Abstract
Characterizing how the brain appraises the psychological dimensions of reward is one of the central topics of neuroscience. It has become clear that dopamine neurons are implicated in the transmission of both rewarding information and aversive and alerting events through two different neuronal populations involved in encoding the motivational value and the motivational salience of stimuli, respectively. Nonetheless, there is less agreement on the role of the ventromedial prefrontal cortex (vmPFC) and the related neurotransmitter release during the processing of biologically relevant stimuli. To address this issue, we employed magnetic resonance spectroscopy (MRS), a non-invasive methodology that allows detection of some metabolites in the human brain in vivo, in order to assess the role of the vmPFC in encoding stimulus value rather than stimulus salience. Specifically, we measured gammaaminobutyric acid (GABA) and, with control purposes, Glx levels in healthy subjects during the observation of appetitive and disgusting food images. We observed a decrease of GABA and no changes in Glx concentration in the vmPFC in both conditions. Furthermore, a comparatively smaller GABA reduction during the observation of appetitive food images than during the observation of disgusting food images was positively correlated with the scores obtained to the body image concerns sub-scale of Body Uneasiness Test (BUT). These results are consistent with the idea that the vmPFC plays a crucial role in processing both rewarding and aversive stimuli, possibly by encoding stimulus salience through glutamatergic and/or noradrenergic projections to deeper mesencephalic and limbic areas.
Keywords: Magnetic resonance spectroscopy (MRS), Neurotransmitter, Mesotelencephalic pathways, Food, Salience
INTRODUCTION
Because of the relevance of pleasure and other psychological dimensions of reward in daily life, understanding how the brain appraises gratification is one of the main goals of neuroscience (for a review see Berridge and Kringelbach, 2008). While the powerful responses to rewards are mostly ascribable to midbrain dopamine neurons functioning whose critical role in appetitive contexts is well-known (for a review see Bromberg-Martin et al., 2010), it has become progressively clearer that these neurons are also implicated in the transmission of salient but non-rewarding information, related to aversive and alerting events (Horvitz, 2000; Faure et al., 2008; Wang and Tsien, 2011; Hayes et al., 2014). Positive and negative events can be handled both on the basis of their value (rewarding vs aversive), or with regard to their salience, which indicates the absolute importance of the considered events (Bromberg-Martin et al., 2010).
However, neural mechanisms and related neurotransmitter release underlying these processes have not been fully unveiled yet, as witnessed by a host of studies that yielded contradictory results. In general, it has been widely demonstrated that the ventromedial prefrontal cortex (vmPFC) is implicated in the encoding of salient stimuli. Nonetheless, whereas in some cases findings are consistent with the idea that this area mediates the encoding of stimulus value (e.g., Knutson et al., 2003; Chib et al., 2009; Lin et al., 2015), other studies support the proposal that it encodes salience (e.g. Kensinger and Schacter, 2006; Ventura et al., 2007; Puglisi-Allegra and Ventura, 2012).
Effectively, a study carried out by Matsumoto and Hikosaka (2009) showed that whereas some dopamine neurons in the ventral tegmental area (VTA) were excited by rewarding stimuli and were inhibited by aversive stimuli, as predicted by the value hypothesis, a larger amount of dopamine neurons in the same area were excited by both these stimuli, independently of the value hypothesis, suggesting that these neurons are divided into different groups according to their distinct roles in processing different stimulus features. Despite that, there is less agreement on the functional role of the vmPFC and the related neurotransmitter release during the processing of the stimulus value rather than the stimulus salience.
As a consequence of the contrasting results produced by the studies mentioned above, our aim is to give a response to the question of whether gamma-aminobutyric acid (GABA) concentration within vmPFC is related to the encoding of stimulus salience or valence. To address this issue, we employ magnetic resonance spectroscopy (MRS), a non-invasive methodology that allows the direct detection of some endogenous metabolites in the human brain in vivo, such as GABA and Glx complex (glutamate +glutamine; e.g., Delli Pizzi et al., 2016). The hypothesis is based on the fact that the activation of GABAergic systems in the prefrontal cortex can decrease glutamatergic excitatory activity directed to subcortical structures, including the nucleus accumbens (NAc) and the VTA (e.g., Jayaram and Steketee, 2004). VTA dopaminergic system activation, in turn, increases the DA release in the NAc. This latter area also receives direct glutamatergic efferents from the mPFC and projects to the midbrain DA neurons in the VTA, where it either directly inhibits or indirectly activates DA neurons (e.g., Cooper, 2002; Stuber et al., 2012). Using an event-related block-design protocol we measured GABA in the vmPFC, together with Glx as a control both at baseline level and during the presentation of visual images of positivevalue stimuli (appetitive foods) and negative-value stimuli (rotten foods). In case of a role of vmPFC in salience encoding, we expect herein a decrease of GABA and concomitantly no effects on Glx levels (control measure), being both appetitive and rotten food images salient stimuli. Conversely, in case of a role of vmPFC in valence encoding, the decrease should be confined to appetitive food images, still in the presence of no variations in Glx concentration, having only appetitive food images positive valence. Therefore, the measurement of GABA levels in the vmPFC should contribute to reconstruct part of the neural circuitry underlying reward in humans (Carr and Sesack, 2000; Harte and O’Connor, 2005).
EXPERIMENTAL PROCEDURES
Subjects
Fifteen subjects (seven females and eight males; mean age = 24.3, SD = ±3.8; mean years of education = 13.5, SD = ±1.4) recruited through posted advertisements, participated in the experiment. They had normal or corrected-to-normal vision. Female participants were matched according to their menstrual cycle phase (50% follicular and 50% luteal). Participants were instructed to fast for at least 15 h prior to arriving in the laboratory, but were permitted to drink water. Prior to participating in the experiment, subjects were pre-screened to ensure that they were not overweight (mean BMI = 21.47, SD = ±2.1), not on a diet, or not planning to go on a diet. Subsequently, they were pre-screened by the psychiatrist and, upon arrival at the laboratory, they received general information about the experiment and were asked to complete the questionnaires (listed below). Then, subjects underwent MR imaging.
All the exclusion criteria were as follows: prior history of major medical or psychiatric disorders; head injury or neurological problems; current pregnancy or breastfeeding; history of substance abuse; eating disorders, food allergies and/or intolerances; all kind of medications; tobacco addiction; any contraindications to MRI scanning, including metal implants and claustrophobia. Likewise, participants’ state of mind was assessed by a psychiatrist (GS) to exclude any DSM-5 psychiatric disorder (APA, 2013). Subjects were asked to fill psychological questionnaires: the State-Trait Anxiety Inventory (STAI-Y; Spielberger, 1983); the Barratt Impulsiveness Scale (BIS-11; Patton and Stanford, 1995); the Beck Depression Inventory II (BDI-II; Beck et al., 1996) and the Body Uneasiness Test (BUT; Cuzzolaro et al., 2006). Participants remained naïve as to the purpose of the study until debriefing and were compensated for their participation. All participants gave written informed consent. All research procedures were approved by the Local Institutional Ethics Committee and were performed according to the Declaration of Helsinki (1997) and subsequent revisions.
Stimuli and procedure
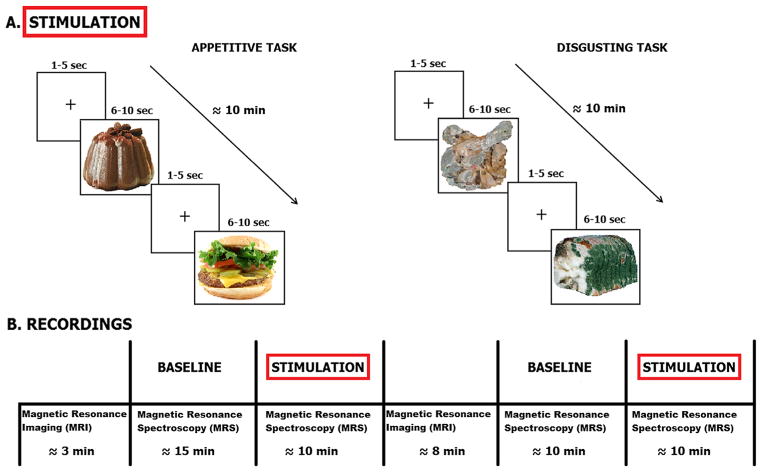
Twenty transformed food images (appetitive stimuli) and 20 rotten food images (aversive stimuli), selected from the FoodCast research image database (FRIDa; Foroni et al., 2013), were used as stimuli. As depicted in Fig. 1, two separate blocks of appetitive and aversive stimuli were presented in counterbalanced order between subjects, so that half of the subjects (N = 8) received the appetitive block first, and the other half (N = 7) received the aversive block first. Each set of stimuli was presented in random order for 10 min. Each image was displayed two times in a block, both in its original arrangement and flipped horizontally (40 images per block), for a duration variable from 6 to 10 s, followed by a 3-s mean inter-stimulus interval (ranging from 1 to 5 s). In order to prevent the intervention of many uncontrollable and unwanted psychological processes (e.g., memory, attention, etc.) we only asked our participants to look at the pictures all the time and, after the recording session, we asked them to report their preferred appetitive images and their most disliked disgusting images (of note, the consistency between participants’ responses suggests that they attended the task with reasonable accuracy). Participants were tested individually and performed the experimental task in one session. The paradigm was completely automated using a software written in E-prime (Psychology Software Tools, Inc.). Stimuli were presented on a computer display that was projected onto a mirror in the MRI scanner. The entire data acquisition lasted approximately one hour.
Fig. 1.
Experimental protocol. (A) Stimulation protocol. Left: appetitive task, right: disgusting task. (B) Magnetic resonance recordings. MRS was recorded during both baseline and stimulation periods. Before baseline structural MRI was also acquired.
MR protocol
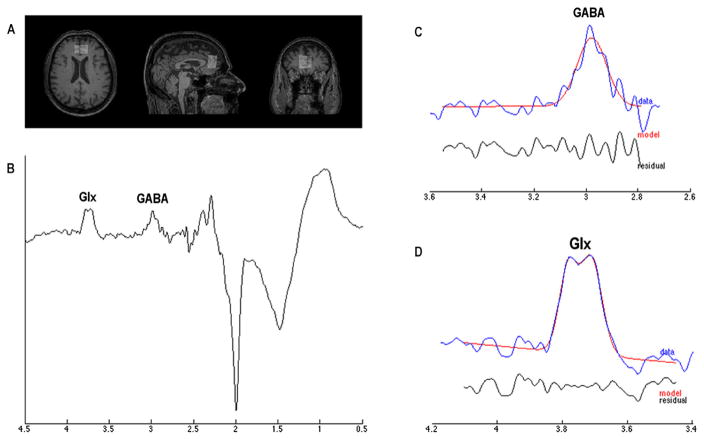
All MR data were acquired with a Philips Achieva 3-T scanner (Philips Medical Systems, Best, The Netherlands) equipped with an 8-channel receiver coil. T1-weighted images were acquired by using a 3D Turbo Field Echo (TR/TE = 11/5 ms, slice thickness of 0.8 mm). According with the experimental design, four 1H MRS spectra were acquired from a voxel of 2.0 (anterior-posterior) × 3.0 (left–right) × 3.0 (craniocaudal) mm3 placed on ventro-medial prefrontal cortex (Fig. 2A). A MEshcher-GArwood Point RESolved Spectroscopy (MEGA-PRESS) sequence (TR/TE = 2000/68 ms, 320 averages) was used to acquire 1024 points within a spectral width of 2000 Hz. MEGAPRESS generates two sub-spectra, with the editing pulse on in one and off in the other. Specifically, an editing pulse is applied to GABA spins at 1.9 ppm in order to selectively refocus the evolution of J-coupling to the GABA spins at 3.02 ppm (ON spectra). In the other, the inversion pulse is applied elsewhere so that the Jcoupling evolves freely throughout the TE (OFF spectra). Subtracting scans acquired without these pulses (OFF spectra) from scans acquired with the editing pulses (ON spectra) removes overlying creatine signals from the edited spectrum, revealing the GABA signal in the difference spectrum (Fig. 2B) (Rothman et al., 1993; Mescher et al., 1998; Mullins et al., 2014). Point-resolved spectroscopy (PRESS) sequence (TR/TE = 2000/68 ms, 32 averages) with and without water suppression was additionally acquired by using chemically shift-selective (CHESS) pulses.
Fig. 2.
Proton Magnetic Resonance Spectroscopy (1H MRS). (A) a voxel (2.0 × 3.0 × 3.0 mm3) was placed into the ventro-medial prefrontal cortex by using T1-weighted image as anatomical reference; (B) representative raw GABA difference spectrum; (C, D) respectively report the representative GANNET-edited spectra (in blue) with estimated GABA and Glx models indicated in red. Residue was shown in black. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
1H MRS analysis
GANNET, a MATLAB-based tool (Edden et al., 2014), was used to assess the GABA+/tCr and the Glx/tCr in each spectrum using default parameters, including frequency and phase correction of time-resolved data using spectral registration (Near et al., 2015). Specifically, the Glx signal was quantified from the 3.75 ppm as pseudodoublet peaks in the GANNET-edited spectrum. Because the signal detected at 3.02 ppm is also expected to include contributions from both macromolecules and homocarnosine, in the rest of the manuscript this signal is labeled as GABA+ rather than GABA, to underline the potential presence of these other compounds (Rothman et al., 1997; Gao et al., 2013). GANNET-estimated signal for GABA and Glx is shown in Fig. 2C and Fig. 2D, respectively.
By using jMRUI (Naressi et al., 2001), the analysis of OFF spectra was performed to investigate the N-acetylaspartate (NAA)/tCr and tCr/water (see Table 2; Naressi et al., 2001). In detail, spectra with water suppression were filtered for removal of residual water by using the Hankel–Lanczos Singular Values Decomposition (HLSVD) algorithm. After autophasing, baseline and frequency shifts correction, a priori knowledge database (N-acetyl aspartate = NAA, 2.02 ppm; total creatine = tCr, 3.03 ppm) was created to put constraints on the Advanced Magnetic Resonance (AMARES) fitting algorithm within the jMRUI package. Using the PRESS sequence, the water signal was quantified to use it as internal reference standard to calculated the absolute tCr quantification (Christiansen et al., 1993;Delli Pizzi et al., 2012, 2013, 2015, 2016). Peak shifts were restricted to ±5 ppm of the theoretical location. Spectra with artifact and metabolites fits with Cramer Rao Lower Bounds above 20% were excluded.
Table 2.
Two-tailed t-test and corresponding significance of tCr levels across all recording blocks (i.e., appetitive baseline and task, and disgusting baseline and task)
| tCr/Water | T14 | Sig. |
|---|---|---|
| Appetitive baseline vs Appetitive task | .444 | .664 |
| Appetitive baseline vs Disgusting baseline | −.217 | .832 |
| Appetitive baseline vs Disgusting task | −.499 | .626 |
| Appetitive task vs Disgusting baseline | −.613 | .551 |
| Appetitive task vs Disgusting task | −.747 | .468 |
| Disgusting baseline vs Disgusting task | −.579 | .572 |
To verify the correspondence between the first and second blocks (baseline+task), two experienced operators visually inspected the location of the MRS voxel on T1-image.
Data analyses
Statistical analyses were performed using StatSoft STATISTICA 8.0.550. Three repeated measures analyses of variances (ANOVAs) were performed in order to assess whether the metabolites/tCr levels differed according the factors Stimulation (baseline vs. task) and Block (positive vs. negative). Moreover, Pearson correlation analyses were used to assess the presence of linear associations between the amount of GABA+/tCr and Glx/tCr during appetitive minus aversive tasks and the obtained scores of the psychometric tests. The difference between GABA+/tCr (baseline-corrected) during appetitive task and GABA+/tCr (baseline-corrected) during aversive task was thus computed. The same procedure was used to calculate the difference between the Glx/tCr and NAA/tCr concentrations in the two conditions. This procedure allowed to correlate the differential salience of appetitive versus aversive food images with individual differences. From the questionnaires we analyzed the total scores, except than for the four BUT sub-scales: weight phobia (WF), body image concerns (BIC), avoidance (Av) and compulsive self-monitoring (CSM) according to the kind of stimuli we presented (food pictures). P-values were considered significant only after Bonferroni correction for multiple comparisons (corrected significance level for correlations: p = 0.00625).
RESULTS
GABA+/tCr, Glx/tCr, and NAA/tCr
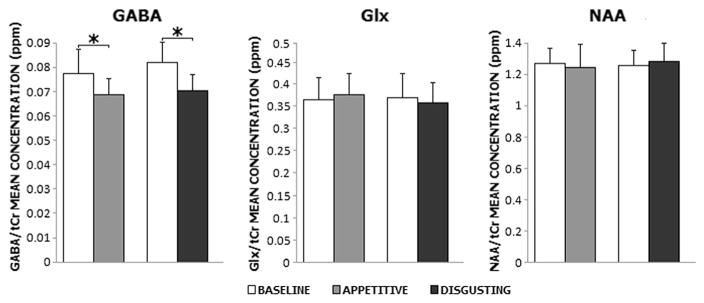
As shown in Fig. 3, a significant effect of Stimulation was observed, with lower average levels of GABA+/tCr levels during task compared to baseline (F(1,13) = 12.645, p = .004). No significant difference was observed between blocks (F(1,13) = 1.366, p = .263). The interaction Stimulation x Block was not significant (F(1,13) = .294, p = .597), with Duncan’s post hoc showing that average levels of GABA+/tCr were lower during the task than during the baseline condition for both blocks (appetitive: p = .02; disgusting: p = .005), and that the appetitive and disgusting conditions differed neither during baseline (p = .199) nor during task (p = .601). No effects of gender were found (F(1,13) = .121, p = .734).
Fig. 3.
Means and standard errors of GABA+/tCr, Glx/tCr (an index of glutamate levels) and NAA/tCr concentrations during baseline (white) and task (gray: appetitive foods; black: disgusting foods). Asterisks indicate statistically significant differences (p <.05)
The average Glx/tCr and NAA/tCr concentrations did not differ according to either Stimulation (Glx/tCr: F(1,13) = .019, p = .893; NAA/tCr: F(1,13) = .000, p = .990) or Block (Glx/tCr: F(1,13) = .286, p = .602; NAA/tCr: F(1,13) = .534, p = .478). The interaction Stimulation x Block was not significant (Glx/tCr: F(1,13) = 1.486, p = .245; NAA/tCr: F(1,13) = 3.784, p = .074). No gender effect emerged (Glx/tCr: F(1,13) = .594, p = .455; NAA/tCr: F(1,13) = .062, p = .807). Table 1 reports mean and standard deviation values for the 3 metabolites.
Table 1.
Mean and standard deviation (st. dev.) metabolite values in appetitive and disgusting food image view at baseline and during tasks
| Metabolites | Appetitive | Disgusting | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Baseline | Task | Baseline | Task | |||||
|
|
|
|
|
|||||
| Mean | St. dev. | Mean | St. dev. | Mean | St. dev. | Mean | St. dev. | |
| GABA/tCr | 0.0774 | 0.0187 | 0.0685 | 0.0072 | 0.0819 | 0.0129 | 0.0703 | 0.0138 |
| Glx/tCr | 0.3636 | 0.0629 | 0.3737 | 0.0598 | 0.3694 | 0.0749 | 0.3581 | 0.0762 |
| NAA/tCr | 1.2679 | 0.1372 | 1.2412 | 0.1316 | 1.2566 | 0.1549 | 1.2808 | 0.1489 |
Finally, tCr stability across all conditions was verified by means of t-tests. These analyses are reported in Table 2.
Correlations with psychometric tests
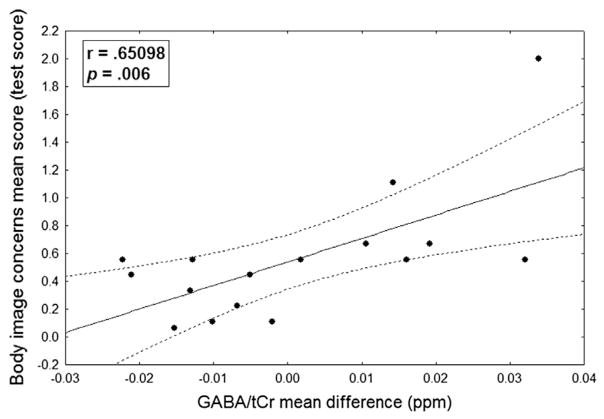
The correlation analysis shown in Fig. 4 highlighted the presence of a positive correlation between the difference in GABA+/tCr (baseline-corrected) during the appetitive task minus GABA+/tCr (baseline-corrected) during aversive task and the scores obtained in the body image concerns sub-scale of BUT (r = .651, p = .006). In contrast to GABA+, the difference Glx/tCr-baseline during appetitive task minus Glx/tCr-baseline during aversive task showed no significant correlation.
Fig. 4.
Correlation between GABA concentration differences and body image concerns score. Scatterplot displaying correlation between GABA concentration differences (appetitive minus disgusting task) and body image concerns score.
DISCUSSION
In the present study, we applied for the first time a noninvasive block-design MRS paradigm to quantify the GABA (and Glx) concentrations in the brain of healthy subjects during the presentation of appetitive and disgusting (rotten) food images. Based on the results obtained in the vmPFC, we observed a decrease of GABA (and no change in Glx) concentrations in both conditions, i.e. observation of appetitive or disgusting food images.
GABA is localized in vmPFC inhibitory interneurons; therefore, we hypothesize that within the vmPFC, low GABA concentration leads to increased activity of pyramidal neurons, which could enhance their excitatory drive to the dopaminergic VTA neurons (Carr and Sesack, 2000; Harte and O’Connor, 2005). Thus, these findings seems to be in accordance with previous studies that claimed an intervention of the medial prefrontal cortex (mPFC) in encoding salience attribution to biological relevant stimuli, such as appetitive and rotten food. Otherwise a decrease in GABA concentration would have been observed while viewing high-value pleasurable images, but not low-value or aversive images. Effectively, other studies carried out by stimulating, lesioning or injecting drugs into the medial prefrontal cortex or into the VTA have associated increases in vmPFC activity (Au-Young et al., 1999; Tzschentke and Schmidt, 2000) with enhanced midbrain activity and dopamine release. In addition, it has been established that chemical or electrical stimulation of mPFC causes an increase in extracellular DA levels in NAc septi (Tzschentke, 2000) Of note, recent evidence has also demonstrated a negative correlation between GABA concentration and BOLD response amplitude (Duncan et al., 2014), suggesting a possible coupling also between lower GABA content and increased vmPFC activity.
A second result is the unchanged concentration in glutamate levels (Glx signal). We take this finding as a control measure for GABA variations, with the intent to ensure that possible general effects due to the mere presentation of images could affect spectral power globally. The observation of no effects on Glx complex levels in the presence of an effect on GABA levels conveys to the latter more reliability. Of note, effects on Glx should be interpreted with caution, as the Glx complex describes the contributions of two substances, i.e. glutamate and glutamine wherein glutamate concentration in the brain is up to 45% higher than glutamine concentration (Jang et al., 2005). Moreover, glutamate is not only the primary excitatory neurotransmitter in the brain, but it is also implicated in several metabolic roles such as in the aminoacid synthesis of GABA (Michels et al., 2012; Rae, 2014). Thus, given that the MRS Glx signal encloses contributions from several glutamate pools, it was not possible to separate the spectral contributions resulting from the neurotransmitter population of glutamate from those resulting from the other glutamate pools. It should be also considered that animal evidence indicated possible alternative explanations based on the observation that mesoaccumbens dopamine release might be facilitated not only by corticoaccumbal glutamatergic projections but also from prefrontal norepinephrine projections to VTA (Ventura et al., 2007). Indeed, Ventura and co-workers demonstrated that the prefrontal norepinephrine transmission, besides mediating the rewarding properties of common drugs of abuse, is also necessary for attributing salience to both appetitive and aversive stimuli.
Considerable work from decision making literature has recognized the vmPFC as an area of the brain that is involved in the representation of the value of a stimulus (Arana et al., 2003; Blair et al., 2006, 2013; Kable and Glimcher, 2007). Despite that, such studies implemented sophisticated decision making tasks, leading to the involvement of additional brain circuits related to attention, emotion encoding, working memory and selection of behavioral responses. On the contrary, our study was based on passive image viewing preventing the intervention of many uncontrolled psychological processes. Thus, it could be possible that results from other studies at least partially arose from the recourse to complex tasks, which imply the involvement of high-order processing functions. In this respect, previous literature reported contradictory results regarding the functional role of vmPFC in food processing. For instance, Killgore and coworkers (2003) suggested that while the amygdala may be responsive to a general category of biologically relevant stimuli such as food, separate ventromedial prefrontal systems may be activated according to the perceived reward value or salience of the same stimuli. Another study (Beaver et al., 2006) carried out with the purpose of analyzing differences in a fronto–striatal–amyg dala–midbrain network activation while viewing appetizing and disgusting food, also found a common activation in the ventral striatum and in the ventral portion of the prefrontal cortex.
One possible account of our results could be represented by the processing of salience, which is ascribable to both appetizing and disgusting food images views. It could be supposed a potential involvement of the vmPFC in salience attribution, thus encoding the salience of the stimuli presented rather than their value. Indeed, we can consider appetitive and rotten food images as opposite poles along the dimension of value; on the contrary, we should assign them similar salience, due to their comparable significance. Thus, it is possible that the reduction of GABA concentration while viewing both appetitive and aversive stimuli is attributable to the processing of their similar salience. A fMRI study (Siep et al., 2009) carried out to investigate the effects of attention, hunger and calorie content on food reward processing, also revealed a significant activation of medial prefrontal cortex, following the presentation of high calorie food when participants were hungry, independently of the focus of the attention. In accordance with the authors’ suggestion, this attention-independent activity in the mPFC might represent the hungry participants’ “awareness” of relevant and salient stimuli, also in conditions in which subjects were asked to pay attention to other stimuli. Moreover, Hasler and co-workers (2010) found a specific GABA reduction in the mPFC when healthy human subjects received occasional shocks, that is during potential aversive events. The same area, with the anterior cingulate cortex, has been recognized as part of the “salience network” (Kullmann et al., 2012). In this regard, a possible functional role of the vmPFC in processing stimulus salience is a fascinating alternative explanation that needs to be verified, considering also the underlying biochemical processes.
Furthermore, a positive correlation was found between the difference in GABA concentration between appetitive (positive) and aversive (negative) tasks and the scores obtained in the body image concerns subscale of BUT (Cuzzolaro et al., 2006). In other words, a greater positive difference between GABA/tCr (baseline-corrected) during the positive task and GABA/tCr (baseline-corrected) during the negative task, that is a comparatively smaller GABA reduction during the positive task, was positively correlated with higher body image concerns or worries related to physical appearance. It has been demonstrated that the BUT-A score significantly contributes to the prediction of general body dissatisfaction, even after controlling for gender and BMI (Pokrajac-Bulian et al., 2014), and that it is useful in the assessment of both clinical and general populations. In general, an unenthusiastic body image can be depicted on a continuum ranging from modest forms without any significant negative consequences for physical health, to clinical manifestations such as anorexia and bulimia nervosa (Cash, 1996; Paxton and McLean, 2010). Body image disorders have proven not only to be a symptom of anorexia and bulimia nervosa, but also important predictors for the development, maintenance and degeneration process of these disorders (Killen et al., 1996; Stice, 2002). Additionally, it has been proposed that body dissatisfaction may be deemed as a mediator between restrained eating style and the development of eating disorders (Ricciardelli et al., 1997) and that food may be particularly reinforcing in food-restricted individuals (Avena et al., 2013). In line with these observations, it could be possible to suggest that people with higher body image concerns are also more prone to restrained eating behaviors. Thus, as regards the present results, we suggest that these people, which consider high-energy rich food images as more salient and arousing stimuli compared to rotten food images, tend to moderate their actual desire to eat those foods. This inhibition could be related to the comparatively smaller GABA reduction in the appetitive vs. aversive task.
To summarize, the present results suggest that viewing appetitive and aversive images caused a decrease in GABA concentration in the vmPFC. Furthermore, a comparatively smaller GABA reduction during the positive task than during the negative task is positively correlated with body image concerns. These results seem to be consistent with the idea that the vmPFC plays a crucial role in processing both appetitive and aversive stimuli, casting new light on its probable function in encoding stimulus salience through sending glutamatergic and/or noradrenergic projections (Ventura et al., 2007) to deeper mesencephalic and limbic areas. Additional studies, however, are needed to clarify how neurotransmitter systems activated by salient rewarding and aversive stimuli could help to offer a substratum in explaining the functioning of the underlying neural systems. Such a new insight could help to develop more specific intervention strategies for individuals belonging to specific clinical population, such as obese and anorexic patients.
Acknowledgments
This study applies tools developed under NIH R01 EB016089 and P41 EB015909; RAEE also receives salary support from these grants.
Abbreviations
- BUT
Body Uneasiness Test
- MRS
magnetic resonance spectroscopy
- NAc
nucleus accumbens
- PRESS
Point RESsolved spectroscopy
- vmPFC
ventromedial prefrontal cortex
- VTA
ventral tegmental area
References
- American Psychiatric Association. DSM. 5. Washington: APA; 2013. [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23(29):9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au-Young SM, Shen H, Yang CR. Medial prefrontal cortical output neurons to the ventral tegmental area (VTA) and their responses to burst-patterned stimulation of the VTA: neuroanatomical and in vivo electrophysiological analyses. Synapse. 1999;34(4):245–255. doi: 10.1002/(SICI)1098-2396(19991215)34:4<245::AID-SYN1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Avena NM, Murray S, Gold MS. Comparing the effects of food restriction and overeating on brain reward systems. Exp Gerontol. 2013;48(10):1062–1067. doi: 10.1016/j.exger.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26(19):5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Marsh AA, Morton J, Vythilingam M, Jones M, Mondillo K, Pine DC, Drevets WC, Blair JR. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J Neurosci. 2006;26(44):11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Otero M, Teng C, Jacobs M, Odenheimer S, Pine DS, Blair RJR. Dissociable roles of ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (rACC) in value representation and optimistic bias. NeuroImage. 2013;78:103–110. doi: 10.1016/j.neuroimage.2013.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38(2):114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Cash TE. The treatment of body image disturbances. In: Thompson JK, editor. Body image, eating disorders, and obesity: an integrative guide for assessment and treatment. Washington, DC: APA; 1996. [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O’Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J Neurosci. 2009;29(39):12315–12320. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen P, Henriksen O, Stubgaard M, Gideon P, Larsson HBW. In vivo quantification of brain metabolites by 1H-MRS using water as an internal standard. Magn Reson Imaging. 1993;11(1):107–118. doi: 10.1016/0730-725x(93)90418-d. [DOI] [PubMed] [Google Scholar]
- Cooper DC. The significance of action potential bursting in the brain reward circuit. Neurochem Int. 2002;41(5):333–340. doi: 10.1016/s0197-0186(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Cuzzolaro M, Vetrone G, Marano G, Garfinkel PE. The Body Uneasiness Test (BUT): development and validation of a new body image assessment scale. Eat Weight Disord. 2006;11(1):1–13. doi: 10.1007/BF03327738. [DOI] [PubMed] [Google Scholar]
- Declaration of Helsinki. Recommendation guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- Delli Pizzi S, Madonna R, Caulo M, Romani GL, De Caterina R, Tartaro A. MR angiography, MR imaging and proton MR spectroscopy in-vivo assessment of skeletal muscle ischemia in diabetic rats. PLoS One. 2012;7(9):e44752. doi: 10.1371/journal.pone.0044752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Pizzi S, Rossi C, Di Matteo V, Esposito E, Guarnieri S, Mariggiò MA, Franciotti R, Caulo M, Thomas A, Onofrj M, Tartaro A, Bonanni L. Morphological and metabolic changes in the Nigro-striatal pathway of synthetic proteasome inhibitor (PSI)-treated rats: a MRI and MRS study. PLoS One. 2013;8(2):e56501. doi: 10.1371/journal.pone.0056501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Pizzi S, Franciotti R, Taylor JP, Thomas A, Tartaro A, Onofrj M, Bonanni L. Thalamic involvement in fluctuating cognition in dementia with Lewy bodies: magnetic resonance evidences. Cereb Cortex. 2015;25(10):3682–3689. doi: 10.1093/cercor/bhu220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Pizzi S, Padulo C, Brancucci A, Bubbico G, Edden RA, Ferretti A, Franciotti R, Manippa V, Marzoli D, Onofrj M, Sepede G, Tartaro A, Tommasi L, Puglisi-Allegra S, Bonanni L. GABA content within the ventromedial prefrontal cortex is related to trait anxiety. Soc Cogn Affect Neurosci. 2016;11(5):758–766. doi: 10.1093/scan/nsv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans – a review of multimodal imaging studies. Neurosci Biobehav Rev. 2014;47:36–52. doi: 10.1016/j.neubiorev.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40(6):1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28(28):7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroni F, Pergola G, Argiris G, Rumiati RI. The food cast research image database (FRIDa) Front Hum Neurosci. 2013;7:51. doi: 10.3389/fnhum.2013.00051. http://dx.doi.org/10.3389/fnhum.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. NeuroImage. 2013;78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte M, O’Connor WT. Evidence for a selective prefrontal cortical GABA B receptor-mediated inhibition of glutamate release in the ventral tegmental area: a dual probe microdialysis study in the awake rat. Neuroscience. 2005;130(1):215–222. doi: 10.1016/j.neuroscience.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Grillon C, Drevets WC, Shen J. Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy. Am J Psychiatry. 2010;167(10):1226–1231. doi: 10.1176/appi.ajp.2010.09070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DJ, Duncan NW, Xu J, Northoff G. A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neurosci Biobehav Rev. 2014;45:350–368. doi: 10.1016/j.neubiorev.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Jang DP, Lee JM, Lee E, Park S, Kim JJ, Namkoong K, et al. Interindividual reproducibility of glutamate quantification using 1.5 T proton magnetic resonance spectroscopy. Magn Reson Med. 2005;53:708–712. doi: 10.1002/mrm.20387. [DOI] [PubMed] [Google Scholar]
- Jayaram P, Steketee JD. Effects of repeated cocaine on medial prefrontal cortical GABAB receptor modulation of neurotransmission in the mesocorticolimbic dopamine system. J Neurochem. 2004;90(4):839–847. doi: 10.1111/j.1471-4159.2004.02525.x. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: effects of valence and arousal. Cogn Affect Behav Neurosci. 2006;6(2):110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Killen JD, Taylor CB, Hayward C, Haydel KF, Wilson DM, Hammer L, Kraemer H, Blair-Greiner A, Strachowski D. Weight concerns influence the development of eating disorders: a 4- year prospective study. J Consult Clin Psychol. 1996;64(5):936–940. doi: 10.1037//0022-006x.64.5.936. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high-versus low-calorie foods. NeuroImage. 2003;19(4):1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Pape AA, Heni M, Ketterer C, Schick F, Häring HU, Fritsche A, Preissl H, Veit R. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cereb Cortex. 2012;23(5):1247–1256. doi: 10.1093/cercor/bhs124. [DOI] [PubMed] [Google Scholar]
- Lin WJ, Horner AJ, Bisby JA, Burgess N. Medial prefrontal cortex: adding value to imagined scenarios. J Cogn Neurosci. 2015;27(10):1957–1967. doi: 10.1162/jocn_a_00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. (EPFL-ARTICLE-177509) [DOI] [PubMed] [Google Scholar]
- Michels L, Martin E, Klaver P, Edden R, Zelaya F, Lythgoe DJ, Luchinger R, Brandeis D, O’Gorman RL. Frontal GABA levels change during working memory. PLoS One. 2012;7(4):e31933. doi: 10.1371/journal.pone.0031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Edden RA Cardiff Symposium on MRS of GABA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, De Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12(2–3):141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015;73(1):44–50. doi: 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paxton SJ, McLean SA. Treatment for body-image disturbances. The treatment of eating disorders: a clinical handbook. 2010:471–486. [Google Scholar]
- Pokrajac-Bulian A, Tončić M, Anić P. Assessing the factor structure of the Body Uneasiness Test (BUT) in an overweight and obese Croatian non-clinical sample. Eat Weight Disord. 2014;20(2):215–222. doi: 10.1007/s40519-014-0166-8. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Ventura R. Prefrontal/accumbal catecholamine system processes emotionally driven attribution of motivational salience. Rev Neurosci. 2012;23:509–526. doi: 10.1515/revneuro-2012-0076. [DOI] [PubMed] [Google Scholar]
- Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39(1):1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- Ricciardelli LA, Tate D, Williams RJ. Body dissatisfaction as a mediator of the relationship between dietary restraint and bulimic eating patterns. Appetite. 1997;29(1):43–54. doi: 10.1006/appe.1997.0093. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. PNAS. 1993;90(12):5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Prichard JW, Petroff OA. Homocarnosine and the measurement of neuronal pH in patients with epilepsy. Magn Reson Med. 1997;38:924–929. doi: 10.1002/mrm.1910380611. [DOI] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198(1):149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory STAI (Form Y) Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- Stice E. Risk and maintenance factors for eating pathology: a meta-analytic review. Psychol Bull. 2002;128(5):825–848. doi: 10.1037/0033-2909.128.5.825. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry. 2012;71(12):1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids. 2000;19(1):211–219. doi: 10.1007/s007260070051. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14(2):131–142. [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward-and aversion-related stimuli. PNAS. 2007;104(12):518–6. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DV, Tsien JZ. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS One. 2011;6(2):e17047. doi: 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]