Abstract
The anaphylaxis response is frequently associated with food allergies, representing a significant public health hazard. Recently, exposure to tick bites and production of specific IgE against α-galactosyl (α-Gal)-containing epitopes has been correlated to red meat allergy. However, this association and the source of terminal, non-reducing α-Gal-containing epitopes have not previously been established in Brazil. Here, we employed the α-1,3-galactosyltransferase knockout mouse (α1,3-GalT-KO) model and bacteriophage Qβ-virus like particles (Qβ-VLPs) displaying Galα1,3Galβ1,4GlcNAc (Galα3LN) epitopes to investigate the presence of α-Gal-containing epitopes in the saliva of Amblyomma sculptum, a species of the Amblyomma cajennense complex, which represents the main tick that infests humans in Brazil. We confirmed that the α-1,3-galactosyltransferase knockout animals produce significant levels of anti-α-Gal antibodies against the Galα1,3Galβ1,4GlcNAc epitopes displayed on Qβ-virus like particles. The injection of A. sculptum saliva or exposure to feeding ticks was also found to induce both IgG and IgE anti-α-Gal antibodies in α-1,3-galactosyltransferase knockout mice, thus indicating the presence of α-Gal-containing epitopes in the tick saliva. The presence of α-Gal-containing epitopes was confirmed by ELISA and immunoblotting following removal of terminal α-Gal epitopes by α-galactosidase treatment. These results suggest for the first known time that bites from the A. sculptum tick may be associated with the unknown etiology of allergic reactions to red meat in Brazil.
Keywords: Amblyomma sculptum, Alpha-Gal, Tick saliva, IgE, Red meat allergy
1. Introduction
Ticks are efficient ectoparasites that feed on a wide range of hosts such as mammals, reptiles, birds and amphibians. During blood feeding, ticks may introduce many pathogenic microorganisms such as protozoan parasites, viruses or bacteria (Ramamoorthi et al., 2005; Randolph, 2009). In addition, the many salivary proteins introduced by ticks into their host can inhibit hemostasis, decrease inflammation processes and modulate the immune system (Brossard and Wikel, 2004; Valenzuela, 2004; Francischetti et al., 2009; Kotal et al., 2015). The effects of tick bites on the immune response are still poorly understood. Here we focus on the production of IgE antibody in response to the cross-reactive carbohydrate determinant galactose-α-1,3-galactose-β-1,4-N-acetylglucosamine (Galα1,3Galβ1,4GlcNAc, or Galα1,3LacNac) as a potential mediator of red meat allergy (Commins et al., 2009). This antigen is found in glycoproteins and glycolipids of many mammalian species as well as other organisms, but it is not present in Old World non-human primates and humans due to the loss of the α1,3-galactosyltransferase (α1,3-GalT) responsible for its synthesis (Galili et al., 1987; Galili and Swanson, 1991). Therefore, the α-galactosyl (α-Gal) epitope constitutes a potent non-self marker in human immunology and is a major cause of xenotransplant rejection (Galili, 2005).
Animal models of food allergy have been explored in attempts to clarify mechanisms of sensitization to food proteins; much attention has been focused on the immune response associated with the production of antigen-specific immunoglobulin IgE and hypersensitivity responses upon allergen challenge (Berin and Mayer, 2009). An α-1,3-galactosyltransferase knockout (α1,3-GalT-KO) mouse has been developed and used primarily in the xeno-transplantation field (Thall et al., 1995; Tearle et al., 1996). These animals are also of interest for studies of carbohydrate immunogenicity, since exceptionally high titers of anti-α-Gal IgG antibodies can be elicited upon immunisation (Abdel-Motal et al., 2009a,b, 2010). They therefore represent a unique model among the nonhuman primates, being similar to humans in the context of α-Gal epitope expression. Here we describe the use of this animal model to test the generation of anti-α-Gal antibody production by both natural (tick saliva containing α-Gal epitopes) and unnatural (bacteriophage Qβ virus-like particles (Qβ-VLPs) displaying the α-Gal epitope) sources. The latter experiments relate to both the use of carbohydrate-bearing VLPs as analytical reagents and as immunogenic platforms for the display of tumour-associated carbohydrate antigens (Yin et al., 2013).
The Galα1,3LacNac epitope was recently identified in the intestinal tract of the European tick Ixodes ricinus, and this finding was correlated with red meat allergy in Sweden (Hamsten et al., 2013a,b). Although allergic reactions to red meat are not common, cases of allergic late phase reaction in patients with IgE to the α-Gal epitope have recently been reported in the U.S. (Commins et al., 2011; Commins and Platts-Mills, 2013), Australia (Van Nunen et al., 2009), Germany (Jappe, 2012), France (Morisset et al., 2012), and Japan (Sekiya et al., 2012). In 2006, patients also were reported with severe anaphylactic reactions induced by high IgE antibody titers against the monoclonal antibody cetuximab, which bears the α-Gal epitope (O’Neil et al., 2007). The major allergenic foods studied in Brazil are associated with fish, egg, milk, wheat, peanut, soy and corn (Boye, 2012). In other parts of the world, bites from tick species I. ricinus and Amblyomma americanum have been identified as a major cause of this sensitization (Commins et al., 2011; Hamsten et al., 2013a). In Brazil, individuals frequently exposed to Amblyomma sculptum may react with IgE production against the tick saliva, as reported for other species, providing some evidence for a link between red meat allergy and tick bites in this part of the world. The related A. sculptum, used in this work, was recently classified as one of the species of the Amblyomma cajennense complex. This species is widely distributed in central and southern Brazil, Paraguay and northern Argentina, and it is the leading species that humans are frequently exposed to in the study area (Beati et al., 2013; Estrada-Pena et al., 2014; Nava et al., 2014). As ixodid ticks constitute an assorted group of more than 720 species (Barker and Murrell, 2004; Nava et al., 2014), it is common for their parasitism to extend to a wide range of animals including humans. A rural lifestyle, common in Brazil, elevates the risk of exposure to ticks (Farlow et al., 2004), making humans accidental hosts for different species of ticks. We suspect that, similar to A. americanum, bites from A. sculptum may also produce high titers of IgE and induce anaphylaxis. Here we report the existence of the terminal α-Gal-containing epitope(s) in the saliva of the Brazilian A. sculptum tick, and the capacity of this epitope to induce specific IgE antibodies in the α1,3-GalT-KO mouse previously sensitised with injected tick saliva or by the tick bite.
2. Materials and methods
2.1. Ticks
Tick saliva was obtained by inducing partially and fully engorged adult females of A. sculptum to salivate using the pilocarpine induction method (Tatchell, 1967) with modification. Briefly, A. sculptum ticks engorging naturally on the horses maintained at the experimental farm of the Federal University of Minas, located at Pedro Leopoldo city, Minas Gerais, Brazil, were carefully harvested, rinsed in distiled water, and fixed to glass microscope slides with double-sided tape. Salivation was induced by injecting 2 μL of pilocarpine (2% in PBS, Sigma–Aldrich, MO, USA) into the hemocoel of the tick using a 50 μL syringe (Hamilton, USA) connected to a manual repeater dispenser (Hamilton). Ticks were incubated at 35 °C in a humid chamber and saliva was collected with a 10 μL micropipette every 5 min until salivation ceased (2–3 h). Volumes ranged from 2.5 to 50 μL per tick. The total protein content of the saliva was measured by the bicinchoninic acid assay (BCA) method (Protein Reagent kit, PierceTM, USA).
2.2. Mice
All animals and experiments were handled in strict accordance with the guidelines of the Research Ethics Committee of the Federal University of Minas Gerais, (UFMG), Belo Horizonte, Brazil and approved under the protocol number 137/2011. Female C57Bl/6 mice (6–8 weeks old), having disrupted alleles of the α1,3-GalT gene (Thall et al., 1995; Milland et al., 2006) (α1,3GalT-KO), were used. These mice have the H-2b genetic background and are bred and maintained at the animal facility of UFMG.
2.3. α-Gal antigen linked to Qβ-VLP and conjugate preparation
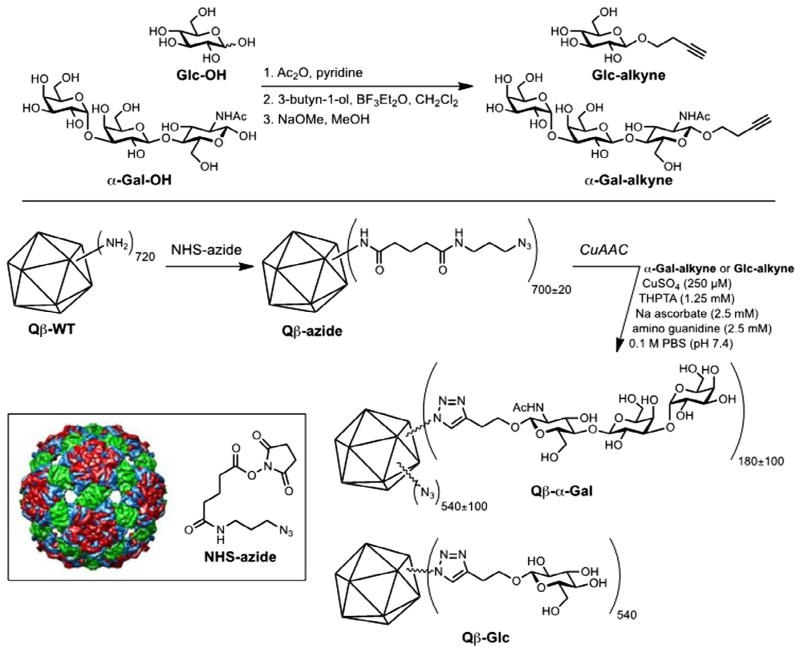
Qβ-VLPs were prepared and purified as described previously (Hong et al., 2009; Fiedler et al., 2010). All particles were characterised by size-exclusion chromatography, dynamic light scattering (DynaPro, Wyatt Technology, USA), microfluidic gel electrophoresis (Agilent Bioanalyzer 2100, using Protein 80 chips), and electrospray ionization mass spectrometry on an accuratemass time-of-flight instrument (Agilent G6230B); representative samples were further examined by transmission electron microscopy and multi-angle light scattering (Viscotec, Malvern Instruments, UK). In all cases, standard properties of size and composition were observed, with the particles showing narrow size distributions and high protein purity (less than 5% protein impurities detected). Protein concentrations in solution were measured with the BCA method (Protein Reagent kit, PierceTM, USA), standardised with BSA. For conjugate preparation, α-Gal trisaccharide (α-Gal-OH, Carbosynth US, LLC, San Diego, CA, USA) and glucose were converted to their respective alkyne derivatives by Lewis acid-mediated glycosylation of 3-butyn-2-ol. Each alkyne was attached to Qβ-VLPs by a two-step procedure in which the protein nanoparticle was first acylated with an azide-terminated N-hydroxysuccinimide ester and then addressed by copper-catalysed azide-alkyne cycloaddition.
2.4. Mice sensitization for antibody detection
We initially verified the competence of these α1,3-GalT-KO mice, previously immunised, to produce antibodies (primarily IgG) against α-Gal epitopes. Immunisation was performed using the following protocol: a group of 10 mice were s.c. injected with 10 μg per dose, four doses, one per week, of the antigen consisting of the bacteriophage Qβ-VLP to which approximately 540 copies of the Galα3LN epitope (Qβ(Galα3LN)540) were attached by covalent chemical ligation as described in Section 2.3. A control group of 10 mice was immunised with unmodified Qβ-VLP. Mice were sensitised by tick saliva using two methods. A standard protocol for tick feeding on mice using feeding chambers as described by Bouchard and Wikel in 2005 (Bouchard and Wikel, 2005) was slightly modified, as follows. Groups of 10 mice were anesthetised i.p. with 100 mg/kg of ketamine and 10 mg/kg of xylazine (Uniao Quimica, Brazil), using a tuberculin syringe (BD Safety-LokTM, USA). Once fully anesthetised, a feeding chamber was assembled on each mouse’s back and two ticks (one male and one female) per mouse were placed into the chamber for 9 days feeding. For artificial inoculation, we injected tick saliva collected as above (20 μg protein per s.c. dose) once per week for 4 weeks. All mice were humanely euthanised 72 h after the last immunisation and the sera were collected for the antibody detection by ELISA, as described in Section 2.6.
2.5. Purification of mouse IgG anti-α-Gal antibody and IgE enrichment
IgG anti-α-Gal antibodies were isolated by the procedure of Galili et al. (1988). A 1 mL pool of sera obtained from α1,3GalT-KO mice immunised with Qβ(Galα3LN)540 was run over a column of silica beads covalently bound with the αGal epitope (Synsorb, Chembiomed, Edmonton, Alberta, Canada) or melibiose-agarose (M-5889, Sigma–Aldrich). The column was then washed extensively with PBS, and bound antibodies were eluted with Gly–HCl buffer, pH 2.8. Purified antibody was applied into the affinity chromatography column over Sepharose-immobilised protein G (Sigma–Aldrich) for IgG purification. The same procedure was performed for IgE enrichment by removing IgG from sera of sensitised mice. The eluent was adjusted to pH 7.0 using 15–20 mL of 0.5% NaOH, diluted 1:1 with PBS-Tween 20 (0.05%) and analysed by ELISA and SDS–PAGE.
2.6. ELISA for α-Gal epitope and anti-α-Gal antibody detection
To aid in the identification of the α-Gal epitope in A. sculptum tick saliva, we used the purified polyclonal mouse IgG anti-α-Gal antibody obtained as described in Section 2.5. Mushroom Marasmius oreades (MOA) lectin-horseradish peroxidase (HRP) conjugate (EY Laboratories, USA), which binds specifically to blood group B and terminal Gal-α1,3Gal residues (Winter et al., 2002), was also used to confirm α-Gal-epitope presence in the A. sculptum saliva. High-binding ELISA plates (NUNC, Thermo Scientific, USA) were coated (overnight at 4 °C, or 1 h at room temperature) with 10 μg/mL of diluted tick saliva from fully or partially engorged ticks in 50 mM carbonate-bicarbonate buffer pH 9.5. The Qβ (Galα3LN)540 antigen (5 μg/mL) was used as a positive control; Qβ(Glc)540 was used as a negative control. After overnight incubation, free microplate binding sites were blocked with 2% BSA (Sigma Aldrich) in PBS, pH 7.4. The coated plates with the antigens were incubated with purified mouse IgG anti-α-Gal antibody (2 μg/mL) or MOA-HRP conjugate (5 μg/mL). Plates were then sequentially incubated with 50 μL of biotinylated anti-mouse IgG (1:2500 dilution for purified mouse anti-α-Gal) (Amersham, GE Healthcare Life Sciences, UK) in PBS-BSA, and 50 μL of streptavidin-HRP conjugate (1:4000 dilution, for both purified mouse anti-α-Gal IgG and MOA-HRP agglutinin conjugate) (Amersham) in PBS-BSA 2%. All incubation steps were performed at 37 °C for 1 h. The reaction was developed with 100 μL of peroxidase substrate SigmaFastTM OPD (o-phenylenediamine dihydrochloride and urea hydrogen peroxide, Sigma–Aldrich) and the reaction stopped by addition of 2 N sulphuric acid. The absorbance unit measurements were performed in a Multiskan GO instrument, using SkanIt 3.2 software (Thermo Scientific). An ELISA assay also was performed as described above for anti-α-Gal (IgG/IgE) antibody detection against the Qβ-α-Gal particles and the tick saliva α-Gal epitope.
To evaluate the production of specific antibodies in response to the α-Gal epitope present in the saliva of A. sculptum, serum samples collected from the various immunised groups (mice receiving tick bites directly, mice receiving s.c. injection of tick saliva and mice receiving the Qβ(Galα3LN)540 particles) were evaluated by ELISA as described above against Qβ(Galα3LN)540 or Qβ(Glc)540 particles deposited on the plate. In this case, however, the secondary antibody was mouse monoclonal anti-IgE and anti-IgG (Amersham) at a dilution of 1:2000 for IgE and IgG detection. To determine antibody specificity, the VLP conjugates were placed on 96 wells plate and treated overnight at 28 °C with 0.1 U/well of α-galactosidase enzyme (from green coffee beans, Sigma–Aldrich, G8507). After the incubation, the ELISA was performed as described.
2.7. SDS–PAGE and protein immunoblotting
SDS–PAGE analyses (12.5% gels) were carried out using the Mini-Protean® 3 cell (Bio-Rad, USA) according to the manufacturer’s instructions with silver staining using the Dodeca Silver Stain Kit (Bio-Rad). For Western blotting, 10 μg of protein of tick saliva were electrotransferred from one-dimensional gel to nitro-cellulose membranes at 400 mA for 90 min. Blots were blocked overnight with 3% BSA in PBS, and then washed with PBS containing 0.05% Tween 20. After blocking, transferred proteins were incubated for 2 h at 37 °C with pooled sera of sensitised α1,3GalT-KO mice fed on by ticks (previous protocol) or with α1,3GalT-KO naïve mouse sera at 1:100 dilution. After washes, the blots were incubated with a HRP-labelled anti-mouse IgE (Sigma–Aldrich) at 1:2,500. Incubations were performed at 37 °C for 1 h, and the washes were carried out at room temperature for 15 min per wash for a total of three washes per step. Immunoreaction was developed with ECL substrate (Thermo Scientific) and their images were scanned with an ImageScanner (Amersham Biosciences).
2.8. Statistical analysis
All data analyses were carried out using Graphpad Prism 5. Single comparisons were made using a Mann–Whitney test, multiple comparisons with a Kruskal–Wallis with Dunn’s multiple comparison test (non-parametric). The level of significance was set at P < 0.05.
3. Results
3.1. Anti-α-Gal IgG production by α-GalT-KO mice immunised with Qβ-α-Gal particle
While the α-Gal epitope has been displayed in a variety of ways for different applications (Abdel-Motal et al., 2009b, 2010; Galili et al., 2010), here we used the bacteriophage Qβ-VLP (Strable and Finn, 2009; Fiedler et al., 2012) to display a large number of the trisaccharide units (an average of 540 per particle) as shown in Fig. 1.
Fig. 1.
Glycoconjugate and nanoparticle preparation. α-Gal trisaccharide (Galα(1,3)Galβ(1,4)GlcNAcβ) or, simply, α-Gal-OH (Carbosynth US, LLC, San Diego, CA) and glucose were converted to their respective alkyne derivatives. Each alkyne was attached to bacteriophage Qβ virus-like particles by a two-step procedure in which the protein nanoparticle was first acylated with an azide-terminated N-hydroxysuccinimide ester and then addressed by copper-catalysed azide-alkyne cycloaddition.
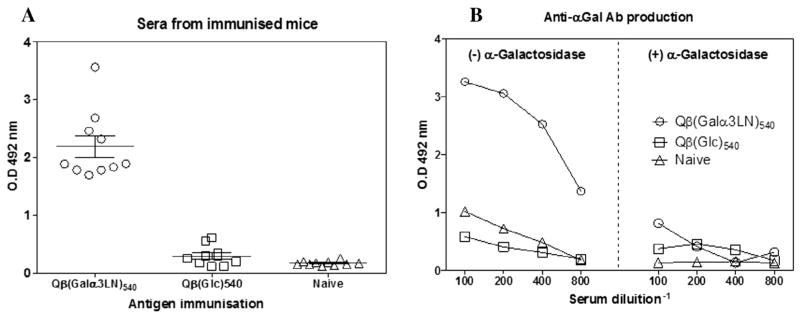
In humans, a large quantity of anti-α-Gal antibody is produced in response to antigenic stimulation by gastrointestinal Gram-negative bacteria expressing the α-Gal-containing epitopes on their surface lipopolysaccharides (Galili, 1988). We used a α1,3-GalT-KO mouse (Thall et al., 1995) to mimic the human sensitivity to the trisaccharide and test the ability of Qβ(Galα3LN)540 particles to induce high titers of anti-α-Gal antibodies (primarily IgG) after immunisation. A group of 10 α1,3-GalT-KO mice was immunised four times, once per week with 10 μg of Qβ(Galα3LN)540. The sera were collected 1 week after the last immunisation. Anti-α-Gal antibody titers were found to be much higher than the naïve group or the group receiving the negative control particle Qβ(Glc)540 (Fig. 2A), overwhelming the usually significant response to the Qβ capsid protein, represented here by the response to the Glcdecorated particle. To assess the specificity of the response, the VLP-α-Gal conjugate immobilised on the ELISA plate was treated overnight at 28 °C with α-galactosidase from green coffee beans, followed by analysis of the serial diluted pooled sera of α1,3-GalT-KO mice as before. This resulted in a loss of approximately 90% of IgG binding (Fig. 2B), verifying that a strong and specific anti-α-Gal immune response was generated. These immunised mouse groups were also tested for IgE antibody production, showing undetectable titers of IgE against Qβ(Galα3LN)540 particles (data not shown).
Fig. 2.
Anti-α-Gal antibody (IgG) produced by α-1,3-galactosyltransferase knockout (α-GalT-KO) mice. (A) Anti-α-Gal antibody detection from individual α1,3-GalT-KO mice immunised with the antigens Galα(1,3)Galβ(1,4)GlcNAc, named Qβ(Galα3LN)540 or glucose, designated as (Qβ-Glc)540, and naïve non-immunised control animals. The antigen immobilised on the plate was the assembled bacteriophage Qβ-α-Gal virus like particle. (B) Pooled sera of immunised mice with the Qβ(Galα3LN)540 or (Qβ-Glc)540 antigen were titrated down to 1:800 dilution. Immobilised antigens were then treated with the enzyme green coffee bean α-galactosidase overnight at 28 °C for α-Gal epitope removal. Groups consisted of 7–10 mice per group and three independent experiments were performed.
3.2. Identification of α-Gal-containing epitopes in the A. sculptum saliva
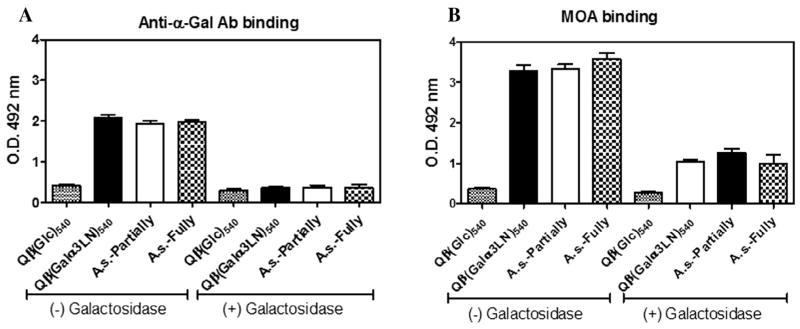
The α-Gal-containing epitopes from salivary proteins of Ixodes holocyclus have previously been characterised as allergenic and have been linked to production of specific IgE in sera of individuals allergic to this tick (Gauci et al., 1988a,b). We sought to identify α-Gal-containing epitope(s) in the tick saliva of A. sculptum by ELISA using the collected tick saliva of both partially and fully engorged ticks. To detect the α-Gal epitope, we used two reagents: anti-α-Gal polyclonal antibodies purified by affinity chromatography from the sera of mice inoculated with Qβ(Galα3LN)540, as described in Section 2, and the lectin MOA, a type B blood group-specific lectin with high specificity for the blood group B-like determinant (Gal-α1,3-Gal-β1,4-GlcNAc), which is widely expressed in mammalian species but absent in humans and catarrhines (Old World non-human primates) (Elo and Estola, 1952; Galili, 1988; Winter et al., 2002; Cordara et al., 2011). ELISA analysis revealed very strong binding of both reagents to tick saliva using purified IgG anti-α-Gal antibody (Fig. 3A), or MOA lectin, (Fig. 3B) indicating the presence of α-Gal-like epitope(s). Consistent with this, pretreatment with green coffee bean α-galactosidase largely abolished binding in either circumstance (Figs. 3A, B).
Fig. 3.
α-Gal epitope detected by ELISA in Amblyomma sculptum tick saliva. (A) Purified mouse IgG anti-α-Gal antibody (2 μg/mL) and (B) Marasmius oreades agglutinin (5 μg/ mL), which binds specifically to terminal, non-reducing α-Gal epitopes, were used in the ELISA to identify the α-Gal epitope in the A. sculptum tick saliva that was obtained from fully and partially engorged A. sculptum female ticks (A. sculptum-Fully and A. sculptum-Partially, respectively). Both experiments were tested for anti-α-Gal-binding specificity by previous overnight incubation with green coffee bean α-galactosidase. O.D. at 492 nm. Qβ-glucose (Qβ-Glc)540 also was used as a control. All experiments were performed in triplicate.
3.3. IgE detection in sera of α1,3-GalT-KO sensitised by A. sculptum tick saliva
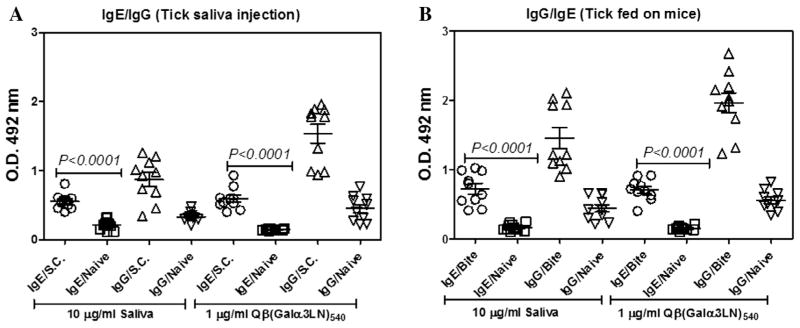
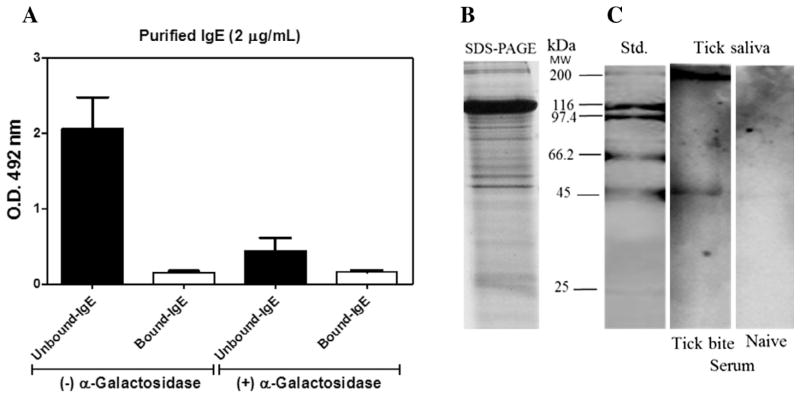
IgE mediates anaphylaxis responses that are pathogenic in allergic diseases such as allergic rhinitis, asthma, atopic dermatitis and food allergy (Gould and Sutton, 2008). Here we evaluated the capacity of α1,3-GalT-KO mice to produce IgE antibodies by tick saliva sensitization using two protocols. First, a group of 10 α1,3-GalT-KO mice were sensitised (four weekly s.c. injections of A. sculptum saliva, 20 μg per dose), and sera were collected 72 h after the final boost. Second, A. sculptum ticks were allowed to feed on α1,3-GalT-KO mice for 9 days. In each case, sera were collected after the exposure or inoculation regimen and analysed for anti-α-Gal IgE and IgG responses with Qβ(Galα3LN)540 as the probe reagent on the ELISA plate. Significant serum IgE levels, and even higher IgG levels, were observed for sera from both groups (s.c. injected tick saliva is shown in Fig. 4A and tick-feeding is shown in Fig. 4B) at 1:50 serum dilution. Interestingly, we observed a significant (P < 0.0001) increase in IgE production under both protocols compared with the naïve animal group (Fig. 4A, B). While these αGalT-KO mice therefore showed the type of specific IgE response expected for a model of allergic reactions, we attempted to improve the sensitivity of IgE ELISA detection by removing the IgG antibodies by affinity chromatography over Sepharose-immobilised protein G (Sigma–Aldrich). This IgG removal resulted in a three to fivefold enhancement of the IgE antibody signal against the α-Gal-containing epitope present in tick saliva (Fig. 5A). Even under these more sensitive conditions, we were unable to detect an IgE response when saliva samples were incubated overnight with α-galactosidase enzyme (Fig. 5A). The profile of tick saliva proteins revealed by SDS–PAGE is shown in Fig. 5B. IgE was observed by immunoblotting only for sera from sensitised mice. Two protein bands with relative molecular masses at approximately 200 and 45 kDa emerged as the major saliva components recognised by the serum of α1,3-GalT-KO mice subjected to blood feeding by A. sculptum ticks (Fig. 5C).
Fig. 4.
Detection of anti-α-Gal antibodies. (A) α-1,3-Galactosyltransferase knockout (α1,3-GalT-KO) mice were sensitised by s.c. injection of four doses, once per week, of 10 μg of Amblyomma sculptum saliva and serum was collected 72 h later. (B) Two ticks were placed on the back of α1,3-GalT-KO mice to feed for 9 days. After feeding, ticks were detached and mouse sera collected 72 h later for ELISA. In both protocols, individual sera were tested against 10 μg/mL of tick saliva or 1 μg/mL of Qβ(Galα3LN)540 particle immobilised on the plate. Each group contained 6–10 mice and independent experiments were performed in triplicate. IgE/s.c. was compared with IgE/Naïve by a two-tailed Mann Whitney test performed using Prism Graph Pad. O.D. at 492 nm.
Fig. 5.
IgG removal and tick saliva pattern of recognition by IgE. (A) ELISA assay using 2 μg/mL of purified IgE. IgG antibody was removed from pooled sera by affinity chromatography and unbound IgE was measured against 10 μg/mL of tick saliva. For the anti-α-Gal binding specificity, tick saliva was previously incubated overnight at 28 °C with α-galactosidase. (B) Profile of proteins from Amblyomma sculptum tick saliva as detected by silver-stained SDS–PAGE. (C) Immunoblotting revealing the pattern of the tick saliva proteins recognised by specific IgE produced in a pool of sera collected from α-1,3-galactosyltransferase knockout (α1,3-GalT-KO) mice sensitised by tick blood feeding. Unbound-IgE and bound-IgE correspond to the flow-through and retained samples over a Sepharose-immobilised protein G column, respectively. All experiments were performed in triplicate.
4. Discussion
While information regarding the prevalence and incidence of food allergies and other food sensitivities in developing countries is growing (Boye, 2012), more research is clearly needed. In Brazil, food allergies from several different sources (mainly fish, egg and milk) are thought to produce similar clinical symptoms, including constant cutaneous manifestation. In one study, fish was the major source of allergen, with higher titers of IgE antibody detected in patients (79%) compared with controls (28%) (Naspitz et al., 2004; Sanchez and Sanchez, 2015). Allergies induced by tick bites, in contrast, have only recently been appreciated as a world-wide problem (van Nunen, 2015). The connection between red meat allergy and tick bites was first described in Australia in 2009, in which study the authors correlated 24 of 25 patients with a history of tick bite followed by the development of an allergic response to red meat (Van Nunen et al., 2009).
Galactose-α-1,3-galactose (Galα1,3Gal) has been identified as the major antigen from tick salivary protein responsible for triggering IgE production, causing a delayed anaphylactic reaction to red meat (Commins et al., 2011). Recently, the epitope Galα3LN was identified in gastrointestinal extract of I. ricinus, and this Galα3LN epitope was recognised by sera from patients previously reported with red meat allergies (Hamsten et al., 2013a). Since A. sculptum has been reported as the main species that infests both humans and dogs in central and southeastern states of Brazil (Labruna et al., 2002), we sought to confirm our hypothesis that the Galα3LN structure, and particularly the trisaccharide Galα(1,3 )Galβ(1,4)GlcNAc or similar epitope(s), could be responsible for A. sculptum-mediated allergic response.
The studies described here rely on the antigenic display of the Galα3LN carbohydrate on the modified surface of the bacteriophage Qβ-VLP (Kaltgrad et al., 2007; Yin et al., 2013). Approximately 540 molecules of the carbohydrate were efficiently attached by the copper-catalysed azide-alkyne bioconjugated reaction to the VLP, representing a fairly dense array of the glycan epitope. Immunisation with the resulting particle elicited a potent IgG anti-α-Gal response in α-GalT-KO mice, which was found to overwhelm the response to the coat protein. The α1,3-GalT-KO mouse was also therefore validated as an excellent source for anti-α-Gal antibody production. A polyclonal preparation of such antibodies was used to conclusively detect α-Gal-like or α-Gal-containing epitope( s) in A. sculptum tick saliva, validated with a known blood group B-like lectin and the loss of binding after treatment with an α-galactosidase. However, since we have not carried out the structural characterisation of these a-Gal-containing epitope(s), the precise chemical structure(s) of the glycan(s) remain to be determined.
During blood-feeding most ixodid ticks produce cement proteins that facilitate attachment to the host, followed by the synthesis of a complex set of proteins in the salivary gland (Bowman and Sauer, 2004). The mechanisms of action of tick saliva proteins have attracted much attention recently, although two common allergens (28 and 35 kDa) were identified previously by radio-immunoassay and Western blot analysis (Gauci et al., 1988a). In a study performed in 2006, an allergen from Argas at the 44 kDa band also was recognised by sera from 19 patients previously reported to have been bitten by the European pigeon tick, Argas reflexus, demonstrating the sensitization of these patients and the IgE production against this protein band (Kleine-Tebbe et al., 2006). In our case, 200 and 45 kDa proteins, as yet unidentified, have been found as the probable major α-Gal carriers in the saliva of A. sculptum ticks.
We also showed that sensitization of the α1,3-GalT-KO mouse with tick saliva using two different methods led to the production of a robust IgE response against Galα3LN. Interestingly, we were unable to observe detectable levels of IgE antibodies against Galα3LN in α1,3-GalT-KO mice sensitised with Qβ(Galα3LN)540 particles (data not shown), in contrast to the potent IgG response in these animals. This suggests that the salivary protein(s) bearing the α-Gal-like antigen(s) modulate(s) the immune response in a different way than VLP display, since the former induces robust IgE production.
The link between tick bites and red meat allergy has been confirmed in Australia, Europe and the U.S., where sera from allergic patients was shown to recognise α-Gal-containing epitope(s) in tick saliva as the allergen respondent. Identifying this type of epitope in the saliva of the A. sculptum tick, a common species in Brazil, leads us to hypothesise that related cases of red met allergy may also be associated with tick bites. This work will continue with studies of sera from allergic patients, and with the identification of the α-Gal-bearing protein in tick saliva, to confirm or refute this hypothesis.
Acknowledgments
This study was supported in part by grants (A.F.M and RNA), from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ # 470737/2013-1), Brazil, Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG), Brazil, and by the National Institutes of Health (NIH), USA (R01 CA149451). ICA is partially supported by NIH/NIMHD grant 2G12MD007592, and is a Special Visiting Researcher of the Science Without Borders Program, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
References
- Abdel-Motal UM, Wigglesworth K, Galili U. Intratumoral injection of alpha-gal glycolipids induces a protective anti-tumor T cell response which overcomes Treg activity. Cancer Immunol Immunother. 2009a;58:1545–1556. doi: 10.1007/s00262-009-0662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Motal UM, Wigglesworth K, Galili U. Mechanism for increased immunogenicity of vaccines that form in vivo immune complexes with the natural anti-Gal antibody. Vaccine. 2009b;27:3072–3082. doi: 10.1016/j.vaccine.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Abdel-Motal UM, Wang S, Awad A, Lu S, Wigglesworth K, Galili U. Increased immunogenicity of HIV-1 p24 and gp120 following immunization with gp120/p24 fusion protein vaccine expressing alpha-gal epitopes. Vaccine. 2010;28:1758–1765. doi: 10.1016/j.vaccine.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SC, Murrell A. Systematics and evolution of ticks with a list of valid genus and species names. Parasitology. 2004;129(Suppl):S15–S36. doi: 10.1017/s0031182004005207. [DOI] [PubMed] [Google Scholar]
- Beati L, Nava S, Burkman EJ, Barros-Battesti DM, Labruna MB, Guglielmone AA, Caceres AG, Guzman-Cornejo CM, Leon R, Durden LA, Faccini JL. Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae), the Cayenne tick: phylogeography and evidence for allopatric speciation. BMC Evol Biol. 2013;13:267. doi: 10.1186/1471-2148-13-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berin MC, Mayer L. Immunophysiology of experimental food allergy. Mucosal Immunol. 2009;2:24–32. doi: 10.1038/mi.2008.72. [DOI] [PubMed] [Google Scholar]
- Bouchard K, Wikel SK. Care, maintenance, and experimental infestation of ticks in the laboratory setting. In: Marquart WC, editor. Biology of Disease Vectors. Elsevier Academic Press; San Diego: 2005. pp. 705–711. [Google Scholar]
- Bowman AS, Sauer JR. Tick salivary glands: function, physiology and future. Parasitology. 2004;129(Suppl):S67–S81. doi: 10.1017/s0031182004006468. [DOI] [PubMed] [Google Scholar]
- Boye JI. Food allergies in developing and emerging economies: need for comprehensive data on prevalence rates. Clin Transl Allergy. 2012;2:25. doi: 10.1186/2045-7022-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossard M, Wikel SK. Tick immunobiology. Parasitology. 2004;129(Suppl):S161–S176. doi: 10.1017/s0031182004004834. [DOI] [PubMed] [Google Scholar]
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354–359. doi: 10.1097/ACI.0b013e3283624560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, Woodfolk JA, Platts-Mills TA. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–433. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TA. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–1293. e1286. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordara G, Egge-Jacobsen W, Johansen HT, Winter HC, Goldstein IJ, Sandvig K, Krengel U. Marasmius oreades agglutinin (MOA) is a chimerolectin with proteolytic activity. Biochem Biophys Res Commun. 2011;408:405–410. doi: 10.1016/j.bbrc.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Elo J, Estola E. Phytagglutinins present in Marasmius oreades. Ann Med Exp Biol Fenn. 1952;30:165–167. [PubMed] [Google Scholar]
- Estrada-Pena A, Tarragona EL, Vesco U, Meneghi D, Mastropaolo M, Mangold AJ, Guglielmone AA, Nava S. Divergent environmental preferences and areas of sympatry of tick species in the Amblyomma cajennense complex (Ixodidae) Int J Parasitol. 2014;44:1081–1089. doi: 10.1016/j.ijpara.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63:1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- Fiedler JD, Brown SD, Lau JL, Finn MG. RNA-directed packaging of enzymes within virus-like particles. Angew Chem Int Ed Engl. 2010;49:9648–9651. doi: 10.1002/anie.201005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler JD, Higginson C, Hovlid ML, Kislukhin AA, Castillejos A, Manzenrieder F, Campbell MG, Voss NR, Potter CS, Carragher B, Finn MG. Engineered mutations change the structure and stability of a virus-like particle. Biomacromolecules. 2012;13:2339–2348. doi: 10.1021/bm300590x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci (Landmark Ed) 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U. The natural anti-Gal antibody, the B-like antigen, and human red cell aging. Blood Cells. 1988;14:205–228. [PubMed] [Google Scholar]
- Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–686. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- Galili U, Swanson K. Gene sequences suggest inactivation of alpha-1,3-galactosyltransferase in catarrhines after the divergence of apes from monkeys. Proc Natl Acad Sci USA. 1991;88:7401–7404. doi: 10.1073/pnas.88.16.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1–3Gal epitope in primates. Proc Natl Acad Sci USA. 1987;84:1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- Galili U, Wigglesworth K, Abdel-Motal UM. Accelerated healing of skin burns by anti-Gal/alpha-gal liposomes interaction. Burns. 2010;36:239–251. doi: 10.1016/j.burns.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Gauci M, Stone BF, Thong YH. Detection in allergic individuals of IgE specific for the Australian paralysis tick, Ixodes holocyclus. Int Arch Allergy Appl Immunol. 1988a;85:190–193. doi: 10.1159/000234501. [DOI] [PubMed] [Google Scholar]
- Gauci M, Stone BF, Thong YH. Isolation and immunological characterisation of allergens from salivary glands of the Australian paralysis tick Ixodes holocyclus. Int Arch Allergy Appl Immunol. 1988b;87:208–212. doi: 10.1159/000234674. [DOI] [PubMed] [Google Scholar]
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- Hamsten C, Starkhammar M, Tran TA, Johansson M, Bengtsson U, Ahlen G, Sallberg M, Gronlund H, van Hage M. Identification of galactose-alpha-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy. 2013a;68:549–552. doi: 10.1111/all.12128. [DOI] [PubMed] [Google Scholar]
- Hamsten C, Tran TA, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, van Hage M. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol. 2013b;132:1431–1434. doi: 10.1016/j.jaci.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong V, Presolski SI, Ma C, Finn MG. Analysis and optimization of copper-catalyzed azide-alkyne cycloaddition for bioconjugation. Angew Chem Int Ed Engl. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jappe U. Update on meat allergy: alpha-Gal: a new epitope, a new entity? Hautarzt. 2012;63:299–306. doi: 10.1007/s00105-011-2266-y. [DOI] [PubMed] [Google Scholar]
- Kaltgrad E, Sen Gupta S, Punna S, Huang CY, Chang A, Wong CH, Finn MG, Blixt O. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. ChemBioChem. 2007;8:1455–1462. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- Kleine-Tebbe J, Heinatz A, Graser I, Dautel H, Hansen GN, Kespohl S, Rihs HP, Raulf-Heimsoth M, Vater G, Rytter M, Haustein UF. Bites of the European pigeon tick (Argas reflexus): risk of IgE-mediated sensitizations and anaphylactic reactions. J Allergy Clin Immunol. 2006;117:190–195. doi: 10.1016/j.jaci.2005.08.056. [DOI] [PubMed] [Google Scholar]
- Kotal J, Langhansova H, Lieskovska J, Andersen JF, Francischetti IM, Chavakis T, Kopecky J, Pedra JH, Kotsyfakis M, Chmelar J. Modulation of host immunity by tick saliva. J Proteomics. 2015;128:58–68. doi: 10.1016/j.jprot.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna MB, de Paula CD, Lima TF, Sana DA. Ticks (Acari: Ixodidae) on wild animals from the Porto-Primavera Hydroelectric power station area, Brazil. Mem Inst Oswaldo Cruz. 2002;97:1133–1136. doi: 10.1590/s0074-02762002000800012. [DOI] [PubMed] [Google Scholar]
- Milland J, Christiansen D, Lazarus BD, Taylor SG, Xing PX, Sandrin MS. The molecular basis for galalpha(1,3)gal expression in animals with a deletion of the alpha1,3galactosyltransferase gene. J Immunol. 2006;176:2448–2454. doi: 10.4049/jimmunol.176.4.2448. [DOI] [PubMed] [Google Scholar]
- Morisset M, Richard C, Astier C, Jacquenet S, Croizier A, Beaudouin E, Cordebar V, Morel-Codreanu F, Petit N, Moneret-Vautrin DA, Kanny G. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67:699–704. doi: 10.1111/j.1398-9995.2012.02799.x. [DOI] [PubMed] [Google Scholar]
- Naspitz CK, Sole D, Jacob CA, Sarinho E, Soares FJ, Dantas V, Mallozi MC, Wandalsen NF, Borges W, Rocha Filho W. Sensitization to inhalant and food allergens in Brazilian atopic children by in vitro total and specific IgE assay. Allergy Project–PROAL. J Pediatr (Rio J) 2004;80:203–210. [PubMed] [Google Scholar]
- Nava S, Beati L, Labruna MB, Caceres AG, Mangold AJ, Guglielmone AA. Reassessment of the taxonomic status of Amblyomma cajennense with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n sp and Amblyomma patinoi n sp., and reinstatement of Amblyomma mixtum, and Amblyomma sculptum (Ixodida: Ixodidae) Ticks Tick Borne Dis. 2014;5:252–276. doi: 10.1016/j.ttbdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- O’Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–3648. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, Fikrig E. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE. Tick-borne disease systems emerge from the shadows: the beauty lies in molecular detail, the message in epidemiology. Parasitology. 2009;136:1403–1413. doi: 10.1017/S0031182009005782. [DOI] [PubMed] [Google Scholar]
- Sanchez J, Sanchez A. Epidemiology of food allergy in Latin America. Allergol Immunopathol (Madr) 2015;43:185–195. doi: 10.1016/j.aller.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Sekiya K, Fukutomi Y, Nakazawa T, Taniguchi M, Akiyama K. Delayed anaphylactic reaction to mammalian meat. J Investig Allergol Clin Immunol. 2012;22:446–447. [PubMed] [Google Scholar]
- Strable E, Finn MG. Chemical modification of viruses and virus-like particles. Curr Top Microbiol Immunol. 2009;327:1–21. doi: 10.1007/978-3-540-69379-6_1. [DOI] [PubMed] [Google Scholar]
- Tatchell RJ. A modified method for obtaining tick oral secretion. J Parasitol. 1967;53:1106–1107. [PubMed] [Google Scholar]
- Tearle RG, Tange MJ, Zannettino ZL, Katerelos M, Shinkel TA, Van Denderen BJ, Lonie AJ, Lyons I, Nottle MB, Cox T, Becker C, Peura AM, Wigley PL, Crawford RJ, Robins AJ, Pearse MJ, d’Apice AJ. The alpha-1,3-galactosyltransferase knockout mouse. Implications for xenotransplantation Transplantation. 1996;61:13–19. doi: 10.1097/00007890-199601150-00004. [DOI] [PubMed] [Google Scholar]
- Thall AD, Maly P, Lowe JB. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem. 1995;270:21437–21440. doi: 10.1074/jbc.270.37.21437. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG. Exploring tick saliva: from biochemistry to ‘sialomes’ and functional genomics. Parasitology. 2004;129(Suppl):S83–S94. doi: 10.1017/s0031182004005189. [DOI] [PubMed] [Google Scholar]
- van Nunen S. Tick-induced allergies: mammalian meat allergy, tick anaphylaxis and their significance. Asia Pac Allergy. 2015;5:3–16. doi: 10.5415/apallergy.2015.5.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190:510–511. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- Winter HC, Mostafapour K, Goldstein IJ. The mushroom Marasmius oreades lectin is a blood group type B agglutinin that recognizes the Galalpha 1,3Gal and Galalpha 1,3Galbeta 1,4GlcNAc porcine xenotransplantation epitopes with high affinity. J Biol Chem. 2002;277:14996–15001. doi: 10.1074/jbc.M200161200. [DOI] [PubMed] [Google Scholar]
- Yin Z, Comellas-Aragones M, Chowdhury S, Bentley P, Kaczanowska K, Benmohamed L, Gildersleeve JC, Finn MG, Huang X. Boosting immunity to small tumor-associated carbohydrates with bacteriophage qbeta capsids. ACS Chem Biol. 2013;8:1253–1262. doi: 10.1021/cb400060x. [DOI] [PMC free article] [PubMed] [Google Scholar]