrial fibrillation duration or inducibility with shunt opening.
Conclusions
This canine model of mitral regurgitation demonstrated that acute left atrial volume overload did not increase the inducibility of atrial arrhythmias in contrast with experimental and clinical findings of chronic left atrial volume overload. This suggests that the substrates for AF in patients with mitral regurgitation are a result of chronic remodeling.
Keywords: Animal Model, Atrial Fibrillation, Electrophysiology, Mitral Regurgitation
Atrial fibrillation (AF) is the most common arrhythmia in the world, occurring in about 1% of the population.(1) Despite its wide prevalence, the underlying mechanisms leading to the development of AF have been difficult to characterize. Left atrial dilatation (LA) has been associated with an increased incidence of AF in many studies.(2, 3) This has been observed in both experimental animal models and clinical conditions leading to LA dilation such as mitral regurgitation (MR). Up to 30% of patients with MR presenting for a mitral valve procedure have a history of AF.(4) This relationship between LA dilation and AF has been hypothesized by some to be due to the mechano-electrical feedback induced by LA stretch.(5, 6) In order to better understand the complex mechanisms by which LA dilatation induces AF, several animal models reproducing left atrial dilation have been developed.
Ex vivo Langendorff-perfused models have been used to study the relationship between atrial electrophysiology and atrial dilation. In rabbit hearts, it has been shown that acute dilatation decreased atrial effective refractory period (AERP) and increased the inducibility of AF.(7) However, Langendorff-perfused dog hearts have shown no change in AERP, but also revealed an increased inducibility of AF.(8) These studies implicating a definite role of acute left atrial dilatation in AF induction have several limitations which confound the clinical interpretation of these data. These shortcomings include the use of non-physiological pressures and dilatation, and the ex vivo, crystalloid preparations which are far removed from the clinical setting.
Few studies exist describing left atrial dilation in acute intact animal models and these have yielded conflicting results. Sideras and colleagues have shown increased AERP with no change in AF induction percentage through volume overload by rapid saline infusion.(9) Satoh and Zipes have shown increased AERP with increased AF inducibility with saline infusion.(10) Wijffels et al. have shown no change in AERP and no change in the ability to induce AF after Haemaccel infusion.(11) These studies all attempted to characterize atrial electrophysiology by increasing the circulating volume with crystalloid solutions and do not account for the cyclic changes in LA volume. In contrast, the overall circulating volume is fixed during MR.
Part of the inconsistency in intact models may have been due to differences in the methodology used to create atrial dilatation. Some studies have examined MR induced atrial dilatation and AF in a chronic in vivo chordae cut model. These models are clinically relevant, however, the chordae cut models result in varying and uncontrollable amounts of MR and are not reversible.(12)
To date, there have been no studies describing the mechanisms of AF in a reversible setting of acute MR in an intact animal. This study used a titratable atrioventricular shunt to produce acute LA volume overload in an intact canine model.(13–15) We wanted a model that would accurately reproduce the regurgitant fraction seen in moderate to severe MR clinically and specifically avoided the use of unphysiological conditions that have plagued previous ex-vivo models. In addition, the shunt model produces the same changes in volume and pressures, both in magnitude and phase, as clinical MR. The goal of this study was to define the electrophysiological changes in the right and left atria following acute LA volume overload, as seen clinically in the setting of MR, and to examine their reversibility. These findings have great clinical relevance, since if acute stretch induces AF, mitral valve repair or replacement alone should restore sinus rhythm, while if the etiology of AF in these patients is due to chronic remodeling, this would require a concomitant ablation procedure.
MATERIAL AND METHODS
Surgical Procedure
The study protocol was approved by the Washington University School of Medicine Animal Studies Committee. All animals involved in this study received human care in compliance with the 2011 “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Science and Published by the National Institutes of Health.(16)
Ten mongrel canines weighing between 23 and 29.5 kg were used in this study. Anesthetic induction was achieved using IV Propofol (7mg/kg). Dogs were intubated with a 7.5–8.5 cuffed endotracheal tube, and mechanically ventilated with volume-controlled ventilation. Anesthesia was maintained using 1–3% inhaled isoflurane. Monitoring included a limb-lead electrocardiogram (ECG) and pulse oximetry. An esophageal temperature probe and heating pad were used to keep temperatures >37.5°C. Through a subinguinal incision, the femoral artery and vein were cannulated to monitor arterial and central venous pressure (CVP) respectively. A 7fr catheter catheter was inserted through a femoral venous sheath to the level of the right atria (RA) to monitor CVP. All dogs received a continuous infusion of normal saline and a 1L bolus at the start of each case to achieve a CVP of 6–8 mmHg. Arterial blood gas samples were drawn every 30 minutes to determine oxygen tension, acid-base balance, and electrolyte levels and normalized as needed. Phenylephrine was initiated for systolic blood pressures <70 mmHg and aggressively weaned.
A median sternotomy was performed and pericardium opened. A 16–20mm flow probe connected to a TS420 Perivascular Flow Module (Transonic, Ithaca, NY) was placed around the ascending aorta to determine cardiac output. The animals were heparinized with a dose 150 units/kg for a goal activated clotting time >350 seconds which was checked every 30 minutes. The intraatrial groove was dissected and using a double pledgeted 4-0 Prolene pursestring suture, a custom shunt made from 8mm polyester conduit (Maquet, Rastatt, Germany) joined to two 24fr angled venous cannula tips attached at both ends (Figure 1), was inserted into the left atria (LA) medial to the right pulmonary veins. The shunt was occluded using a saline occlusion balloon and a 12mm flow probe was placed around the shunt to determine shunt flow. Two separate pledgeted 4-0 Prolene pursestring sutures were placed at the left ventricular (LV) apex and the ventricular side of the shunt was inserted after making a stab incision using an 11-blade. The shunt was de-aired using a 23-gauge needle. An M-Turbo ultrasound machine and TEEx 8-3MHz transesophageal echocardiography (TEE) probe (Sonosite, Bothell, Washington) were used to confirm cannula placement and aid in deairing of the LA and LV. Two separate 5fr Mikro-Tip pressure catheters (Millar, Houston, Texas) were advanced into the LA and RA through the left superior pulmonary vein and superior vena cava respectively and secured with pursestring sutures. Three custom contoured epicardial plaques with 250 bipolar electrodes and interelectrode distance of 5mm were sutured to the anterior and posterior surfaces of both atria.
Figure 1.
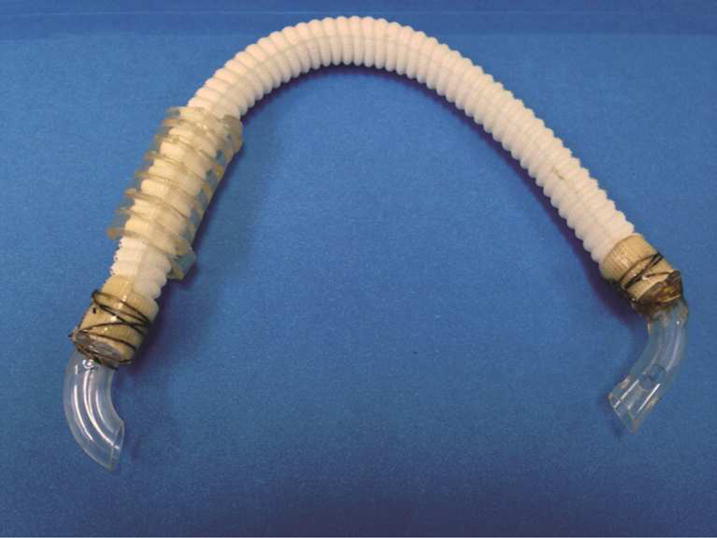
Atrioventricular shunt using woven collagen embedded polyester and venous cannula. The shorter venous cannula (left side) is placed into the LA at the right superior pulmonary venous confluence. The longer perforated venous cannula (right side) is placed into the LV apex. Kinks are prevented using a coiled piece of cardiopulmonary bypass tubing for radial support.
Electrograms were recorded simultaneously from all 250 electrodes on a custom data acquisition system. Electrograms were amplified at a gain of 250, filtered from 0.5–500Hz and digitized 1000Hz. Activation times were determined by the time of the maximum –dV/dt of the electrogram. Activation times were determined at all 250 sites. These activation times were displayed as isochronous maps on a three-dimensional model of the atria.
Experimental Protocol
Atrial pressures, volumes, and diameters were recorded at baseline with the shunt in place but occluded. Atrial pressures were recorded as both a maximum and minimum pressures for a given cardiac cycle. Atrial volumes and diameters were measured in accordance with the 2005 American Society of Echocardiography’s guidelines for chamber quantification and obtained by a single cardiothoracic anesthesiologist.(17) LA volume was calculated as the mean of 4-chamber and 2-chamber measurements obtained with TEE using Simpson’s rule. RA volume was calculated from an epicardial apical 4-chamber view obtained with a P21× 5-1MHz ultrasound transducer (Sonosite, Bothel, Washington) using Simpson’s rule. LA diameter was recorded as the distance between the posterior LA wall and mitral valve annulus and RA diameter was recorded as the distance between the posterior RA wall and tricuspid valve annulus. Both volumes and diameters were measured at end ventricular systole, just prior to mitral valve opening.
Baseline AERPs and pacing thresholds were recorded from 6 points: the anterior RA appendage (RAA), the anterior LA appendage (LAA), the posterior base of LAA, the posterior base of RAA, the posterior LA along the inferior vena cava (IVC), and the posterior RA along the IVC. Pacing was performed at twice the threshold. Eight stimuli with a basic cycle length of 300ms (S1) followed by a single extrastimulus at decreasing cycle lengths (S2) were used to obtain AERP. After determining AERP at all 6 points, 4 attempts were made to induce sustained AF by burst pacing with a cycle lengths from 50 to 100ms or 15 seconds from the site with the lowest recorded AERP. Sustained AF was defined as any episode lasting longer than 180 seconds. All episodes of AF that occurred during the study were recorded and their duration noted. Electrograms were recorded during normal sinus rhythm and pacing. Activation times were determined from the maximum amplitude of the bipolar deflection. These were used to create activation maps during NSR and while pacing at baseline, with the shunt open, and following closure of the shunt.
Once all baseline data were collected, the saline occlusion band was deflated causing systolic retrograde flow from the LV to the LA. This was confirmed using TEE (Figure 2). A goal shunt fraction (Shunt fraction=shunt flow/(cardiac output+shunt flow)) of 40–50% was created by titrating the degree of occlusion. Once the desired shunt fraction was achieved, a 20-minute stabilization period was allowed. Atrial pressure and volume measurements, as well as electrophysiologic data, were again recorded with the shunt open. The shunt flow and CO were monitored throughout the study and remained stable during the recording period. The shunt was then occluded, and following a 20-minute stabilization period all data were recorded a third time with the shunt closed.
Figure 2.
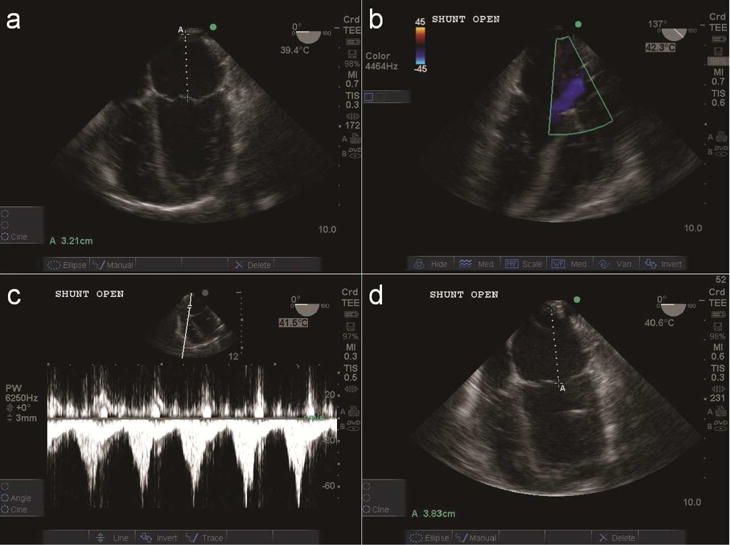
Representative echocardiogram a) Measuring LA diameter of 3.21cm at baseline b) Shunt open with Doppler c) Shunt velocity at atrial cannula d) Enlargement of LA diameter to 3.83cm after shunt opening.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or as median with range. Categorical variables were expressed as frequencies and percentages with outcomes compared using the χ2 or the Fisher exact test. Continuous variables were compared using a repeated measures analysis of variance. Multiple comparisons were made using contrasts and were corrected for multiple comparisons. All data analyses were performed using SYSTAT 13 software (Systat Software, Inc., Chicago, IL).
RESULTS
Atrioventricular Shunt Model Stability and Hemodynamics
Vitals during the study are listed in Table 1. Heart rate at baseline was 123±16 bpm and did not change with shunt opening or closure (p=0.644). Mean systolic and diastolic blood pressures similarly did not change with shunt opening or closure (p=0.723 and p=0.789 respectively). A shunt flow of 1.3±0.5 L/min was achieved, which resulted in a shunt fraction of 43±6%. Cardiac output at baseline was 2.2±0.6 L/min and dropped significantly to (1.6±0.5 L/min, p=0.017) with the shunt open and did not recover once the shunt was closed (1.5±0.3 L/min, p=0.007) when compared to baseline. There was a small increase in baseline temperature (37.3±1.1°C) following shunt opening (38.4±0.7°C) which persisted through closure (38.4± 0.9°C). Oxygen saturation was 98±3% and did not change throughout the study.
Table 1.
Atrioventricular Model: Temperature, Oxygenation, and Hemodynamics
| Variable | Baseline | Shunt Open | Shunt Closed | Overall P-value | P - value Baseline - Open | P - value Baseline - Closed | P - value Open - Closed |
|---|---|---|---|---|---|---|---|
| Temperature(°C) | 37.3±1.1 | 38.4±0.7 | 38.4±0.9 | 0.039 | 0.061 | 0.041 | 0.931 |
| O2 Saturation(%) | 98±3 | 99±1 | 99±2 | 0.440 | —— | —— | —— |
| Heart Rate(bpm) | 123±16 | 124±15 | 121±21 | 0.644 | —— | —— | —— |
| Systolic BP(mmHg) | 114±16 | 122±18 | 119±26 | 0.723 | —— | —— | —— |
| Diastolic BP(mmHg) | 56±12 | 59±12 | 55±16 | 0.789 | —— | —— | —— |
| Cardiac Output(L/min) | 2.2±0.6 | 1.6±0.5 | 1.5±0.3 | 0.003 | 0.017 | 0.007 | 0.527 |
| Shunt Flow(L/min) | 0 | 1.3±0.5 | 0 | —— | —— | —— | —— |
| Shunt Fraction(%) | 0 | 43±6 | 0 | —— | —— | —— | —— |
BP=blood pressure
Atrial Pressure and Volume Measurements
Baseline maximum LA pressure was 10.9±2.4 mmHg and increased to 14.1±2.7 mmHg (29%, p=0.001) with shunt opening (Figure 3). Maximum LA pressure returned near baseline with shunt closure (10.3±3.2 mmHg, p=0.631). Minimum LA pressure was 5.8±2.4 mmHg at baseline and did not change throughout the study. Maximum and Minimum RA pressures were 9.5±3.1 mmHg and 6.1±3.2 mmHg respectively and did not change following shunt opening or closure. LA volumes were 21.3±4.4 ml at baseline and increased to 27.1±5.8 ml during shunting (27%, p=0.002) and dropped below baseline after shunt closure (17.9±3.5 ml, p=0.011) (Figure 4). LA diameters at baseline also increased with shunt opening (3.40±0.25 cm vs. 3.72±0.32 cm, p<0.001) and dropped to near baseline upon closure (3.31±0.35 cm, p=0.625). There were no changes in RA volumes or diameters following shunt opening or closure.
Figure 3.
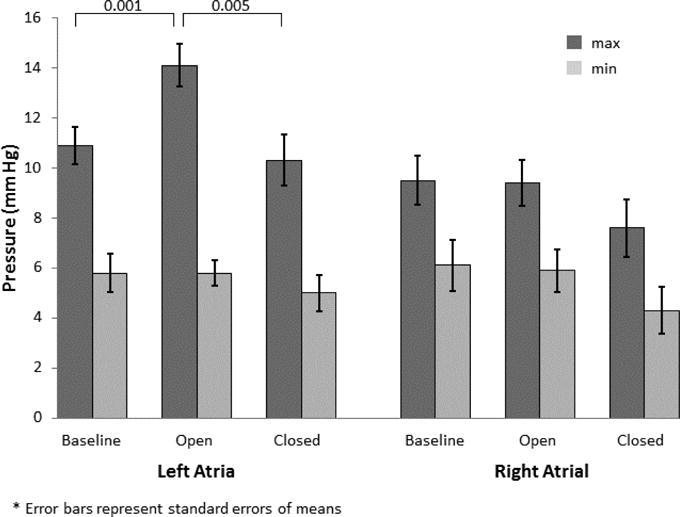
LA ad RA pressures during baseline, while shunt open, and following shunt closure.
Figure 4.
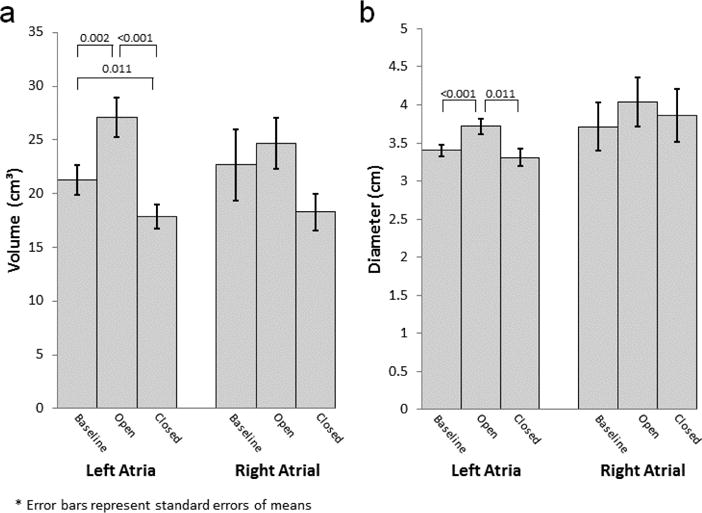
a) LA and RA volumes during baseline, while shunt open, and following shunt closure. b) LA and RA diameters during baseline, while shunt open, and following shunt closure.
Atrial Electrophysiology
AERPs, conduction times, AF durations, and AF induction percentages at baseline, with the shunt open, and following shunt closure are shown in Table 1. AERPs did not change from baseline in any region of the atria during shunt opening or closure. Conduction time increased significantly with shunt opening (77±9ms vs. 84±9ms, p<0.001) and approached baseline after shunt closure (78±9ms, p<0.58). Figure 5 illustrates an increased conduction time when the shunt was opened which decreased when the shunt was closed.
Figure 5.
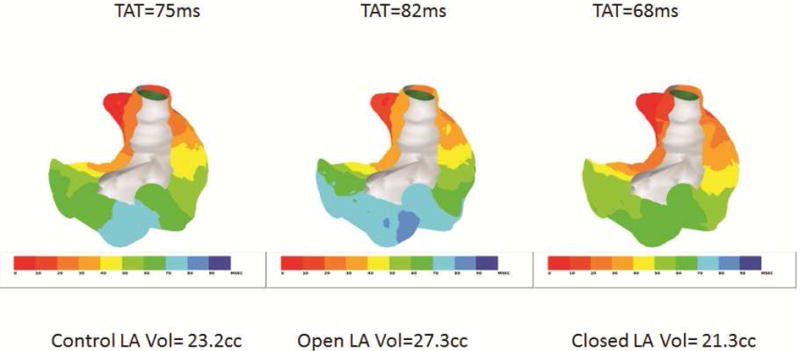
Activation sequence maps of the atria viewed posteriorly. The atria were paced at 300ms cycle length from the RA appendage. The total activation time (TAT) and corresponding LA volumes are shown during control with the shunt closed (left panel), with the shunt opened (center panel) and with the shunt closed (right panel). Each activation map was constructed using 250 electrodes recorded simultaneously.
AF Inducibility
There was no increase AF inducibility with shunt opening, neither was there any noticeable increase in AF duration.
COMMENT
In this study, the effects of acute volume overload in a canine model of reversible mitral regurgitation were examined. Our model of MR allowed for a controllable degree of MR, and also provided a unique opportunity to test the reversibility of the effects of MR.
This model of acute LA volume overload produced from our shunt accurately mimicked moderate to severe MR. The shunt fraction averaged 43% and there was a 27% increase the LA volume. In this physiological model, there was no increase in inducibility of AF with acute volume overload. There were also, no changes in AERP in either atrium. The total conduction time was increased uniformly and significantly by 7ms (~10%) with acute volume overload and returned to baseline when the shunt was closed. These data suggest that the degree of acute stretch seen in the setting of acute MR has little impact the electrophysiology of the normal atria and that the increased incidence of AF observed in patients and in chronic canine models of MR is likely due to the remodeling of the atria and not mechanical-electrical feedback from acute stretch or distention.
In vitro studies have suggested that acute stretch causes significant differences in AF inducibility, refractory period and conduction. In isolated perfused rabbit hearts, Ravelli et al. showed that with increase in the biatrial pressure from 0 to 7 mmHg the inducibility of AF went from 0 to 100%.(7) This same increase in pressure caused the AERP to decrease from 70 to 50ms and returned to the baseline value when the pressure was lowered back to 0 mmHg. The present study is different in several ways. First, the atrial pressures were not held constant like the Ravelli study, in which pressure was increased and held at that level for ten minutes. In the intact heart, the pressure varies during the cardiac cycle under physiological conditions. When the shunt was open in our study, the minimum pressure was 6 mmHg and the maximum was 14 mmHg. There was no change in the AERP with these pressure changes. Second, in the Ravelli study the AERP took 1–2 minutes to stabilize when the pressure increased 7 mmHg. This change in pressure is almost the same as the pulse pressure when the shunt was open. It is possible that AERP in our model did not change because of the rapid increase and decrease in pressure during each cardiac cycle and the delay in the change of AERP with the change in pressure. In addition, there were no changes in the RA pressure or RA volume. Our model is more physiologically relevant to the clinical situation in patients with MR since it is a volume not a pressure overload. Moreover, the intact blood-perfused animal more closely replicates the clinical situation as compared to a crystalloid-perfused isolated heart preparation.
Huang et al, using isolated perfused canine hearts, increased atrial pressure from 0–11 mmHg.(8) They observed no change in AERP with a 38% increase in RA and LA volumes. They noted a slowing of conduction velocity at faster heart rates, increased splitting of waveforms, and areas of low voltages with increased dilation. These were associated with an increase in the dominant frequency of induced AF. This study was similar to the present study in that it showed no changes in AERP, and some slowing of conduction. However, we did not observe the splitting of waveforms. This may have been due to the degree of dilatation that this group used which was out of the physiological realm. It is possible that at some degree of dilatation, the stretch disrupts the cell-to-cell connections causing fractionation of the conduction. However, this is unlikely to be relevant to the volume overload seen in patients with acute MR in which patients develop LA volume overload alone. Lastly, this study was able to induce AF both before and after increasing pressure. This may have been because of the lower and distinctly non-physiological (64–78ms) range of AERP in their study compared to the AERP in our study which ranged from 120–154ms.
In patients with persistent AF undergoing surgery and on cardiopulmonary bypass, Elvan et al. increased and decreased the LA volumes and pressures. The reduction of the volume and pressure resulted in a decrease in AF cycle lengths suggesting that the AERP decreased with decreasing pressure. When they increased the LA volume and pressure, the AF cycle increased suggesting that the AERP increased. This was opposite to what was observed in the rabbit study of Ravelli et al.(7) Elvan et al. also observed increased conduction delay with increased LA volume and pressure and this could contribute to the changes in atrial fibrillation cycle lengths.(18) The atria of these patients however were already likely to have undergone remodeling making this study more relevant to chronic MR and not acute MR. In our study which mimics acute MR, the total conduction time increased but there was no evidence of local conduction block as the increase was uniform over the entire LA. This increase may have been due to a general slowing of conduction.
The degree of dilation and increase in pressure in these studies is greater than that which occurs clinically in moderate to severe MR and therefore they have limited physiologic and clinical relevance. Moreover, a number of these studies equalized RA and LA pressures, as opposed to our study, which replicated physiological MR which increases LA volume but not RA volume.
In chronic animal models of moderate to severe MR, Verheule et al. demonstrated an increased AERP with increased heterogeneity of conduction.(12) These findings were associated with increased areas of LA fibrosis and inducibility of AF. Compared with our acute study, these data suggest that the underlying mechanism for AF in MR may be a result of the chronic remodeling and not directly as a result of the stretch of the LA.
Limitations
Our study has several limitations. The experiments were done in an open chest with an open pericardium. As a result, some of the changes in volume and pressure could be different than those observed in an intact chest with a closed pericardium. However, in an intact animal, the changes in volumes would probably be even less than those observed with the open chest and pericardium. There was a decrease in the cardiac output over the course of the study suggesting that the extended period of cardiac anesthesia may have had an effect on the mechanics and electrophysiology. However, this effect was probably not significant since all the volumes, pressures and electrophysiological parameters returned to near baseline values when the shunt was closed. The flow pattern into the LA, in this model, is different from clinical MR. However, the shear stresses due to the different flow patterns are much smaller than the wall stress generated by the chamber pressure. Since blood is a Newtonian fluid at physiologic pressures, the changes in volume and pressure should be independent of the direction the blood enters the LA. In this acute study we were able to control the shunt fraction, however in a chronic study changes in the compliance of the LA and changes in LV and LA pressure would dynamically alter the shunt fraction. But this would also occur in clinical MR.
Conclusion
This study demonstrated that the acute dilation of the atria that occurs with moderate to severe MR does not directly increase the inducibility of AF. In this model, the conduction times were prolonged uniformly in the atria, but there were no changes in AERP. Both the increased volume, pressures, and conduction times were acutely reversible with elimination of the MR. The model provides a unique opportunity to test the electrophysiological consequences and the reversibility of the changes in the atria that occur with MR. Future studies are planned in a chronic model to further elucidate the mechanisms of arrhythmogenesis in volume-overloaded left atria.
Table 2.
Pressure-Volume Data
| Variable | Baseline | Shunt Open | Shunt Closed | Overall P-value | P - value Baseline - Open | P - value Baseline - Closed | P - value Open - Closed |
|---|---|---|---|---|---|---|---|
| LAP max(mmHg) | 10.9±2.4 | 14.1±2.7 | 10.3±3.2 | 0.002 | 0.001 | 0.631 | 0.005 |
| LAP min(mmHg) | 5.8±2.4 | 5.8±1.6 | 5.0±2.3 | 0.336 | —— | —— | —— |
| RAP max(mmHg) | 9.5±3.1 | 9.4±2.9 | 7.6±3.6 | 0.065 | —— | —— | —— |
| RAP min(mmHg) | 6.1±3.2 | 5.9±2.7 | 4.3±2.9 | 0.063 | —— | —— | —— |
| CVP(mmHg) | 6.9±2.3 | 6.5±2.6 | 5.8±2.6 | 0.110 | —— | —— | —— |
| LA Volume(ml) | 21.3±4.4 | 27.1±5.8 | 17.9±3.5 | <0.001 | 0.002 | 0.011 | <0.001 |
| LA Diameter(cm) | 3.40±0.25 | 3.72±0.32 | 3.31±0.35 | 0.001 | <0.001 | 0.625 | 0.011 |
| RA Volume(ml) | 22.7±10.5 | 24.7±7.6 | 18.3±5.4 | 0.109 | —— | —— | —— |
| RA Diameter(cm) | 3.71±1.00 | 4.04±1.01 | 3.86±1.03 | 0.124 | —— | —— | —— |
LA=left atrial, RA= right atrial, LAP=left atrial pressure, RAP=right atrial pressure, CVP=central venous pressure
Table 3.
Atrial Electrophysiology
| Variable | Baseline | Shunt Open | Shunt Closed | Overall P-value | P - value Baseline - Open | P - value Baseline - Closed | P - value Open - Closed |
|---|---|---|---|---|---|---|---|
| AERP | |||||||
| Anterior RA(ms) | 127±12 | 123±18 | 133±28 | 0.405 | —— | —— | —— |
| Anterior LA(ms) | 128±16 | 128±14 | 124±16 | 0.520 | —— | —— | —— |
| Posterior LAA(ms) | 131±17 | 128±28 | 123±17 | 0.818 | —— | —— | —— |
| Posterior LA IVC(ms) | 141±24 | 134±22 | 120±16 | 0.161 | —— | —— | —— |
| Posterior RAA(ms) | 149±17 | 149±21 | 154±17 | 0.193 | —— | —— | —— |
| Posterior RA IVC(ms) | 140±15 | 136±20 | 135±23 | 0.819 | —— | —— | —— |
| Total CT(ms) | 77±9 | 84±9 | 78±9 | <0.001 | <0.001 | 0.58 | <0.001 |
| Induced AF(%) | 28% | 15% | 37% | 0.210 | —— | —— | —— |
| AF Duration(sec)* | 2[0–180] | 0[0–82] | 6[0–120] | 0.891 | —— | —— | —— |
Median [Range]
AERP=atrial effective refractory period, AF=atrial fibrillation, LA=left atrial, RA= right atrial, CT=conduction time LA=left atrial, RA= right atrial, LAA=left atrial appendage, RAA=right atrial appendage, IVC=inferior vena cava
Acknowledgments
The authors would like to acknowledge the technical assistance of Naomi R. Still and P. Diane Toeniskoetter.
This research was supported by National Institute of Health grants R01 HL032257 and T32 HL007776 and the Barnes-Jewish Hospital Foundation. RJD receives research grants and educational funding from AtriCure and Edwards.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke; a journal of cerebral circulation. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Larson MG, Levy D, Evans JC, Benjamin EJ. Distribution and categorization of echocardiographic measurements in relation to reference limits: The framingham heart study: Formulation of a height- and sex-specific classification and its prospective validation. Circulation. 1997;96(6):1863–1873. doi: 10.1161/01.cir.96.6.1863. [DOI] [PubMed] [Google Scholar]
- 3.Damiano RJ, Jr, Schwartz FH, Bailey MS, Maniar HS, Munfakh NA, Moon MR, Schuessler RB. The cox maze iv procedure: Predictors of late recurrence. The Journal of thoracic and cardiovascular surgery. 2011;141(1):113–121. doi: 10.1016/j.jtcvs.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gammie JS, Haddad M, Milford-Beland S, Welke KF, Ferguson TB, Jr, O’Brien SM, Griffith BP, Peterson ED. Atrial fibrillation correction surgery: Lessons from the society of thoracic surgeons national cardiac database. The Annals of thoracic surgery. 2008;85(3):909–914. doi: 10.1016/j.athoracsur.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 5.Nazir SA, Lab MJ. Mechanoelectric feedback and atrial arrhythmias. Cardiovascular research. 1996;32(1):52–61. [PubMed] [Google Scholar]
- 6.Eckardt L, Kirchhof P, Breithardt G, Haverkamp W. Load-induced changes in repolarization: Evidence from experimental and clinical data. Basic research in cardiology. 2001;96(4):369–380. doi: 10.1007/s003950170045. [DOI] [PubMed] [Google Scholar]
- 7.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated langendorff-perfused rabbit heart. Circulation. 1997;96(5):1686–1695. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 8.Huang JL, Tai CT, Lin YJ, Ting CT, Chen YT, Chang MS, Lin FY, Lai WT, Chen SA. The mechanisms of an increased dominant frequency in the left atrial posterior wall during atrial fibrillation in acute atrial dilatation. Journal of cardiovascular electrophysiology. 2006;17(2):178–188. doi: 10.1111/j.1540-8167.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 9.Sideris DA, Toumanidis ST, Thodorakis M, Kostopoulos K, Tselepatiotis E, Langoura C, Stringli T, Moulopoulos SD. Some observations on the mechanism of pressure related atrial fibrillation. European heart journal. 1994;15(11):1585–1589. doi: 10.1093/oxfordjournals.eurheartj.a060433. [DOI] [PubMed] [Google Scholar]
- 10.Satoh T, Zipes DP. Unequal atrial stretch in dogs increases dispersion of refractoriness conducive to developing atrial fibrillation. Journal of cardiovascular electrophysiology. 1996;7(9):833–842. doi: 10.1111/j.1540-8167.1996.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 11.Wijffels MC, Kirchhof CJ, Dorland R, Power J, Allessie MA. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: Roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation. 1997;96(10):3710–3720. doi: 10.1161/01.cir.96.10.3710. [DOI] [PubMed] [Google Scholar]
- 12.Verheule S, Wilson E, Everett Tt, Shanbhag S, Golden C, Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation. 2003;107(20):2615–2622. doi: 10.1161/01.CIR.0000066915.15187.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beeri R, Yosefy C, Guerrero JL, Abedat S, Handschumacher MD, Stroud RE, Sullivan S, Chaput M, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA. Early repair of moderate ischemic mitral regurgitation reverses left ventricular remodeling: A functional and molecular study. Circulation. 2007;116(11 Suppl):I288–293. doi: 10.1161/CIRCULATIONAHA.106.681114. [DOI] [PubMed] [Google Scholar]
- 14.Braunwald E, Welch GH, Jr, Sarnoff SJ. Hemodynamic effects of quantitatively varied experimental mitral regurgitation. Circulation research. 1957;5(5):539–545. doi: 10.1161/01.res.5.5.539. [DOI] [PubMed] [Google Scholar]
- 15.Rankin JS, Nicholas LM, Kouchoukos NT. Experimental mitral regurgitation: Effects on left ventricular function before and after elimination of chronic regurgitation in the dog. The Journal of thoracic and cardiovascular surgery. 1975;70(3):478–488. [PubMed] [Google Scholar]
- 16.Guide for the care and use of laboratory animals. 8th. Washington (DC): 2011. [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing G, American Society of Echocardiography’s G, Standards C, European Association of E Recommendations for chamber quantification: A report from the american society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Elvan A, Adiyaman A, Beukema RJ, Sie HT, Allessie MA. Electrophysiological effects of acute atrial stretch on persistent atrial fibrillation in patients undergoing open heart surgery. Heart rhythm: the official journal of the Heart Rhythm Society. 2013;10(3):322–330. doi: 10.1016/j.hrthm.2012.10.041. [DOI] [PubMed] [Google Scholar]


