Abstract
There is a compelling need for more effective vaccine adjuvants to augment induction of antigen specific adaptive immune responses. Recent reports suggested the bacterial second messenger bis-(3′–5′)-cyclic-dimeric-guanosine monophosphate (c-di-GMP) acts as an innate immune system modulator. We recently incorporated a Vibrio cholerae diguanylate cyclase (DGC) into an adenovirus (Ad) vaccine, fostering production of c-di-GMP as well as pro-inflammatory responses in mice. Here, we recombined a more potent DGC, VCA0848, into a non-replicating adenovirus serotype 5 (AdVCA0848) that produces elevated amounts of c-di-GMP when expressed in mammalian cells in vivo. This novel platform further improved induction of type I interferon β (IFN-β) and activation of innate and adaptive immune cells early after administration into mice as compared to control vectors. Co-administration of the extracellular protein ovalbumin (OVA) and the AdVCA0848 adjuvant significantly improved OVA-specific T cell responses as detected by IFN-γ and IL-2 ELISPOT, while also improving OVA-specific humoral B cell adaptive responses. Additionally, we found that co-administration of AdVCA0848 with another Ad5 vector expressing the HIV-1 derived antigen Gag (AdGag) or the Clostridium difficile-derived Toxin B (AdToxB), resulted in significant inhibitory effects on the induction of Gag and ToxB-specific adaptive immune responses. As a proof of principle, these data confirm that in vivo synthesis of c-di-GMP stimulates strong innate immune responses that correlate with enhanced adaptive immune responses to concomitantly administered extracellular antigen, which can be utilized as an adjuvant to heighten effective immune responses for protein-based vaccine platforms against microbial infections and cancers.
Keywords: c-di-GMP, DGC, AdVCA0848, HIV-1 Gag, Ovalbumin, IFN-β, IFN-γ, IL-2
Introduction
With a limited number of adjuvants approved for human administration, there is a pressing need for the development and testing of vaccine adjuvants that can improve the efficacy and maintain the safety profile of vaccines against resilient infectious diseases and cancers (1). The addition of adjuvants to vaccine formulations can serve to significantly improve vaccine efficacy when using less immunogenic antigens (2), to decrease vaccine toxicity by diminishing the need for higher vaccine dosages, or reduce the need for repeated boosting (3).
Many significant cellular functions in bacteria, including regulation of motile/sessile phenotypes, virulence capabilities, and global gene expression are mediated by the second messenger bis-(3′–5′)-cyclic-dimeric-guanosine monophosphate (c-di-GMP) (4). C-di-GMP is generated by diguanylate cyclase (DGC) enzymes combining two guanosine-5′-triphosphate (GTP) molecules (5). In the mammalian cytosol, the presence of c-di-GMP molecules can be detected by nucleotide sensors, including absent in melanoma 2 (AIM2) (6), the DEAD box-containing helicase (DDX41) (7), and stimulator of interferon genes (STING), each of which directly binds to c-di-GMP, resulting in the increased expression of type I interferons (IFNs) and other innate immune responses (8, 9).
The direct administration of c-di-GMP has been shown to induce innate immune responses that can enhance protection of mice against challenges with Klebsiella pneumoniae (10), Staphylococcus aureus (11), methicillin-resistant S. aureus (MRSA) (12), Bordetella pertussis (13), Streptococcus pneumoniae (14), and avian influenza A/H5N1 (15, 16). Specifically, following intranasal challenge with B. pertussis in BALB/c mice, c-di-GMP induced production of cytokines such as IFN-γ, TNF-α, IL-6, and the chemokine MCP-1 in lung tissue (13). Recently, the ability of c-di-GMP to cause robust induction of IFN-β has been shown to attenuate experimental autoimmune encephalitis (EAE) progression and onset through the induction of T regulatory (Treg) cells, which suppress helper/effector T cell responses (17, 18).
The ability of c-di-GMP to trigger mammalian inflammatory responses has recently been harnessed for potential use as a promising vaccine adjuvant (19). Several studies suggest that inclusion of c-di-GMP in vaccine formulations can improve vaccine efficacy so as to provide immune protection against various bacterial infections (13, 20), and cancers (21–23). Local co-administration (intranasal and sublingual) of H5N1 virosomes and c-di-GMP to BALB/c mice resulted in strong H5N1-specific B cell and T cell adaptive immunity, but the intramuscular (i.m.) route of vaccination resulted in significantly less protection (15). A liposome-based delivery system that improved c-di-GMP cell uptake in vivo resulted in IFN-β induction and enhanced tumor-specific cytotoxic T cell activity associated with regression of tumor growth in mice (21). However, this study suggests that pure extracellular c-di-GMP does not efficiently enter target cells.
V. cholerae encodes upwards of 40 unique DGCs, many of which have been shown to synthesize c-di-GMP in this bacterium (24–26). These DGCs have highly divergent synthesis activities (27). Approximately half of these DGCs are thought to be integral inner membrane proteins, while the other half are cytoplasmic. Each contains a unique N-terminal sensory domain that is predicted to be regulated by environmental or host derived cues (28). Tens of thousands of DGCs have been identified across bacterial genomes (29). Thus, these genes offer a wide-range of unique enzymes possessing different properties that can be transduced by vectors to potentially modulate immune responses.
We have recently developed a novel strategy to synthesize high concentrations of c-di-GMP in vivo by transducing a Vibrio cholerae-encoded DGC, VCA0956, via a replication-deficient adenovirus vector in mammalian cells. Transduction of VCA0956 and subsequent production of c-di-GMP stimulated several innate immune responses, including significant induction of IFN-β and other cytokines as well as the expression of several IFN-responsive genes. When co-administered with an adenovirus-based vector carrying the Clostridium difficile-derived Toxin A (TA) antigen, the Ad5 vaccine expressing VCA0956 also enhanced, although moderately, the induction of adaptive immune responses against the TA antigen in mice (30).
In this study, we examined a second DGC encoded by the gene VCA0848 that synthesizes higher levels of c-di-GMP in V. cholerae (unpublished work). This DGC was recombined into the non-replicative Ad5 vector to create the AdVCA0848 transducing particle. AdVCA0848 significantly enhances c-di-GMP production compared to our previous platform, AdVCA0956 by producing ~ 400-fold more c-di-GMP in vivo. Delivery of AdVCA0848 improved the induction of innate immune responses including dramatic inductions of IFN-β. Importantly, co-injection of AdVCA0848 with ovalbumin (OVA) protein resulted in significant enhancement of OVA-specific T cell and B cell adaptive immune responses. However, we also found that inclusion of AdVCA0848 with a second adenovirus-based vaccine transducing either the HIV-1-derived Gag antigen (AdGag) or the C. difficile-derived Toxin B antigen (AdToxB) resulted in the decreased induction of Gag or ToxB-specific adaptive immune responses relative to animals only vaccinated with AdGag or AdToxB. Therefore, in vivo produced c-di-GMP can both enhance and inhibit adaptive immune responses depending on the nature of the co-administered antigen.
Materials and Methods
Vector construction
Adenovirus-based vectors used in this study were all replication-deficient. AdNull and AdGag were constructed as previously described (31, 32). AdVCA0848 was constructed similarly to AdVCA0956 as previously described (30). Briefly, the V. cholerae gene VCA0848 gene (GeneBank sequence: CP007635.1) was sub-cloned into pShuttle-CMV as previously described (33). Primers used for AdVCA0848 construction were: forward: 5′-ATAGGTACCCCACCATGAATGACAAAGTGCT-3′ and reverse: 5′-ATACTCGAGTTAGAAAAGTTCAACGTCATCAGAA-3′. The mutant version of AdVCA0848, AdVCA0848mut, carrying the following amino acid changes: GGEEF > AAEEF in the GGDEF domain of VCA0848 allele was mutated using the QuikChange Lightning site-directed mutagenesis kit (Agilent) with the primer 5′-GTCTTCTCAACTATTTCGCTTTGCTGCTGAAGAGTTCGTGATTATTTTTT-3′. AdToxB was constructed as previously described (34). Briefly, a synthetic gene was designed based on the Clostridium difficile toxin B sequence data from previous studies (35, 36) and ordered from GENEART (Regensburg, Germany). The synthetic gene representing the C-terminal portion of Toxin B, including 617 amino acids (residues 1750–2366), was sub-cloned into pShuttle-CMV as previously described (33). Primers used for AdToxB construction: forward: 5′-GCTACTACGAGGACGGCCTG-3′ and reverse: 5′-CTCATCGATGATCAGCTTGCC-3′. The C-terminal region of the new synthetic gene did not contain the enzymatic domain and recombination and viral propagation were carried out as previously described (30, 33, 37). Constructs were confirmed to be replication-competent adenovirus (RCA) negative using RCA PCR and direct sequencing methods as previously described (32, 38). All procedures with recombinant adenovirus constructs were performed under BSL-2 conditions.
Animal Procedures
The Michigan State University Institutional Animal Care and Use Committee (IACUC) approved the animal procedures conducted in this study. Care was provided to mice in this study in accordance with PHS and AAALAC standards. Mice were purchased from Taconic Biosciences, (Germantown, NY).
To determine the amount of c-di-GMP produced by the AdVCA0848 vector, male 6–8 weeks old Balb/c mice, were intravenously (i.v.) injected (retro-orbitally) with AdNull (n=3), AdVCA0956 (n=4), or AdVCA0848 (n=4) in 200 μl of a phosphate-buffered saline solution (PBS, pH 7. 4) containing 2×1011 viral particles (vps)/mouse; or not injected (naïves) (n=3) as previously described (30). The same viral dose was also used for additional experiments in which mice were injected with AdVCA0848, AdVCA0848mut, or not injected (naïves). At 24 hours post-injection (hpi), mice were sacrificed and liver samples were collected, immediately snap frozen, and used later for c-di-GMP quantification as described below.
For innate immunity studies, 6–10 weeks old male C57BL/6 mice (n=4) were i.v. injected (retro-orbitally) with AdNull or AdVCA0848 in 100 μl of a phosphate-buffered saline solution (PBS, pH 7. 4) containing 1×1010 vps/mouse or not injected (Naïve). The same viral dose was also used for additional experiments in which mice were injected with AdVCA0848, AdVCA0848mut, or not injected (naïves). At 6 hpi, mice were sacrificed. Blood samples were collected and used for ELISA analysis and splenocytes were harvested, counted and used for immune cell surface staining. Liver samples were immediately stored at −80° C for c-di-GMP quantification.
To determine the effect of AdVCA0848 on adaptive immune responses against OVA, male 8–10 weeks old C57BL/6 mice (n=4) were co-injected with AdVCA0848 or AdNull in 30 μl of a phosphate-buffered saline solution (PBS, pH 7. 4) containing 1×1010 vps/mouse via i.m. injection and 100 μg/mouse OVA via intraperitoneal (i.p.) injection, with an additional group of mice which were not injected (naïves). At 6 days post-injection (dpi), retro-orbital bleeding was used to collect blood samples for ELISA analysis. At 14 dpi, mice were sacrificed, peripheral blood samples collected and spleen was harvested in 2% FBS RPMI media.
To determine the effect of AdVCA0848 on the adaptive immune response against the HIV-1-derived Gag antigen, we initially conducted a dose-dependent study to determine the optimum AdVCA0848 dose that would significantly modulate adaptive immunity specific to the co-injected 5×106 vps/mouse dose of AdGag. 6–8 weeks old male BALB/c mice (n= 4) were intramuscularly (i.m.) co-injected in the tibialis anterior with viral particles in a phosphate-buffered saline solution in 30 μl (PBS, pH 7. 4) containing a dose of 5×106 vps of AdGag along with 3 different doses of 5×107, 5×108, or 5×109 vps/mouse of either AdNull or AdVCA0848. An additional group of mice were not injected (naive). Additional experiments were conducted in which mice were co-injected with AdGag at 5×106 vps/mouse and 5×109 vps/mouse of AdVCA0848 or AdVCA0848mut, or not injected (naïves). At 14 dpi, mice were sacrificed, peripheral blood samples collected and spleen was harvested in 2% FBS media. To determine the effect of AdVCA0848 on the adaptive immune response against C. difficile-derived Toxin B antigen, female 6–8 weeks old C57BL/6 mice (n=4) were i.m. co-immunized in the tibialis anterior with viral particles of AdToxB (5×108 vps/mouse) along with 5×108 vps/mouse of either AdGFP or AdVCA0848. At 21 dpi, mice were terminally sacrificed, and blood samples were collected for B cell analysis with ELISA. To verify the expression of Gag protein in the injected mice, 6–8 weeks old male BALB/c mice were i.v. injected with 1×1011 vps/mouse of AdGag only (n=3), or co-injected of 1×1011 vps/mouse of AdGag along with 1×1011 vps/mouse of either AdNull or AdVCA0848. At nearly 24 hpi, mice were humanely sacrificed and liver samples were obtained and frozen at −80° C until analysis by western blot for Gag protein levels.
Quantification of in vivo c-di-GMP synthesis
Liver samples were harvested from mice injected with 2×109 vps/mouse AdVCA0848, or 2×1011 vps/mouse of AdVCA0848, AdVCA0848mut, AdVCA0956, AdNull, or not injected (naïves) as described in the animal procedures. 20 mg from each liver sample was placed in 500 μL PBS and homogenized using an Omni Tissue Homogenizer (Omni International). 300 μL of homogenate was added to an equal volume of equilibrated Phenol Solution (Sigma-Aldrich, St. Louis, MO). The homogenate-phenol solution was then vortexed and centrifuged at 15,000 rpm for 10 minutes. The aqueous phase was removed and added to 500 μL chloroform. The mixture was vortexed and then centrifuged at 15,000 rpm for 10 minutes. The aqueous phase was removed and stored at −80° C until analysis. Quantification of c-di-GMP was conducted by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) at Michigan State University spectrometry & metabolomics core facility as previously described (39).
Western blot for Gag protein
Liver samples from mice injected with AdGag alone, or co-injected with AdGag and AdNull or AdVCA0848 as described above were harvested, and later were homogenized in ice cold lysis buffer containing 1% Triton and complete protease Inhibitor. Supernatant was collected and analyzed for protein concentration (BCA protein kit; Sigma-Aldrich, St. Louis, MO). Total protein of 15 μg was heated at 100° C for 5 min with Laemmli sample buffer (Sigma Aldrich, St. Louis, MO), and samples were loaded on 1 mm-thick 10% gel Mini-Protean TGX Precast Gels (BIO-RAD, Hercules, CA, USA). Transfer was completed overnight at 4° C using a 0.2 um Nitrocellulose membrane (Millipore, Billerica, MA). The membrane was blocked for 1 h in Odyssey® Blocking Buffer (Licor Biosciences - U.S., Lincoln, NE), then incubated for 1 hour at room temperature with primary monoclonal mouse anti Gag (1:10,000) antibody (183-H12-5C) obtained from the NIH-AIDS research and reference reagent program (gift from Dr. Y-H Zheng, Michigan State University), and mouse anti-ß-actin (1:3000) (#8224; Abcam, Cambridge, MA) diluted in Odyssey Blocking Buffer (#927-40000, Licor, Lincoln, NE). The blot was washed with TBS-T three times, and then incubated with labeled anti-mouse secondary antibody (#926-32210; Licor, Lincoln, NE) diluted in blocking buffer (1:10,000) for 1 hour at room temperature. The blotted membrane was washed and developed on the Licor Odyssey (Licor, Lincoln, NE).
ELISA
Effects of AdVCA0848 on IFN-β induction was determined by quantifying IFN-β using the VeriKine™ mouse IFN-β ELISA kit (PBL Assay Science, Piscataway, NJ) according to the manufacturer’s instructions. To determine the effect of AdVCA0848 on B cell adaptive immune responses specific to antigens delivered by the co-administered AdGag or AdToxB, or the extracellular antigen OVA with the use of AdNull or AdVCA0848mut as a negative control, ELISA-based titering experiments were conducted as previously described (40). Briefly, 5×108 vps/well of inactivated Ad5 particles, 0.2 mg/well of Gag protein, 50 μg/well of OVA, or 100 ng/well of ToxB (each diluted in PBS) was used to coat wells of a 96-well plate overnight at 4° C. Plates were washed with PBS-Tween 20 (0.05%) solution, and blocking buffer (3% BSA in PBS) was added to each well and incubated for 1–3 h at room temperature. For measuring total IgG Abs, plasma from injected mice was serially diluted in PBS buffer. Following dilution, plasma was added to the wells and incubated at room temperature for 1 h. Wells were washed using PBS-Tween 20 (0.05%), and HRP-conjugated rabbit anti-mouse Ab (Bio-Rad, Hercules, CA) was added at a 1:5000 dilution in PBS-Tween 20. Tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO) substrate was added to each well, and the reaction was stopped with 2 N sulfuric acid. Optical density (O.D.) was then obtained by reading the plates at 450 nm in a microplate spectrophotometer.
ELISPOT
Splenocytes were harvested from individual mice and red blood cells were lysed using ACK lysis buffer (Invitrogen, Grand Island, NY). Ninety-six–well Multi-Screen high protein binding Immobilon-P membrane plates (Millipore, Billerica, MA) were wetted with 70% ethanol, coated with mouse anti–IFN-γ or IL-2 capture Abs, incubated overnight, and blocked prior to the addition of 5×105 (AdGag studies) or 1×106 (OVA studies) splenocytes/well. Additional studies were conducted using AdVCA0848mut as a control (AdGag studies) with the use of 1×106 splenocytes/well. Ex vivo stimulation included incubation of splenocytes in 100 μl media alone (unstimulated) or media containing 4 μg/ml Gag-specific AMQMLKETI (AMQ) peptide (GenScript, Piscataway, NJ) for the AdVCA0848 and AdGag studies, or 10 μg/ml OVA or SIINFEKL (MHC class I-restricted OVA-derived peptide (41)) for AdVCA0848 and OVA studies, overnight in a 37° C, 5% CO2 incubator. Staining of plates was completed per the manufacturer’s protocol. Spots were counted and photographed by an automated ELISPOT reader system (Cellular Technology, Cleveland, OH). Ready-SET-Go! IFN-γ and IL-2 mouse ELISPOT kits were purchased from eBioscience (San Diego, CA).
Flow Cytometry Analysis
To investigate innate immune responses following AdVCA0848 vaccination, mice were injected with 1×1010 vps/mouse of AdVCA0848 vector and activation of innate immune cells was evaluated 6 hours following i.v. injection. Splenocytes were stained with various combinations of the following antibodies: PE-CD69 (clone: H1.2F3), allophycocyanin-Cy7-CD3 (clone: 145-2C11), PerCP-Cy5.5-CD19 (clone: 1D3), Pacific Blue-CD8α (clone: 53-6.7), and PE-Cy7-NK1.1 (clone: PK136) (4 μg/ml). To assess the effect of AdVCA0848 on dendritic cells (DCs), splenocytes were stained with combinations of the following antibodies: PE-Cy7-CD11c (clone: HL3), allophycocyanin (APC)-Cy7-CD11b (clone: M1/70), Alexa Fluor 700-CD8a (clone: 53-6.7), FITC-CD40 (clone: HM40-3), PerCP-Cy5.5-CD80 (clone: 16-10A1), and V450-CD86 (clone: GL1) (4 μg/ml). All antibodies were obtained from BD Biosciences. To determine the intracellular cytokine levels 14 dpi of AdVCA0848 and AdGag co-injections, intracellular staining was performed as previously described (37). Briefly, splenocytes (2.5×106/well) were stimulated with Gag-specific AMQ peptide for 6 hours with Brefeldin A (BFA) (Sigma-Aldrich, St. Louis, MO) for 30 minutes and stored at 4° C overnight. Cells were washed twice with FACS buffer and surface stained with APC-CD3, Alexa Fluor 700-CD8a, and CD16/32 Fc-block Abs, fixed with 2% formaldehyde (Polysciences, Warrington, PA), permeabilized with 0.2% saponin (Sigma-Aldrich, St. Louis, MO), and stained for intracellular cytokines with PE-Cy7-TNF-α, and Alexa Fluor 488-IFN-γ (4 μg/ml) (all obtained from BD Biosciences, San Diego, CA). We included a violet fluorescent reactive dye (ViViD; Invitrogen) as a viability marker to exclude dead cells from the analysis. Tetramer staining of splenocytes at 1×106 cell/well was performed using PE-labeled MHC class I tetramer folded with the AMQ peptide (generated at the NIH Tetramer Core Facility (Atlanta, GA)) for 30 minutes at room temperature, and for memory T cell staining, a mixture of the following antibodies (at 2 μg/ml) were used: APC-CD3, Alexa Fluor 700-CD8a, PerCP-Cy5.5-CD127, FITC-CD62L, and CD16/32 Fc-block Abs. All antibodies were purchased from BD Biosciences (San Diego, CA). After washing with FACS buffer, data for stained cells were collected with the use of BD LSR II instrument and analyzed using FlowJo software (Tree Star, San Carlos, CA). Gating strategy was based on negative control results (naïves) that were applied consistently across all samples examined. Representative examples from this gating approach are presented here for activation of innate immunity cells and for the frequency of cytokine-producing CD8+ T cells.
Statistical analysis
Statistically significant differences in innate immune responses were determined using a one-way ANOVA with a Student–Newman–Keuls post hoc test (p value of <0.05 was deemed statistically significant). The ELISPOT and ELISA studies were all analyzed using one-way ANOVA with a Student–Newman–Keuls post hoc test (p value of <0.05 was deemed statistically significant). For flow cytometry, a one-way ANOVA with a Student–Newman–Keuls post hoc test was used (p value of <0.05 was deemed statistically significant). Statistical analyses were performed using GraphPad Prism (GraphPad Software).
Results
AdVCA0848 produces significant amounts of c-di-GMP in vivo in mice
We previously demonstrated the feasibility of in vitro and in vivo production of c-di-GMP in mammalian cells by using Ad5 vectors to transduce DGCs (30). Our prior unpublished studies suggested that use of an alternative DGC, VCA0848, which has greater enzymatic activities, might generate a significantly elevated amount of c-di-GMP in vivo. We constructed an Ad5 vector with a CMV enhancer/promoter element to drive VCA0848 expression in mammalian cells. The use of the AdVCA0848 platform resulted in a significant in vivo c-di-GMP production measured in the liver of injected mice. Injecting with increasing viral loads of 2×109 vps/mouse and 2×1011 vps/mouse of AdVCA0848 resulted in approximately 130 μmol/g and 3000 μmol/g c-di-GMP in the liver, respectively. This confirms that the in vivo c-di-GMP production is entirely due to the enzymatic activity of the delivered VCA0848 as AdVCA0848mut vectors and naïve mice failed to produce detectable levels of c-di-GMP (Figure 1). Additionally, when compared to an earlier DGC-expressing platform that was constructed using the exact same adenovirus vector backbone, the AdVCA0848 platform produces significantly higher levels of c-di-GMP in the mouse liver (~ 400-fold increase) than that produced by an equal viral dose of the AdVCA0956 platform per gram of mouse liver (p<0.05). As expected, similar to AdVCA0848mut control, the AdNull vectors, which lack the DGC gene, did not produce detectable levels of c-di-GMP (Supplemental Figure 1). These results confirm the feasibility of transducing the bacterial DGC VCA0848 using Ad5 to synthesize in vivo larger amounts of c-di-GMP in vivo.
Figure 1. Active VCA0848 produces significant amounts of c-di-GMP in mice.
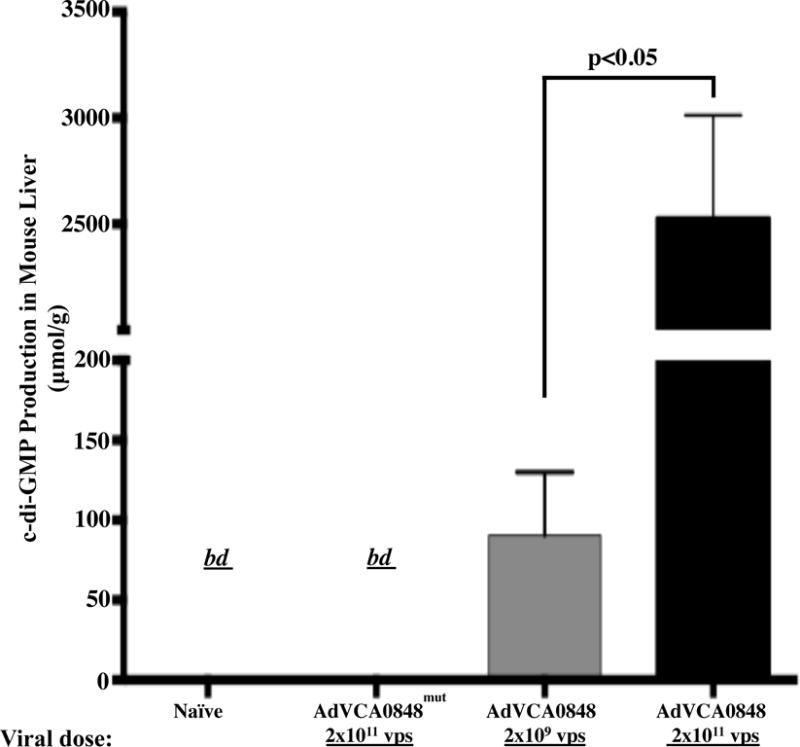
Male 6–8 weeks old BALB/c WT mice were retro-orbitally i.v. injected with 2×109 vps/mouse of AdVCA0848 (n=3); or 2×1011 vps/mouse of AdVCA0848mut (n=3) or AdVCA0848 (n=3). As a control not injected (naïves) mice (n=2) were included. At 24 hpi mice were sacrificed and liver samples were collected, and immediately snap frozen in liquid nitrogen. 20 mg of liver samples were used for c-di-GMP extraction as described in methods section. C-di-GMP production measurements were performed using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Bars represent mean ± SD from different groups. Statistical analysis was completed using One Way ANOVA followed by a Student-Newman-Keuls post-hoc test. A value of p<0.05 was deemed statistically significant. “bd”, below detection.
AdVCA0848 activates innate immune responses
It is thought that activation of beneficial innate immune responses by adjuvants is the underlying mechanism that is critical for achieving effective and long-lived, antigen-specific, adaptive immune responses. Intravenous administration of AdVCA0848 dramatically induced plasma levels of IFN-β (p<0.05) nearly 1000-fold compared to the level produced by the AdNull control (Fig. 2A). Importantly, administration of AdVCA0848mut control produced similar levels of IFN-β, as compared to AdNull, suggesting the increased IFN-β levels following AdVCA0848 is due to the enzymatic activity of the transduced VCA08484 (Supplemental Figure 2A). Also, administration of AdVCA0848 significantly induced DC maturation and NK activation as compared to an identical cell population derived from AdNull controls (p<0.05) (Figure 2B & C). Furthermore, administration of AdVCA0848 resulted in increased numbers of CD69-expressing B cells, CD3+CD8− and CD3+CD8+ T cells, as compared to the use of the AdNull vector in this experiment (p<0.05) (Figure 2D–F). Utilization of AdVCA0848mut control suggested that the activation of immune cells is largely due to the enzymatic activity of the transduced VCA0848 (Supplemental Figure 2B–F). Our results also confirmed previous findings that the Ad5 vector itself results in increased activation of NK cells, macrophages, CD3+CD8− T cells, CD3+CD8+ T cells, and B cells as indicated by the significant expression of the activation marker CD69 (37). Together, these data suggest a significant induction of innate immune responses by AdVCA0848 in the mouse model, surpassing that caused by the adenovirus itself.
Figure 2. AdVCA0848 stimulates strong induction of IFN-β and activates innate and adaptive immune cells.
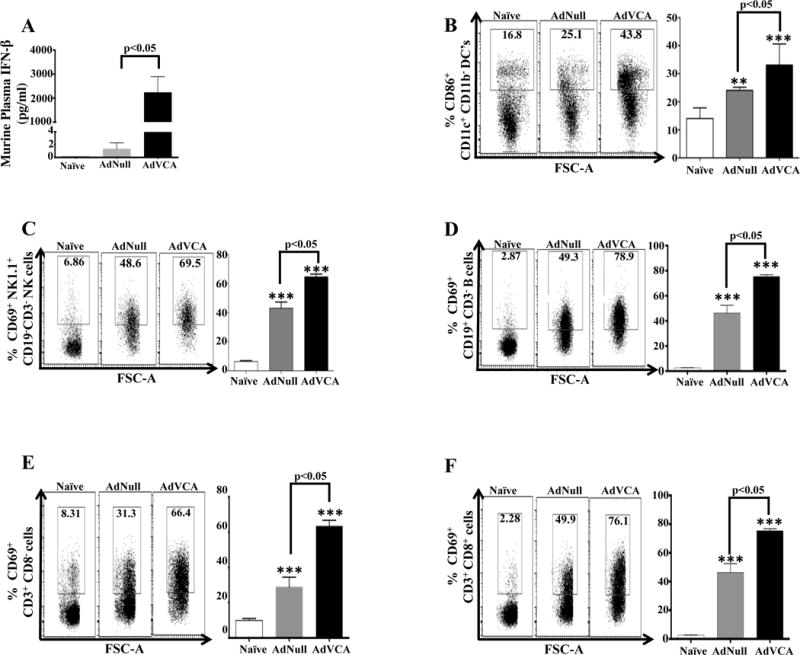
Male 6–10 weeks old C57BL/6 WT mice (n=4) were i.v. injected (retro-orbitally) with 1×1010 vps/mouse of AdNull, AdVCA848, or not injected (naive) as control. At 6 hpi mice were sacrificed and spleens and blood samples were obtained. (A) An ELISA-based assay to determine the amount of IFN-β produced in plasma (diluted 1:2) from naive, mice injected with AdNull, AdVCA0848. Splenocytes harvested and FACS analysis conducted as described in methods and materials. Effects of AdNull and AdVCA0848 (with representative results) on the activation of CD86+ CD11c+CD11b- DCs (B), CD69+ NK1.1+ CD3− NK cells (C), CD69+ CD19+ CD3− B cells (D), CD69+ CD3+ CD8− T cells (E), and CD69+ CD3+ CD8+ T cells (F). Bars with the indicated colors represent mean ± SD. Statistical analysis was completed using One Way ANOVA followed by a Student-Newman-Keuls post-hoc test. A value of p<0.05 was deemed statistically significant. The (**) and (***) denote significance over naïve animals p<0.05 and p<0.001, respectively.
AdVCA0848 enhances induction of antigen-specific adaptive T cell immune responses
Direct administration of the ovalbumin (OVA) protein is a model antigen frequently used to study antigen-specific adaptive immune responses (42, 43). C57BL/6 mice were vaccinated with 100 μg/mL OVA alone, or simultaneously with AdNull or AdVCA0848; and a fourth untreated group served as a naïve control. At 14 dpi, IFN-γ ELISPOT results from the experimental and control animals indicated that OVA-specific T cell responses from mice co-administered with AdVCA0848 and OVA were significantly higher (upon ex vivo stimulation with the entire OVA protein or the OVA-derived MHC class I-restricted peptide SIINFEKL) as compared to splenocytes derived from mice receiving only OVA, or OVA concomitant with the AdNull control vector (p<0.05) (Figure 3A). The simultaneous use of AdVCA0848 with OVA vaccination also increased the number of SIINFEKL and the intact OVA protein-specific IL-2-secreting T cells present in the splenocytes of OVA-treated mice as compared to mice injected with OVA alone, or concomitant with AdNull control (p<0.05) (Figure 3C). The noticeable variability of T cell responses resulted from the ex vivo stimulation with whole OVA protein and the MHC class I-restricted SIINFEKL peptide likely suggest a CD8+ T cell-driven response indicated by higher SIINFEKL-specific IFN-γ producing T cells and smaller SIINFEKL-specific IL-2 producing T cells. Interestingly, splenocytes harvested from mice co-injected with AdVCA0848 and OVA also had dramatically increased numbers of Ad5 capsid-specific IFN-γ-secreting T cells and IL-2 secreting T cells, as compared to mice injected with OVA alone, or concomitant with AdNull control (p<0.05) (Figure 3B and 3D). These results indicate that AdVCA0848 provides enhancement of OVA-specific adaptive T cell immune responses when co-injected with the extracellular antigen OVA.
Figure 3. AdVCA0848 enhances OVA-specific adaptive T cell responses.
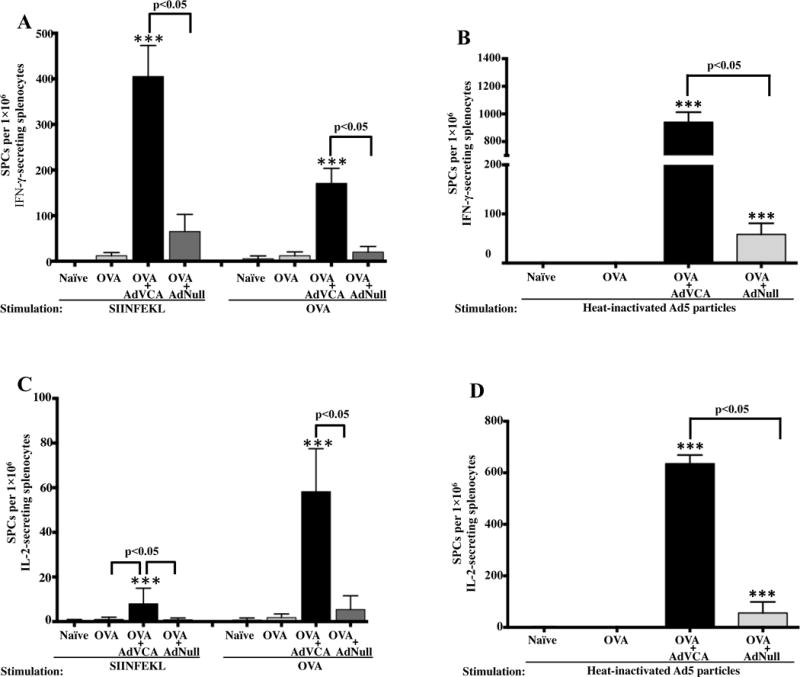
Male 6–10 weeks old C57BL/6 mice (n=5) were injected with OVA alone, OVA + AdVCA0848, OVA + AdNull, or not injected as described in materials and methods. At 14 dpi, mice were sacrificed and splenocytes at1×106 cells/well were ex vivo stimulated with MHC class I-restricted OVA-derived peptide SIINFEKL, OVA protein, heat-inactivated Ad5 particles, or with only media (unstimulated). The ELISPOT assays for IFN-γ (A & B) and IL-2 (C & D) were performed. Bars with the indicated colors represent mean ± SD for samples stimulated with the indicated stimulations. Results are representative of two independent experiments. Statistical analysis was completed using One Way ANOVA followed by a Student-Newman-Keuls post-hoc test. A value of p<0.05 was deemed statistically significant. The (**) and (***) denote significance over naïve animals p<0.05 and p<0.001, respectively.
AdVCA0848 enhances induction of antigen-specific adaptive B cell immune responses
Co-administering AdVCA0848 and OVA also resulted in enhancement of OVA-specific (Figure 4A) and Ad5-specific (Figure 4B) B cell responses 6 dpi. At 14 dpi, OVA-specific B cell response was enhanced compared to mice co-injected with the AdNull control vector (Figure 4C) or when injected with OVA alone (p<0.05) (Supplemental Figure 3). Ad5-specific IgG antibody B cell responses were also detected in those mice that received either of the Ad5 vectors. While the presence of AdVCA0848 significantly increased the Ad5-specific B cell response compared to that exerted by the AdNull control (p<0.05) when measured at 6 dpi, this effect was observed to be minimal when measured at 14 dpi (Figure 4D). Despite the transient enhancement of humoral response against the delivering vector, these results demonstrate the beneficial effects of AdVCA0848 on the OVA-specific adaptive B cell response from a single administration of OVA.
Figure 4. AdVCA0848 enhances OVA-specific adaptive B cell responses.
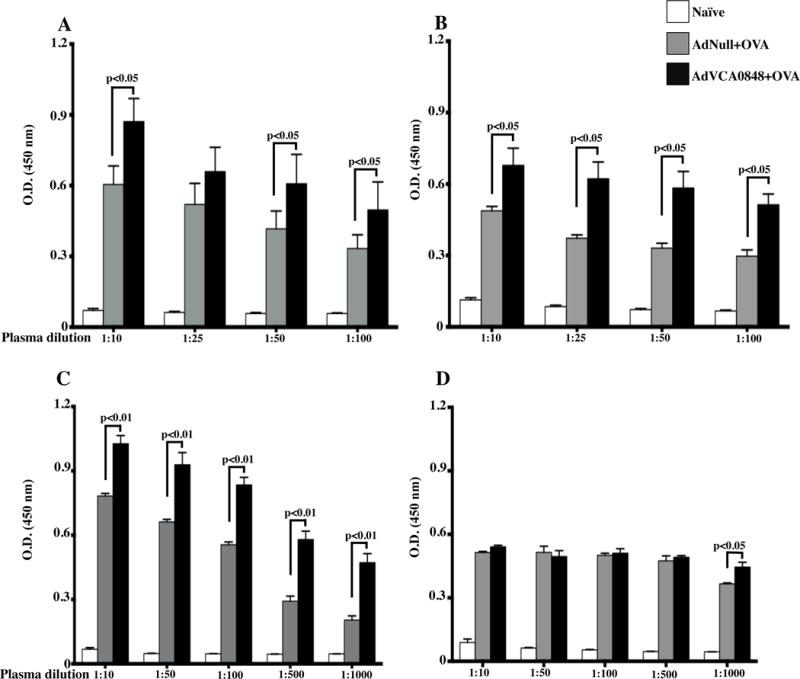
Male 8–10 weeks old C57BL/6 mice (n=5) were injected with OVA + AdNull, OVA + AdVCA0848,, or not injected (naïve) as described in materials and methods. (A & B) At 6 dpi, mice were retro-orbitally bleeded to determine OVA and Ad5-specific B cell response by ELISA-based measurement for total IgG with the indicated plasma dilutions. (C & D) At 14 dpi, mice were sacrificed; blood samples obtained, and plasma samples were prepared and used for ELISA-based measurement for total OVA and Ad5-specific IgG with the indicated plasma dilutions. Bars with the indicated colors represent mean ± SD for samples from different groups. Results are representative of two independent experiments. Statistical analysis was completed using One Way ANOVA followed by a Student-Newman-Keuls post-hoc test. A value of p<0.05 was deemed statistically significant.
Sustained high-level production of c-di-GMP can inhibit T cell responses to antigens expressed from viral vectors
Our previous results indicated a modest, although significant, enhancement of adaptive immune responses specific against antigens expressed from Ad5-based vaccines co-injected with AdVCA0956, a vector expressing a less active DGC (30). Therefore, we assessed whether the enhanced ability of AdVCA0848 to produce c-di-GMP in vivo would also improve adaptive immune responses specific for adenovirus-expressed antigens. We have previously used an adenovirus-based vector to express the Gag protein, an HIV-1-derived antigen, and demonstrated the platform’s ability to induce Gag-specific humoral and cellular immune responses (31, 33, 40, 44). Based on our previous work we administered the AdGag vaccine at the dose of 5×106 vps/mouse along with escalating doses (5 × 107, 5 × 108, or 5 × 109 vps/mouse) of AdVCA0848 or the AdNull control. After 14 days, Gag-specific memory T cell immune responses were evaluated by IFN-γ ELISPOT assay. The results demonstrated that concurrent administration of AdVCA0848 along with the AdGag vaccine inhibited T cell responses to the Gag antigen, which were especially significant at the highest AdVCA0848 dose of 5×109 vps/mouse compared to that seen from the concurrent administration of AdNull control along with AdGag vaccine (p<0.05) (Figure 5A). Similar to our previous observations (45), as the viral load of AdNull co-injected with AdGag increased, the Gag-specific T cell response measured by IFN-γ ELISPOT decreased in a dose-dependent manner (p<0.05). In contrast, ELISPOT assays demonstrated a dramatic enhancement of Ad5-specific IFN-γ-producing T cells at 5×109 vps/mouse of AdVCA0848 compared to the AdNull control group (p<0.05), while the first two doses of 5×107 and 5×108 vps/mouse showed minimal Ad5-specific T cell response (Figure 5B). We confirmed that the inhibitory effects on IFN-γ-secreting T cells was lost in a VCA0848 mutant that cannot synthesize c-di-GMP (Supplemental Figure 4 A).
Figure 5. Co-injecting AdVCA0848 and AdGag results in significant inhibitory effects of Gag-specific T cell responses.

Female 6–8 weeks old BALB/c mice (n=4) were i.m. co-injected in the tibialis anterior with viral particles of AdGag (5×106 vps/mouse) along with 3 different doses (5×107, 5×108, or 5×109 vps/mouse) of either AdNull or AdVCA0848, in the presence of an uninjected group of mice as control naive. At 14 dpi, mice were sacrificed and splenocytes (at 5×105 cells/well) were ex vivo stimulated with the 15-mer HIV/Gag-derived immunogenic peptides AMQ (A), or with UV-inactivated adenoviruses (B) for the IFN-γ ELISPOT assays as described in materials and methods. Bars with the indicated colors represent mean ± SD. Results are representative of two independent experiments. Statistical analysis was completed using One Way ANOVA followed by a Student-Newman-Keuls post-hoc test. A value of p<0.05 was deemed statistically significant. The (**) and (***) denote significance over naïve animals p<0.05 and p<0.001, respectively. The (a) denote significance over AdVCA0848 at the dose of 5×109 vps/mouse (p<0.05).
A multi-parameter tetramer-binding assay showed a significantly decreased number of Gag-specific Tet+CD8+ T cells present in mice co-injected with three different doses of AdVCA0848 along with AdGag as compared to mice co-injected with AdGag and the AdNull control vector (p<0.05) (Figure 6A), confirming the negative impact of AdVCA0848 on the induction of Gag-specific CD8+ T cells. We also performed intracellular staining (ICS) and FACS analysis to evaluate the impact of AdVCA0848 on the numbers of Gag-specific CD8+ T cells upon ex vivo stimulation with the Gag-specific peptide, AMQ. The number of IFN-γ and TNF-α-producing CD8+ T cells specific for this potent Gag peptide were significantly inhibited in mice co-injected with AdVCA0848 as compared to equal viral loads of AdNull (p<0.05) with the highest dose of AdVCA0848 of 5×109 vps/mouse showing the strongest inhibitory effects (Figure 6 B & C). We also looked at the effect of AdVCA0848 on Gag-specific IFN-γ, TNF-α and IL-2-producing CD4+ T cells and observed no significant effect (data not shown). Together, these data strongly suggested that despite a strong induction of innate immunity, and improved induction of adaptive immune responses to extracellular proteins such as the OVA protein and the Ad5 capsid, expressing high levels of c-di-GMP using VCA0848 from an Ad5 vector significantly inhibited induction of antigen specific CD8+ T cell responses to antigens expressed intracellularly by another Ad5 vector.
Figure 6. Co-injecting AdVCA0848 and AdGag results in significant inhibitory effects of Gag-specific CD8+T cells.
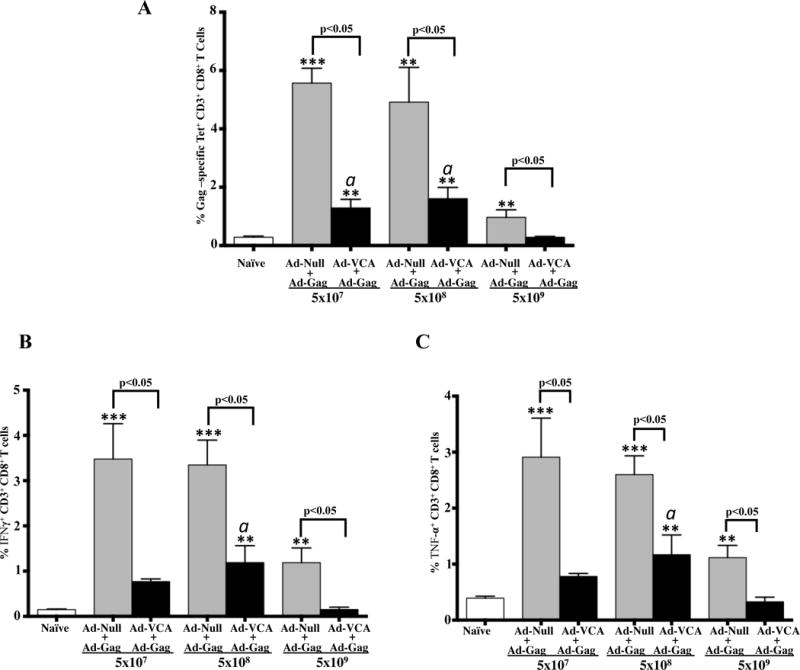
Female 6–8 weeks old BALB/c mice (n=4) were i.m. co-injected in the tibialis anterior with viral particles of AdGag (5×106 vps/mouse) along with 3 different doses (5×107, 5×108, or 5×109 vps/mouse) of either AdNull or AdVCA0848, in the presence of an uninjected group of mice as control naive. At 14 dpi, mice were sacrificed and splenocytes harvested and used at 1×106 cells/well for tetramer staining using PE-labeled MHC class I tetramer folded with the AMQ peptide as described in materials and methods followed by FACS analysis for Tet+ Gag-specific CD8+ T cells (A). Multi-parameter staining was conducted to determine the overall frequency of IFN-γ (B) and TNF-α (C) producing CD8+ T cells followed by FACS analysis conducted on BD LSRII flow cytometer as described in methods and materials. Results are representative of two independent experiments. Bars with the indicated colors represent mean ± SD. Statistical analysis was completed using One Way ANOVA followed by a Student-Newman-Keuls post-hoc test. A value of p<0.05 was deemed statistically significant. The (**) and (***) denote significance over naïve animals p<0.05 and p<0.001, respectively. The (a) denote significance over AdVCA0848 dose of 5×109 vps/mouse (p<0.05).
Sustained high-level production of c-di-GMP can also inhibit B cell responses to antigens expressed from viral vectors
We next evaluated humoral B cell responses following AdVCA0848 co-administration with AdGag. Similar to its effect on T cell responses, the presence of AdVCA0848 resulted in significant inhibition of HIV-1/Gag-specific B cell responses as compared to those mice administered with equal amounts of the AdNull control vector (p<0.05) (Figure 7A). The inhibition of Gag-specific B cell responses by AdVCA0848 was very potent at the doses of 5×107 and 5×108 vps/mouse (compared to AdNull, p<0.05). AdNull exhibited inhibition similar to AdVCA0848 at the highest dose of 5×109 vps/mouse (Fig. 7A). Alternatively, increasing doses of both the AdNull and AdVCA0848 increased B cell responses against the Ad5 vector in a dose-dependent manner (Figure 7B). The inhibitory effects on Gag-specific B cell responses were lost using the AdVCA0848mut that cannot synthesize c-di-GMP (Supplemental Figure 4B). We confirmed the ability of AdVCA0848 to enhance Ad5-specific B cell response compared to that shown by AdVCA0848 mut (Supplemental Figure 4C).
Figure 7. Co-injecting AdVCA0848 resulted in significant inhibition of Gag and ToxB-specific B cell response.
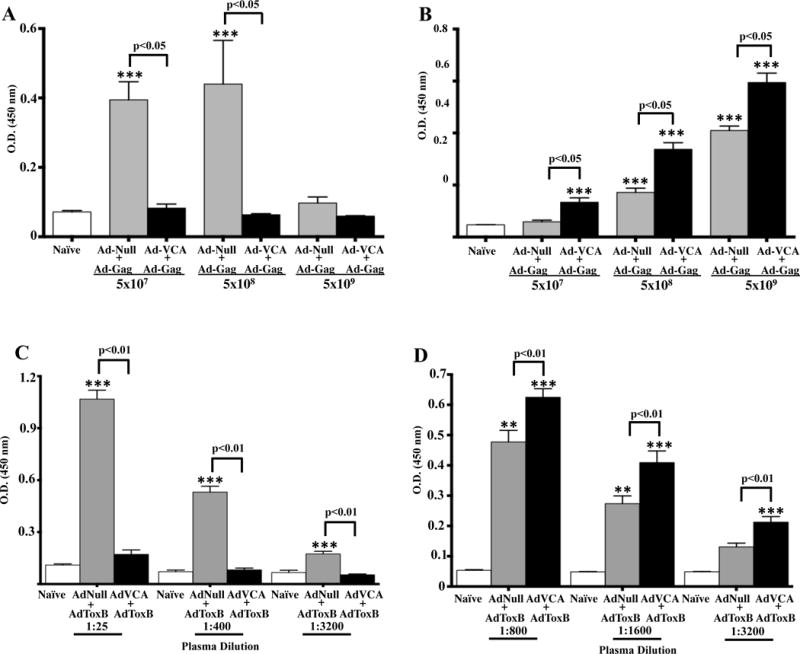
Female 6–8 weeks old BALB/c mice (n=4) were i.m. co-injected in the tibialis anterior with the indicated viral injections and as described in materials and methods of AdVCA0848 along with either AdGag or AdToxB in the presence of uninjected mice control naïves. At 14 dpi, mice were sacrificed and plasma samples collected. Total IgG levels of Gag-specific (plasma dilution 1:25) antibodies (A) or Ad5-specific (plasma dilution 1:400) (B) were measured to determine the effect of indicated does of AdVCA0848 on Gag-specific B cell response by ELISA. ELISA was also used to determine the effect of AdVCA0848 on ToxB-specific (C) and Ad5-specific (D) B cell response by measuring total IgG levels at the indicated plasma dilutions. Results are representative of two independent experiments. Bars with the indicated colors represent mean ± SD. Statistical analysis was completed using One Way ANOVA followed by a Student-Newman-Keuls post-hoc test. A value of p<0.05 was deemed statistically significant. The (**) and (***) denote significance over naïve animals p<0.05 and p<0.001, respectively.
To confirm this interesting observation using a different antigen expressed by an Ad5-based vaccine, we co-administered AdVCA0848 along with an Ad5 vector expressing the truncated form of the C. difficile-derived Toxin B protein (AdToxB). The presence of AdVCA0848 with AdToxB also resulted in significantly reduced ToxB-specific B cell responses as compared to control vaccinations (p<0.001) (Figure 7C). Importantly, we again observed significantly (p<0.01) increased Ad5-specific IgG titers in mice vaccinated with AdVCA0848 and AdToxB, as compared to controls (Figure 7D). These results further confirm the inhibitory effects of the strong c-di-GMP producer, AdVCA0848, on another antigen intracellularly expressed from an adenovirus vector (AdToxB).
Co-administration of AdGag and AdVCA0848 doesn’t inhibit Gag expression
One possible explanation for the inhibition of response to Ad-expressed antigens is that the presence of the AdVCA0848 vector inhibits in trans the in vivo expression of the Ad expressed antigens. However, mice co-injected with AdVCA0848 and AdGag demonstrated the presence of the HIV-1 derived Gag protein whether delivered by the AdGag platform alone, or when co-injected with the AdNull control, or with AdVCA0848, (Figure 8). These results suggest that inhibitory effects exerted by AdVCA0848 on B cell and T cell adaptive immune responses against Gag are not due to lack of Gag expression and translation in vivo.
Figure 8. Co-administration of AdGag and AdVCA0848 does not inhibit the translation of Gag protein.
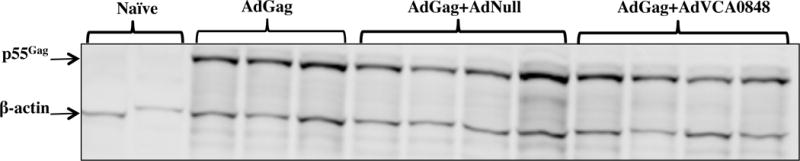
Male 6–8 weeks old BALB/c WT mice were retro-orbitally i.v. injected with 1×10111×1011 vps/mouse of AdGag alone (n=3), or co-injected with 1×1011 vps/mouse AdVCA0848 (n=4), AdNull (n=3), or not injected (naïves) (n=3) as control.
Discussion
Understanding the molecular mechanisms underlying how a putative adjuvant acts to enhance the efficacy of a specific vaccine will help to guide the formulation of newer generation vaccines that efficiently generate specific long-term immunity against difficult antigens derived from pathogens or cancer cells (46). The use of pure c-di-GMP has been demonstrated to be an immune-modulatory molecule with potential therapeutic and prophylactic properties (19). While the presence of nucleic acids can be sensed by AIM2, and signals the activation of caspase-1 (47, 48), the presence of cytosolic c-di-GMP can be sensed by other sensors including the STING and helicase DDX41 pathways, and subsequently lead to the release of IFN-β, primarily from CD11b+ DCs (17). Additionally, c-di-GMP has been shown to stimulate the MYPS/STING-dependent induction of TNF-α and IL-22, not type I IFN, when used as a nasal mucosal adjuvant, suggesting c-di-GMP may have different effects on different innate immunity pathways (49, 50).
In this study, we demonstrated the ability of a potent, bacterial derived DGC to be delivered by an Ad5 vector (AdVCA0848) that produced more than 400-fold more c-di-GMP than our previous Ad5 DGC vector (30), resulting in a robust induction of several innate immune responses, including IFN-β induction. By using a mutant version of VCA0848 delivered by AdVCA0848mut, our data suggests that these significant levels of c-di-GMP are products of the enzymatic activity of the transduced VCA0848. These strong innate immune responses allowed the induction of enhanced adaptive immune responses to an extracellular antigen, i.e. OVA, co-administered with the AdVCA0848, but also suppressed adaptive immune responses to virally expressed antigens. The recent characterization of mammalian endogenous cyclic GMP-AMP (2′3′-cGAMP) synthase (cGAS) (51–53) provided the rationale for testing cGAMP as a vaccine adjuvant, and initial studies demonstrated its usefulness in stimulating innate immune responses and improving antigen-specific adaptive immune responses (54–56). When compared to the bacterial c-di-GMP, cGAMP had higher binding affinity to STING. However, it has also been shown that c-di-GMP results in higher IFN-β induction than that induced by 2′3′-cGAMP or its isomers, suggesting that higher binding affinity to STING does not correlate with IFN-β induction. These results may be attributable to possible differences in biological stability between c-di-GMP and the mammalian cGAMP (53).
The adenovirus-based platforms we utilized in these studies are also expected to activate multiple innate immune responses. The vector is known to activate innate immune responses via interactions with extracellular and intracellular TLRs, and can simultaneously trigger early pro-inflammatory responses such as the induction of IP-10 (57) and the activation of the P13K signaling cascade (58). We and others have also demonstrated that upon penetrating host cells and escaping the endosomal compartment, adenoviral vectors have the ability to ignite the MAPK and NFκB signaling pathways through TLR-dependent (TLR2, 3, 4, and 9) and non-TLR dependent mechanisms (59–61) leading to the induction of several chemokines and cytokines, fostering its utility as a vaccine platform in and of itself. Additionally, the adenoviral dsDNA genome can be sensed by cytoplasmic sensors such as DAI (leading to type I IFN induction) (62) and AIM-2 resulting in activating the inflammasome and the induction of caspase-1-dependent IL-1β (47). Recent data also suggest that STING is central and acts as a major PRR after vaccination with Ad5-based platforms including Ad5 vectors (63). With these facts in mind, it is clear that our results confirm that the additional production of c-di-GMP from an already immunogenic platform such as Ad is significant enough to further promote the induction of pro-inflammatory immune responses beyond that provided by the Ad vector platform itself. Whether expression of DGCs from other vaccine platforms will yield similar results awaits future studies beyond the scope of this manuscript.
The broad impact of the AdVCA0848 platform on innate immune responses clearly demonstrates its promising potential for use as a vaccine adjuvant to enhance adaptive immune responses. For example, relative to enhancing adaptive immune responses to extracellular antigens, plasmacytoid dendritic cell precursors (pDC) are thought to be the major source of IFN-β (64). In agreement with previous reports that demonstrated the stimulatory effects of c-di-GMP on murine and human DCs (13, 19), AdVCA0848 improved the induction of CD11c+CD11b− CD86+ DCs. Ultimately, pDCs can differentiate into typical DCs capable of stimulating naive T cells in an antigen-specific manner (65). IFN-β has also been shown to enhance DC maturation, the efficiency of DC’s to activate the cross-priming of CD8+ T cells, and increase induction of CD4+ Th I differentiation (66). In addition to increasing the number of CD86+CD11c+CD11b− DCs and activating CD69+NK1.1+ NK cells that are involved in regulating innate immune responses, AdVCA0848 activated cells directly involved in adaptive immune responses such as B cells and CD4+ and CD8+ T cells.
AdVCA0848 also enhanced induction of OVA-specific B cell and T cell adaptive responses. These results parallel recent studies evaluating the beneficial effects of direct administration of c-di-GMP as an adjuvant during vaccination with OVA (49, 50), and 4-Hydroxy-3-nitrophenylacetyl-Chicken Gamma Globulin, NP-CGG, in which c-di-GMP was shown to have the capacity to enhance germinal center (GC) development (67). Additionally, the presence of c-di-GMP in an adjuvant formulation containing chitosan (CSN) improved adaptive immune responses to H5N1 antigens (16), and (along with a conventional aluminum salt-based adjuvant) improved adaptive immune responses specific to the hepatitis B surface antigen (HBsAg) (67). Recently, it was demonstrated that nasal administration of c-di-GMP significantly increases the MYPS-mediated uptake of OVA antigen via endocytosis and pinocytosis in vivo. This generates mucosal adjuvant activities that are mediated by type II and type III interferon but not type I interferon suggesting variable c-di-GMP pleiotropic effects on innate immune responses against extracellular antigens. The in vivo production of c-di-GMP by i.m. administration of our AdVCA0848 platform potentially enhanced the OVA uptake and processing by DCs, and subsequently resulted in improved OVA-specific adaptive immune responses (50). As a proof of principle, our results suggest that adenovirus-based platforms expressing DGCs may also be used to promote improved immunity against other disease specific antigens, such as those found in current cholera, diphtheria, and tetanus vaccines, as each are examples of protein-based vaccines. In addition, as our approach also enhances activation of antigen-presenting cells (APCs) and induction of antigen CD8+ cytotoxic T lymphocytes (CTLs), future studies using tumor antigen specific peptides may also enhance the induction of anti-tumor cellular immune responses (21, 22, 68, 69).
Our results also revealed the potential for inhibitory effects on adaptive immune responses to antigens expressed intracellularly, simultaneous with provision of high levels of c-di-GMP. Although, the dose of 5×108 vps/mouse of AdVCA0848 did not show significant inhibition of IFN-γ-secreting splenocytes compared to that shown by the AdNull control, this dose caused significant inhibition of Gag-specific IFN-γ and TNF-α-secreting CD8+ T cells, suggesting that CD8+ T cells may be the specific targets for these inhibitory effects. Furthermore, increasing the AdVCA0848 dose to 5✕109 vps/mouse further inhibited Gag-specific T cell responses. Of note, the use of higher doses of the AdNull control vector also resulted in decreased induction of Gag-specific CD8+ T cell responses. Despite this, the provision of elevated c-di-GMP levels resulted in additional inhibitory effects on Gag-specific adaptive immune responses.
We have previously reported that increasing the dose of AdVCA0956 to 5×109 vps/mouse did not improve B cell responses specific for an antigen delivered by an Ad5 vector in mice (30). Specifically, AdVCA0956 moderately suppressed B cell responses against the C. difficile-derived Toxin A antigen expressed from the co-injected Ad5 vector at the dose of 5×109 vps/mouse. Here, our results suggest that those trends were likely real. Even stronger inhibitory effects were noted after administration of the more potent AdVCA0848 on B cell and T cell adaptive immune responses against the intracellularly expressed Gag and ToxB antigens. These results suggest that in mice the magnitude of inhibitory effects on adaptive immune responses to intracellularly expressed antigens is likely to increase with excessive amounts of c-di-GMP production.
There is also the possibility that the transduced DGC, and ultimately the synthesized c-di-GMP, interferes with the expression of these antigens when using the CMV expression cassette (used in constructing the vectors). We explored this possibility in vitro, and found enhanced GFP expression in HEK293 cells co-infected with AdVCA0848 and an Ad5 vector expressing GFP (AdGFP) from the same CMV enhancer/promoter elements used in these studies (data not shown). Our data also suggest that co-administration of the AdGag vaccine along with the strong c-di-GMP producing AdVCA0848 did not prevent Gag translation. It remains unclear how the significant induction of c-di-GMP and subsequently high levels of type I IFN can inhibit the T cell and B cell responses of an intracellularly expressed antigen (63), and the impact of strong type I IFN induction on the availability of intracellular antigen-loaded APCs requires further investigation. We do note that the production of another bacterial second messenger, c-di-AMP, by the intracellular pathogen Listeria monocytogenes was shown to induce IFN-β in a STING-dependent manner leading to the inhibition of T cell-mediated immunity, similar to our results with excessive production of c-di-GMP (71).
In summary, we demonstrated the feasibility of in vivo synthesis of extremely large amounts of c-di-GMP via an Ad5-based platform expressing a highly potent DGC. While high amounts of c-di-GMP production can inhibit adaptive immune responses to antigens expressed simultaneously with significant increasing c-di-GMP levels, this unique platform appears to preferentially improve antigen specific B cell and T cell adaptive immune responses specific for co-administered extracellular antigens. This approach can be utilized to develop and improve protein-based prophylactic and therapeutic vaccines targeting infectious diseases and cancers.
Supplementary Material
Acknowledgments
We are grateful to Dr. Louis King at the Michigan State University Flow Cytometry facility for his assistance in conducting our FACS experiments, to the Michigan State University Laboratory Animal support facility for their help in the humane care and maintenance of the animals used in this study. We would also like to thank Michigan State University RTSF Mass Spectrometry and Metabolomics Core for their help in c-di-GMP quantification.
Footnotes
This work was supported by NIH-NIAID-R21AI105499 (CMW and AA), and by the Osteopathic Heritage Foundation (AA).
Conflict of Interest statement: The authors have no conflicting financial interest.
References
- 1.Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr Opin Immunol. 2012;24:310–315. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vessely C, Estey T, Randolph TW, Henderson I, Cooper J, Nayar R, Braun LJ, Carpenter JF. Stability of a trivalent recombinant protein vaccine formulation against botulinum neurotoxin during storage in aqueous solution. Journal of pharmaceutical sciences. 2009;98:2970–2993. doi: 10.1002/jps.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed SS, Plotkin SA, Black S, Coffman RL. Assessing the safety of adjuvanted vaccines. Science translational medicine. 2011;3:93rv92. doi: 10.1126/scitranslmed.3002302. [DOI] [PubMed] [Google Scholar]
- 4.Gomelsky M. Cyclic dimeric GMP-mediated decisions in surface-grown Vibrio parahaemolyticus: a different kind of motile-to-sessile transition. J Bacteriol. 2012;194:911–913. doi: 10.1128/JB.06695-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krasteva PV, Giglio KM, Sondermann H. Sensing the messenger: the diverse ways that bacteria signal through c-di-GMP. Protein Sci. 2012;21:929–948. doi: 10.1002/pro.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, Liu ZJ, Modlin RL, Liu YJ, Cheng G. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13:1155–1161. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14:19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 9.McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, Ishii KJ, Akira S, Colonna M, Chen ZJ, Fitzgerald KA, Hayakawa Y, Vance RE. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med. 2009;206:1899–1911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karaolis DK, Newstead MW, Zeng X, Hyodo M, Hayakawa Y, Bhan U, Liang H, Standiford TJ. Cyclic di-GMP stimulates protective innate immunity in bacterial pneumonia. Infect Immun. 2007;75:4942–4950. doi: 10.1128/IAI.01762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brouillette E, Hyodo M, Hayakawa Y, Karaolis DK, Malouin F. 3′,5′-cyclic diguanylic acid reduces the virulence of biofilm-forming Staphylococcus aureus strains in a mouse model of mastitis infection. Antimicrob Agents Chemother. 2005;49:3109–3113. doi: 10.1128/AAC.49.8.3109-3113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu DL, Narita K, Hyodo M, Hayakawa Y, Nakane A, Karaolis DK. c-di-GMP as a vaccine adjuvant enhances protection against systemic methicillin-resistant Staphylococcus aureus (MRSA) infection. Vaccine. 2009;27:4867–4873. doi: 10.1016/j.vaccine.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 13.Elahi S, Van Kessel J, Kiros TG, Strom S, Hayakawa Y, Hyodo M, Babiuk LA, Gerdts V. c-di-GMP enhances protective innate immunity in a murine model of pertussis. PLoS One. 2014;9:e109778. doi: 10.1371/journal.pone.0109778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan H, KuoLee R, Tram K, Qiu H, Zhang J, Patel GB, Chen W. 3′,5′-Cyclic diguanylic acid elicits mucosal immunity against bacterial infection. Biochem Biophys Res Commun. 2009;387:581–584. doi: 10.1016/j.bbrc.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen GK, Ebensen T, Gjeraker IH, Svindland S, Bredholt G, Guzmán CA, Cox RJ. Evaluation of the sublingual route for administration of influenza H5N1 virosomes in combination with the bacterial second messenger c-di-GMP. PLoS One. 2011;6:e26973. doi: 10.1371/journal.pone.0026973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svindland SC, Pedersen GK, Pathirana RD, Bredholt G, Nøstbakken JK, Jul-Larsen Å, Guzmán CA, Montomoli E, Lapini G, Piccirella S, Jabbal-Gill I, Hinchcliffe M, Cox RJ. A study of Chitosan and c-di-GMP as mucosal adjuvants for intranasal influenza H5N1 vaccine. Influenza Other Respir Viruses. 2013;7:1181–1193. doi: 10.1111/irv.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang L, Li L, Lemos H, Chandler PR, Pacholczyk G, Baban B, Barber GN, Hayakawa Y, McGaha TL, Ravishankar B, Munn DH, Mellor AL. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J Immunol. 2013;191:3509–3513. doi: 10.4049/jimmunol.1301419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemos H, Huang L, Chandler PR, Mohamed E, Souza GR, Li L, Pacholczyk G, Barber GN, Hayakawa Y, Munn DH, Mellor AL. Activation of the STING adaptor attenuates experimental autoimmune encephalitis. J Immunol. 2014;192:5571–5578. doi: 10.4049/jimmunol.1303258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E, Philpott D, Schroeder JT, Hyodo M, Hayakawa Y, Talbot BG, Brouillette E, Malouin F. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol. 2007;178:2171–2181. doi: 10.4049/jimmunol.178.4.2171. [DOI] [PubMed] [Google Scholar]
- 20.Fatima M, Rempel H, Kuang XT, Allen KJ, Cheng KM, Malouin F, Diarra MS. Effect of 3′,5′-cyclic diguanylic acid in a broiler Clostridium perfringens infection model. Poult Sci. 2013;92:2644–2650. doi: 10.3382/ps.2013-03143. [DOI] [PubMed] [Google Scholar]
- 21.Miyabe H, Hyodo M, Nakamura T, Sato Y, Hayakawa Y, Harashima H. A new adjuvant delivery system ‘cyclic di-GMP/YSK05 liposome’ for cancer immunotherapy. J Control Release. 2014;184:20–27. doi: 10.1016/j.jconrel.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DK, Gravekamp C. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res. 2014;2:901–910. doi: 10.1158/2326-6066.CIR-13-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, Watkins SC, Sarkar SN, Okada H. STING Contributes to Antiglioma Immunity via Triggering Type I IFN Signals in the Tumor Microenvironment. Cancer Immunol Res. 2014;2:1199–1208. doi: 10.1158/2326-6066.CIR-14-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beyhan S, Odell LS, Yildiz FH. Identification and characterization of cyclic diguanylate signaling systems controlling rugosity in Vibrio cholerae. J Bacteriol. 2008;190:7392–7405. doi: 10.1128/JB.00564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim B, Beyhan S, Meir J, Yildiz FH. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol Microbiol. 2006;60:331–348. doi: 10.1111/j.1365-2958.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- 26.Beyhan S, Yildiz FH. Smooth to rugose phase variation in Vibrio cholerae can be mediated by a single nucleotide change that targets c-di-GMP signalling pathway. Mol Microbiol. 2007;63:995–1007. doi: 10.1111/j.1365-2958.2006.05568.x. [DOI] [PubMed] [Google Scholar]
- 27.Shikuma NJ, Fong JC, Yildiz FH. Cellular levels and binding of c-di-GMP control subcellular localization and activity of the Vibrio cholerae transcriptional regulator VpsT. PLoS Pathog. 2012;8:e1002719. doi: 10.1371/journal.ppat.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galperin MY. Bacterial signal transduction network in a genomic perspective. Environ Microbiol. 2004;6:552–567. doi: 10.1111/j.1462-2920.2004.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter JL, Severin GB, Koestler BJ, Waters CM. The Vibrio cholerae diguanylate cyclase VCA0965 has an AGDEF active site and synthesizes cyclic di-GMP. BMC Microbiol. 2014;14:22. doi: 10.1186/1471-2180-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koestler BJ, Seregin SS, Rastall DP, Aldhamen YA, Godbehere S, Amalfitano A, Waters CM. Stimulation of Innate Immunity by In Vivo Cyclic di-GMP Synthesis Using Adenovirus. Clin Vaccine Immunol. 2014;21:1550–1559. doi: 10.1128/CVI.00471-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldhamen YA, Appledorn DM, Seregin SS, Liu CJ, Schuldt NJ, Godbehere S, Amalfitano A. Expression of the SLAM family of receptors adapter EAT-2 as a novel strategy for enhancing beneficial immune responses to vaccine antigens. J Immunol. 2011;186:722–732. doi: 10.4049/jimmunol.1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seregin SS, Aldhamen YA, Appledorn DM, Hartman ZC, Schuldt NJ, Scott J, Godbehere S, Jiang H, Frank MM, Amalfitano A. Adenovirus capsid-display of the retro-oriented human complement inhibitor DAF reduces Ad vector-triggered immune responses in vitro and in vivo. Blood. 2010;116:1669–1677. doi: 10.1182/blood-2010-03-276949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appledorn DM, Aldhamen YA, Depas W, Seregin SS, Liu CJ, Schuldt N, Quach D, Quiroga D, Godbehere S, Zlatkin I, Kim S, McCormick JJ, Amalfitano A. A new adenovirus based vaccine vector expressing an Eimeria tenella derived TLR agonist improves cellular immune responses to an antigenic target. PLoS One. 2010;5:e9579. doi: 10.1371/journal.pone.0009579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seregin SS, Aldhamen YA, Rastall DP, Godbehere S, Amalfitano A. Adenovirus-based vaccination against Clostridium difficile toxin A allows for rapid humoral immunity and complete protection from toxin A lethal challenge in mice. Vaccine. 2012;30:1492–1501. doi: 10.1016/j.vaccine.2011.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barroso LA, Wang SZ, Phelps CJ, Johnson JL, Wilkins TD. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990;18:4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kink JA, Williams JA. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun. 1998;66:2018–2025. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aldhamen YA, Seregin SS, Schuldt NJ, Rastall DP, Liu CJ, Godbehere S, Amalfitano A. Vaccines expressing the innate immune modulator EAT-2 elicit potent effector memory T lymphocyte responses despite pre-existing vaccine immunity. J Immunol. 2012;189:1349–1359. doi: 10.4049/jimmunol.1200736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seregin SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, Voss T, Wei J, Bujold M, Nance W, Godbehere S, Amalfitano A. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massie JP, Reynolds EL, Koestler BJ, Cong JP, Agostoni M, Waters CM. Quantification of high-specificity cyclic diguanylate signaling. Proc Natl Acad Sci U S A. 2012;109:12746–12751. doi: 10.1073/pnas.1115663109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS, Amalfitano A. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin Vaccine Immunol. 2011;18:150–160. doi: 10.1128/CVI.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahlen G, Strindelius L, Johansson T, Nilsson A, Chatzissavidou N, Sjoblom M, Rova U, Holgersson J. Mannosylated mucin-type immunoglobulin fusion proteins enhance antigen-specific antibody and T lymphocyte responses. PLoS One. 2012;7:e46959. doi: 10.1371/journal.pone.0046959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basto AP, Badenes M, Almeida SC, Martins C, Duarte A, Santos DM, Leitao A. Immune response profile elicited by the model antigen ovalbumin expressed in fusion with the bacterial OprI lipoprotein. Mol Immunol. 2015;64:36–45. doi: 10.1016/j.molimm.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Garulli B, Stillitano MG, Barnaba V, Castrucci MR. Primary CD8+ T-cell response to soluble ovalbumin is improved by chloroquine treatment in vivo. Clin Vaccine Immunol. 2008;15:1497–1504. doi: 10.1128/CVI.00166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Gayle RB, Amalfitano A, Jones FR. A preliminary and comparative evaluation of a novel Ad5 [E1-, E2b-] recombinant-based vaccine used to induce cell mediated immune responses. Immunol Lett. 2009;122:44–51. doi: 10.1016/j.imlet.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuldt NJ, Aldhamen YA, Appledorn DM, Seregin SS, Kousa Y, Godbehere S, Amalfitano A. Vaccine platforms combining circumsporozoite protein and potent immune modulators, rEA or EAT-2, paradoxically result in opposing immune responses. PLoS One. 2011;6:e24147. doi: 10.1371/journal.pone.0024147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rueckert C, Guzman CA. Vaccines: from empirical development to rational design. PLoS Pathog. 2012;8:e1003001. doi: 10.1371/journal.ppat.1003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blaauboer SM, Gabrielle VD, Jin L. MPYS/STING-mediated TNF-α, not type I IFN, is essential for the mucosal adjuvant activity of (3′–5′)-cyclic-di-guanosine-monophosphate in vivo. J Immunol. 2014;192:492–502. doi: 10.4049/jimmunol.1301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blaauboer SM, Mansouri S, Tucker HR, Wang HL, Gabrielle VD, Jin L. The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo. eLife. 2015;4 doi: 10.7554/eLife.06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, Hornung V. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503:530–534. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skrnjug I, Guzman CA, Rueckert C. Cyclic GMP-AMP displays mucosal adjuvant activity in mice. PLoS One. 2014;9:e110150. doi: 10.1371/journal.pone.0110150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tibbles LA, Spurrell JC, Bowen GP, Liu Q, Lam M, Zaiss AK, Robbins SM, Hollenberg MD, Wickham TJ, Muruve DA. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J Virol. 2002;76:1559–1568. doi: 10.1128/JVI.76.4.1559-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verdino P, Witherden DA, Havran WL, Wilson IA. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science. 2010;329:1210–1214. doi: 10.1126/science.1187996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, Amalfitano A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- 60.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Appledorn DM, Patial S, Godbehere S, Parameswaran N, Amalfitano A. TRIF, and TRIF-interacting TLRs differentially modulate several adenovirus vector-induced immune responses. J Innate Immun. 2009;1:376–388. doi: 10.1159/000207194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, Akira S. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 63.Quinn KM, Zak DE, Costa A, Yamamoto A, Kastenmuller K, Hill BJ, Lynn GM, Darrah PA, Lindsay RW, Wang L, Cheng C, Nicosia A, Folgori A, Colloca S, Cortese R, Gostick E, Price DA, Gall JG, Roederer M, Aderem A, Seder RA. Antigen expression determines adenoviral vaccine potency independent of IFN and STING signaling. J Clin Invest. 2015;125:1129–1146. doi: 10.1172/JCI78280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soumelis V, Liu YJ. From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre-dendritic cell differentiation. Eur J Immunol. 2006;36:2286–2292. doi: 10.1002/eji.200636026. [DOI] [PubMed] [Google Scholar]
- 65.Renneson J, Salio M, Mazouz N, Goldman M, Marchant A, Cerundolo V. Mature dendritic cells differentiated in the presence of interferon-beta and interleukin-3 prime functional antigen-specific CD8 T cells. Clinical and experimental immunology. 2005;139:468–475. doi: 10.1111/j.1365-2249.2005.02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huber JP, Farrar JD. Regulation of effector and memory T-cell functions by type I interferon. Immunology. 2011;132:466–474. doi: 10.1111/j.1365-2567.2011.03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gray PM, Forrest G, Wisniewski T, Porter G, Freed DC, DeMartino JA, Zaller DM, Guo Z, Leone J, Fu TM, Vora KA. Evidence for cyclic diguanylate as a vaccine adjuvant with novel immunostimulatory activities. Cell Immunol. 2012;278:113–119. doi: 10.1016/j.cellimm.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Karaolis DK, Cheng K, Lipsky M, Elnabawi A, Catalano J, Hyodo M, Hayakawa Y, Raufman JP. 3′,5′-Cyclic diguanylic acid (c-di-GMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochem Biophys Res Commun. 2005;329:40–45. doi: 10.1016/j.bbrc.2005.01.093. [DOI] [PubMed] [Google Scholar]
- 69.Joshi VB, Geary SM, Gross BP, Wongrakpanich A, Norian LA, Salem AK. Tumor lysate-loaded biodegradable microparticles as cancer vaccines. Expert review of vaccines. 2014;13:9–15. doi: 10.1586/14760584.2014.851606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan NJ, Hensley L, Asiedu C, Geisbert TW, Stanley D, Johnson J, Honko A, Olinger G, Bailey M, Geisbert JB, Reimann KA, Bao S, Rao S, Roederer M, Jahrling PB, Koup RA, Nabel GJ. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med. 2011;17:1128–1131. doi: 10.1038/nm.2447. [DOI] [PubMed] [Google Scholar]
- 71.Archer KA, Durack J, Portnoy DA. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog. 2014;10:e1003861. doi: 10.1371/journal.ppat.1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


