Abstract
Brain dynamic changes associated with schizophrenia are largely equivocal, with interpretation complicated by many factors, such as the presence of therapeutic agents and the complex nature of the syndrome itself. Evidence for a brain-wide change in individual network oscillations, shared by all patients, is largely equivocal, but stronger for lower (delta) than for higher (gamma) bands. However, region-specific changes in rhythms across multiple, interdependent, nested frequencies may correlate better with pathology. Changes in synaptic excitation and inhibition in schizophrenia disrupt delta rhythm-mediated cortico-cortical communication, while enhancing thalamo-cortical communication in this frequency band. The contrasting relationships between delta and higher frequencies in thalamus and cortex generate frequency mismatches in inter-regional connectivity, leading to a disruption in temporal communication between higher-order brain regions associated with mental time travel.
Introduction
Schizophrenia is considered by many to be a state of ‘fractured mind’, with many researchers refining this definition in terms of pathological alterations in brain connectivity [1,2]. Collectively, the results of such studies do not point to a single, unequivocal difference: the situation is complicated by the different antipsychotic regimens used in different patients, the evolving nature of the syndrome, and the heterogeneity of symptom type, load, and putative precipitating factors. In addition, diverse brain-imaging techniques lead to diverse conclusions, perhaps owing to the highly dynamic nature of brain connectivity itself. Functional interactions between regions have been shown to alter in a state- and task-dependent manner more rapidly than the temporal resolution of current fMRI techniques [3].
Therefore, to understand brain-wide network activity changes, we need to first consider the dynamics of small, local populations of neurons: local network oscillations have been shown to provide a substrate for a rich and labile set of functional connectivity motifs based on dynamic interactions between the same and different frequencies (Box 1). Establishing functional connectivity using multiple frequency bands concurrently is a ubiquitous feature of cortical activity (‘spectral processing’ [4]), but makes interpretation of functional connectivity data difficult, not least when attempting to extrapolate between biochemical processes underlying a specific rhythm and suspected primary pathology in schizophrenia. However, in some cases, the relationships, both dynamic and mechanistic, between concurrently expressed network oscillations are sufficiently deterministic to allow the correlation of molecular pathology with large-scale brain activity. In this review, we consider the pitfalls of looking at single network oscillations in single regions and then attempt to reconcile them by considering broader, pathway-specific cross-frequency activity patterns converging on a derangement of large-scale dynamic connectivity favouring thalamocortical over cortico-cortical pathways in schizophrenia.
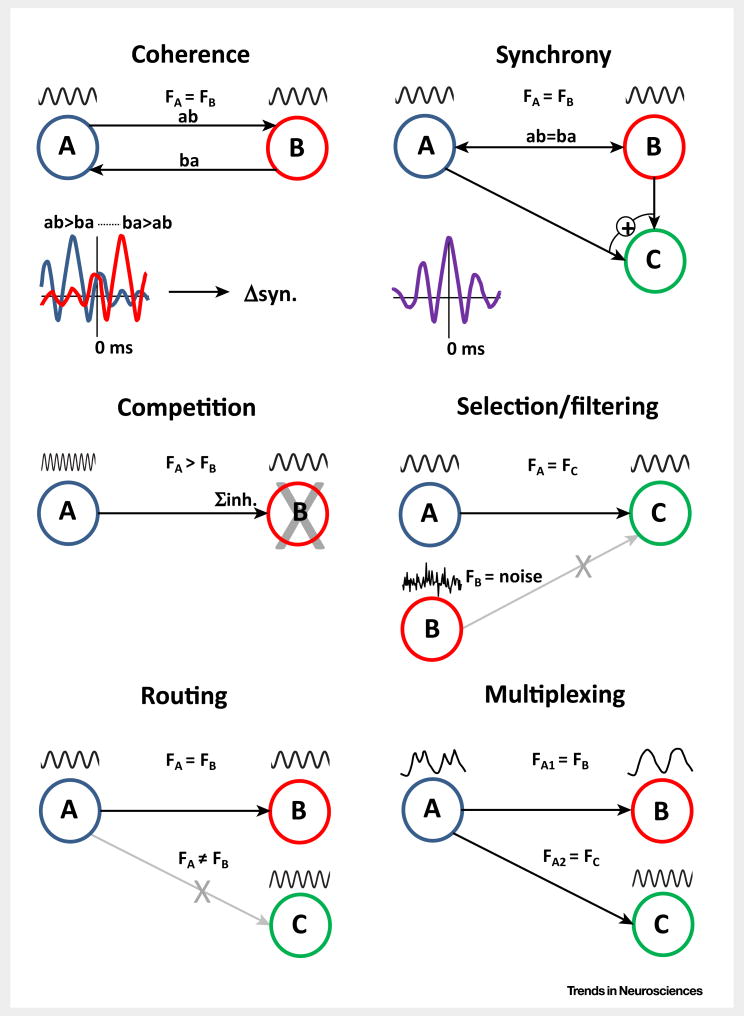
Box 1. Local Network Oscillations Control Larger-Scale Regional Interactions via Multiple Dynamic Mechanisms.
Networks oscillating at approximately the same frequency can communicate through coherence (Figure I), iteratively altering their excitability (probability of outputs) with finite phase relationships [86], with these phase relationships determining the timing of pre- and postsynaptic events upon which changes in synaptic weight critically depend [87]. A ‘special case’ in the coherence state is when all active networks align their phases precisely. This synchronous state among co-active networks provides a strong, collective input to common down-stream regions that is amplified by inherent properties of synaptic transmission in cortex [88]. Regional interactions between locally generated oscillations may also subserve more complex communication strategies: for inhibition-based rhythms (e.g., the gamma rhythm), competition between interconnected networks is potent, with faster frequencies of activity effectively silencing slower partners [89], while the generation of a local network oscillation provides a form of resonance filter allowing selection of the most coherent inputs [90]. An extension of these phenomena is seen in selective ‘routing’ of connectivity, whereby only those downstream network targets sharing the source frequency establish functional connectivity [91]. Finally, single, modal frequencies are rarely seen in cortex. The coexistence of multiple frequencies of oscillation provides a means of multiplexing information within single networks and, thus, allowing multiple, different functionally connected, large-scale networks to exist concurrently.
Diverse Changes in Gamma Rhythm Generation Associated with Schizophrenia and Relevant Models
Perhaps the most widely touted dynamical substrate for cognitive deficits in schizophrenia is the gamma rhythm: an iterative interplay between principal cells and fast-spiking (FS) interneurons via AMPA and GABAA receptor activation in most cortical areas underlying primary sensory processing [5,6]. However, in thalamus, gamma rhythms in thalamocortical cells can arise purely from intrinsic conductances in the absence of synaptic inhibition [7]. Decreases in gamma power and associated cortical communication were first reported at the turn of the century [8,9]. Perhaps the first clear demonstration of a symptom-related reduction in gamma rhythms was the observation of decreased anterior-posterior coherence, manifest between ca. 30 Hz and 50 Hz, in patients with schizophrenia performing a visual Gestalt task [10]. Further studies by these authors also showed a gamma band-related deficit in auditory sensory processing [11]. Other work also supports a reduction in task-dependent gamma oscillations, but at higher frequencies (>50–60 Hz [12]) in schizophrenia, with no replication of the above findings in chronic, medicated patients [12] and drug-naïve, first-episode patients [13].
Further studies add to the confusion by demonstrating that some patients demonstrate increased gamma oscillations and associated connectivity [14–16]. In addition, measurement of gamma rhythms in patients with specific polymorphisms associated with schizophrenia revealed that most correlated with elevated, rather than reduced, gamma power [17]. This equivocality in the literature may relate to the difference between baseline (‘resting state’) gamma rhythms and those activated specifically by task [18], but this is not always the case: both reduced gamma power during resting state [19] and, conversely, excessive gamma rhythms during tasks known to be highly sensitive to schizophrenia pathology [16] have been reported.
Genetic models of schizophrenia-like behavioural and cognitive deficits also show mixed effects on gamma rhythms. Neuregulin, erbB4, and calcineurin mutations all cause schizophrenia-like symptoms and increased gamma rhythm generation [20–22], whereas LPA1 knockout caused either no change or a decrease, depending on the region studied [23]. Different primary mechanisms for disrupted gamma may be at play in these schizophrenia-related control systems, with Neuregulin/erbB4 enhancing excitatory input onto FS interneurons, calcineurin enhancing postsynaptic GABAergic responses via a selective reduction in long-term depression at this locus, and LPA1 coupling NMDA responses to mechanisms of plasticity. However, each of these models has effects on NMDA receptor function (not only in FS interneurons), linking back to one of the earliest acute models of schizophrenia-like symptoms: ketamine administration [24]. More recent work has shown that, in vivo, ketamine enhances frontal cortical gamma power in a highly NR2A subunit-dependent manner [25]. This subunit, in particular, has been implicated in linking deficits in excitation to interneurons with other postmortem markers of schizophrenia, such as parvalbumin immunopositive cell number and GAD67 expression levels (reviewed in [26]). However, GAD67 is also expressed in somatostatin and calretinin-containing interneurons [27], NR2A-containing receptors are also selectively involved in interhemispheric, not intrahemispheric cortical excitation of principal cells [28] and LTP in hippocampus, and GAD67 expression levels do not always change with experimental lesions of pathways reported to be adversely affected in schizophrenia pathology [29]. In addition, effects on NMDA receptor blockade on locally generated, adult cortical gamma rhythms have been shown to be dependent both on the region studied and the source of excitation leading to rhythmogenesis (Figure 1). In particular, in primary auditory cortex, different effects on gamma rhythms were seen with acute ketamine application depending on the presence of cholinergic neuromodulation associated with attention.
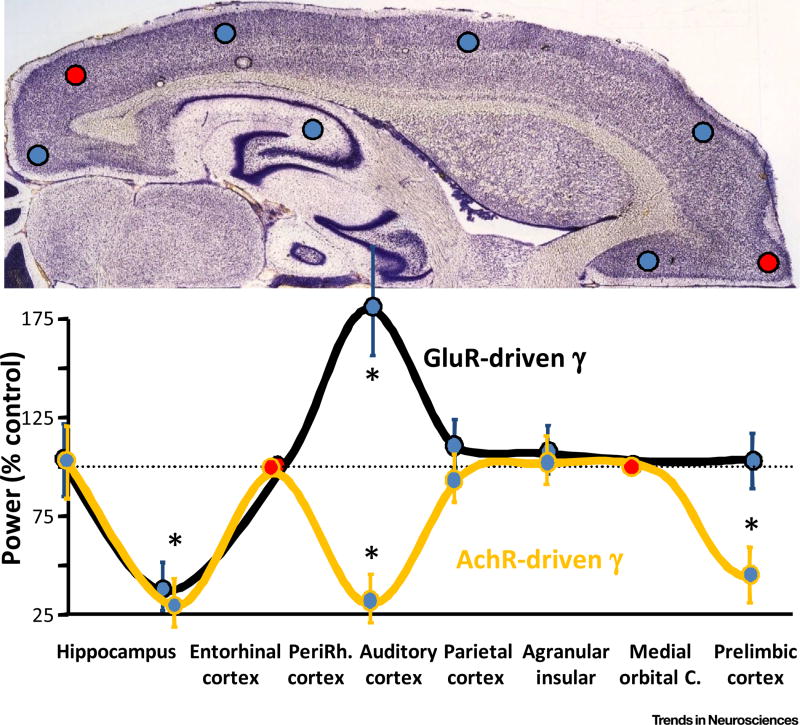
Figure 1. Gamma Frequency Oscillations Respond to Reduced NMDA Receptor-Mediated Excitation Differently Depending on Brain Region and Mechanisms of Generation.
Data from in vitro experiments using isolated slices of individual cortical regions (indicated by the circles in the top panel). Gamma oscillations were evoked by either kainate (gluR-driven gamma) or carbachol (AchR-driven gamma) except in perirhinal and medial or ‘ormital’ = orbitalital cortices, where neither excitation-generated gamma rhythms occurred (red circles). Data show the power of local field potential gamma rhythms in the presence of ketamine relative to control gamma power. Note the overt regional differences in the effect of ketamine in each condition and the contrasting effects of ketamine in auditory cortex depending on the method of gamma rhythm generation. Adapted from [83].
Changes in Theta and Delta Rhythms in Schizophrenia
A meta-analysis of data suggested that elevations in power of lower EEG frequencies during wakefulness (theta and delta band activity, 1–8 Hz) are a more robust electrophysiological marker for schizophrenia [30], a suggestion that fits well with the overt deficits in related sleep structure [31]. However, the literature is again equivocal. Decreases in delta rhythm power and coherence have been reported [14,32,33] almost as frequently as elevations [34–36]. Observations of wake-state theta activity are similarly mixed. General increases in theta activity have been reported in a resting state and in a task/stimulus-dependent manner [34,37] as well as decreases [33,38].
Functionally, the delta rhythm appears to be as important in the wake state as in NREM sleep. For example, 1–4-Hz oscillations coordinate cortical activity during waking movement, ketamine-induced sedation, and natural sleep [39]. Cortico-cortical coherence at delta frequency is a correlate of decision-making [40], modulates responses to repetitive stimuli, and is vital for processing speech [41]. Conversely, thalamic delta rhythms, associated strongly with NREM sleep, appear to reduce communication between cortical areas [42], a signature of disrupted cortical connectivity in schizophrenia [43].
This apparent dichotomy in delta rhythm origin and effect on cortico-cortical versus thalamo-cortical communication may reflect the region-specific mechanism of local-circuit delta rhythm generation: the hippocampus appears not to generate its own, local delta rhythm. The thalamus generates delta frequency outputs at the single cell level in thalamocortical neurons [44]. Burst firing in this neuronal subclass is generated by interplay between intrinsic burst behaviour and a hyperpolarisation-activated conductance (Ih). In neocortex, delta rhythms arise from intrinsically bursting (IB) layer 5 pyramidal cells, coordinated via mutual recurrent excitation and shared GABAB receptor-mediated inhibition (e.g., Figure 2B).
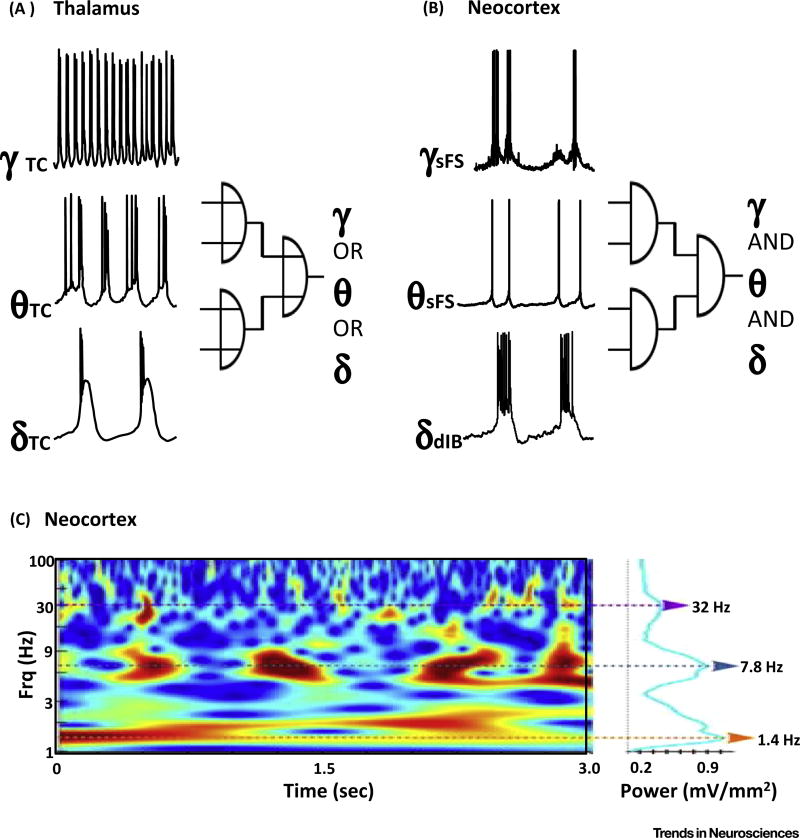
Figure 2. Gamma, Theta and Delta Rhythms Nest Robustly in Local Circuits of Neocortex but Are Mutually Exclusive in Thalamocortical Neurons.
(A) Example thalamocortical neuronal recordings under different neuromodulatory conditions and levels of excitation. Delta rhythms are generated by thalamocortical cells in the absence of cholinergic neuromodulation. Theta rhythms occur under the influence of metabotropic glutamate or acetylcholine receptor activation. Fast rhythmic bursting (FRB) at gamma frequencies arises from these neurons under conditions of reduced BK channel activity in a similar manner to FRB activity underlying persistent gamma rhythms in neocortex [84]. (B) Example neuronal recordings during spontaneous delta rhythms in neocortical slices under dopamine receptor blockade. In these conditions, delta rhythms arise from deep layer (5a) intrinsically bursting neurons (dIB). By contrast, deep layer regular-spiking neurons (dRS) generate packets of theta frequency output temporally aligned (nested) with the dIB cell bursts. dRS neuron theta frequency outputs generate theta frequency and compound excitatory postsynaptic currents (EPSPs) in superficial fast-spiking neurons (sFS), leading to bursts of gamma-frequency output. (C) The coexistence of gamma, theta, and delta rhythms seen in vitro (B) is also commonly seen in invasive recordings from awake, behaving non-human primates. Reproduced, with permission, from [7,44] (A) and [46] (B). Adapted, with permission, from [92] (C).
The importance of GABAB receptors in generating neocortical delta rhythms suggests disruption in this facet of synaptic inhibition in schizophrenia and accompanying NREM sleep deficits. GABBR2 and GABBR1 subunit expression is significantly reduced in patients with schizophrenia [45]. In addition, the recurrent connectivity between IB neurons, required to generate neocortical delta rhythms, is NMDA receptor dependent. NMDA receptor antagonists almost abolish neocortical delta rhythms [46] in vitro. By stark contrast, systemic administration of NMDA receptor antagonists generated a pattern of increased delta rhythms in neocortex in vivo [47]. These neocortical delta rhythms were associated with a hugely elevated delta rhythm projected from thalamus. More recent work by Lisman and colleagues has shown that reduced NMDA function in thalamus, particularly via NR2C-containing receptors known to be reduced in schizophrenia, shifted the excitability state of thalamocortical neurons to one favouring delta rhythm generation [48]. Other NMDA antagonists, such as phencyclidine (PCP), have been shown to have the opposite effect: a decrease in thalamic delta rhythms [49]. However, PCP acts via sigma receptors, which only form functional complexes with certain NMDA subunits. It also has a direct effect on nicotinic acetylcholine receptors (part of the spectrum of neuromodulators thought to be altered in schizophrenia [50]) and has additional effects on potassium channels and IP3 receptors in neurons [51]. Thus, as with gamma rhythms in auditory cortex (Figure 1), a single experimental insult (selective NMDA receptor blockade) has opposing effects on delta rhythm generation in different brain regions.
Theta rhythms have a vital role in temporally coordinating hippocampal-dependent memory functions both within the hippocampus and between the hippocampus and entorhinal and prefrontal areas [52]. In prefrontal neocortex, theta rhythms are strongly associated with decision making and semantic processing [53]. As with gamma and delta rhythms, the theta rhythm arises from different mechanisms in different brain regions. In hippocampus, theta rhythms are not only dependent on theta frequency cholinergic and GABAergic inputs from medial septum/diagonal band, but can also be locally generated through the interplay between basket and oriens lacunosum moleculare (OLM) interneurons and principal cells [54] (Figure 3). In thalamus, thalamocortical neurons have an intrinsic theta rhythmicity that manifests particularly in neuromodulatory states [7]. In neocortex, theta rhythm generation appears to arise from interactions between the intrinsic properties of layer 5 regular spiking (RS) neurons and their superficial layer interneuronal targets [46].
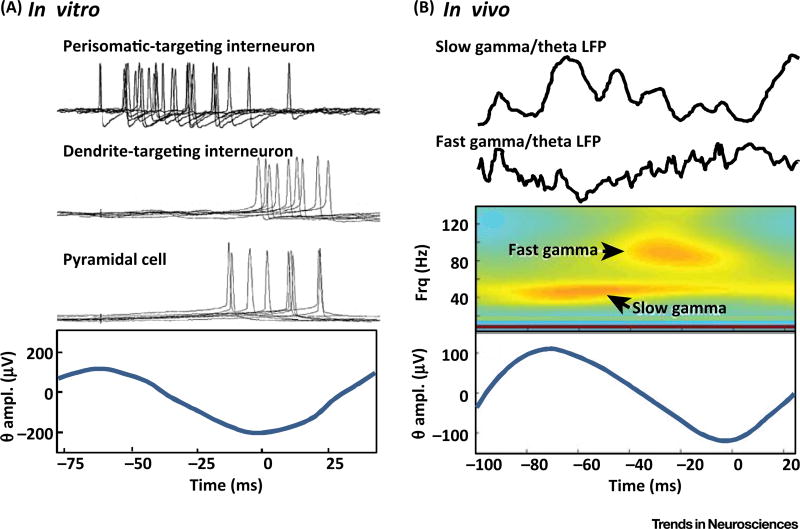
Figure 3. Gamma and Theta Rhythms Nest Robustly in Hippocampus.
(A) In vitro recordings from neurons in rodent hippocampal area CA1 during pharmacologically induced theta rhythms. Note epochs of loosely timed gamma frequency spikes from fast-spiking (FS) interneurons (perisomatic-targeting interneurons) precede spikes on oriens lacunosum moleculare (OLM) interneurons (dendrite-targeting interneurons) and pyramidal cells. Individual intracellular recordings are shown time-locked to the filtered local field potential (LFP) theta rhythm (lower panel). (B) In vivo recordings in awake behaving rats during a spatial learning task showing distinct theta-nested gamma oscillations in two distinct frequency bands. Raw LFP data and a spectrogram are shown relative to the theta-filtered LFP average. Reproduced, with permission, from [54] (A) and [58] (B).
Any direct relationship between theta rhythm generation and recognised primary pathological changes associated with schizophrenia is unclear. In hippocampus, the inherent theta frequency resonance of OLM interneurons is dependent on the expression of a Ih, although this does not appear to be directly associated with the syndrome. Conversely, large changes in markers for dendrite-targeting interneurons in general, such as OLM cells, have been reported in postmortem studies [55]. In general, however, it is difficult to directly relate underlying pathology to any alterations in power of theta rhythms in patients with schizophrenia. To understand this further, we need to consider the relationship between theta rhythms and other locally co-expressed frequencies.
The Gamma–Theta–Delta Cross-Frequency Relationship Points to Essential Synergism in Spatiotemporal Communication
Cross-frequency interactions are common in cortex. Several types of inter-relationship between coexpressed frequency bands have been reported [56] and have been specifically implicated in the coupling of local network oscillations to large-scale cortico-cortical interactions [57] of the type often correlated with schizophrenia pathology (see below). The cross-frequency interaction most relevant to this review is that of nesting: the modulation of the amplitude of a higher frequency by the phase of a lower frequency. This has been studied in detail in hippocampus [52], where gamma rhythms are nested strongly in theta rhythms [58] (Figure 3). Other forms of cross-frequency interaction exist that may be relevant to schizophrenia, particularly involving alpha and gamma rhythms [59], but they are not considered here.
While it is difficult to see a direct relationship between hippocampal theta and known molecular risk factors for schizophrenia, a more robust interaction can be uncovered when considering this gamma–theta nested activity. FS interneuron function (implicated in changes in gamma rhythms, see above) has a positive influence on local theta generation. Gamma frequency FS inhibition of OLM interneurons serves to reset Ih and facilitate theta-frequency spiking in this interneuron subtype [60]. In addition, local NMDA receptor function modulates theta rhythms [54] in a hippocampal subregion-specific manner, and entorhinal FS interneurons, powerfully driven by NMDA receptors, underlie gamma rhythms in this area [61] and send long-range inhibitory projections to hippocampus to facilitate theta rhythm generation therein [62].
Thalamocortical neurons can generate either theta rhythms in a manner dependent on their neuromodulatory state (metabotropic glutamate- or acetylcholine-mediated inputs [7]), or gamma rhythms under conditions of intense excitation (Figure 2). While both rhythms could be generated concurrently in different thalamic nuclei, it is hard to see with this mechanism how local co-activation might occur. By contrast, neocortex generates all three frequencies of interest in a hierarchically nested fashion [63]. The degree of interdependence is high, with delta rhythms, originating from layer 5 IB-containing local circuits, driving intrinsic theta rhythmogenesis in layer 5 RS neurons, which, in turn, send ‘backward-projecting’ inputs to superficial layer FS interneurons to generate bursts of gamma activity (Figure 2 [46]). This pattern of local-network, cross-frequency interaction has been shown to powerfully control information transfer in neocortex [64]. The inherent, bidirectional interlaminar nature of this neocortical nesting motif has also been shown to be important for temporally segregating formation and retrieval of memories [64].
Multiple potential mechanisms for disruption of this triple-frequency nesting behaviour have been shown. Of particular relevance to schizophrenia are the following: reduced GABAA receptor-mediated inhibition potentiates neocortical delta rhythm power, but effectively uncouples the delta rhythm from the nested gamma rhythm [46]. By contrast, reduced GABAB receptor-mediated inhibition almost abolishes this rhythm [46]. The balance between delta and theta rhythm expression is critically dependent on superficial layer inhibitory circuits and, thus, is highly dependent on α7-containing nicotinic acetylcholine receptors [65]. In addition, the backward projection from deep to superficial layers underlying the interlaminar nature of this dynamic signature is largely dependent on NMDA receptor-mediated synaptic excitation.
From the above, we suggest that the variable observations of power of the above individual frequencies reflect different region-specific magnitude changes in individual patients. In addition, observed changes in one frequency may not directly reflect pathology in the core mechanism of that rhythm, but rather an alteration in the degree of nesting with lower frequencies. This is particularly apparent for neocortex (Figure 2), which is the primary source for most EEG/MEG measurements in patients. For example, whether an increase or decrease in delta rhythm power is seen globally would depend on the relative pathology-induced increases in thalamic delta rhythms or decreases in neocortical delta rhythms. Increases or decreases in theta rhythm generation would also depend on the relative magnitude of the neocortical delta rhythm, nesting theta rhythms there, and hippocampal/entorhinal theta generators. Furthermore, changes in gamma rhythm expression would be expected to be positively correlated with neocortical theta rhythms, but negatively correlated with hippocampal theta rhythms. In addition, the exclusive, state-specific nature of the generation of each of these rhythms in thalamocortical cells indicates a mutually antagonistic relationship between each of these frequency bands in the contribution of this region to the observed, brain-wide oscillatory power.
Relation to Brain-Wide Functional Connectivity Changes
The diverse array of changes in oscillations discussed above is also seen in connectivity studies. The field is complicated by the disconnect between structural and functional MRI measures (reviewed in [66]), the complexity of the relationship between classical EEG frequencies and the fMRI signal, and the absence of a clear relationship between structural connectivity and electrophysiological coherence measures. However, in some cases, both network oscillation and structural connectivity changes appear to coincide. This is particularly apparent when considering the default mode network (DMN), its internal connectivity, interaction with hippocampus, and relative connectivity with respect to primary sensory and attentional cortical areas [67,68] (Figure 4). The function of this network has been proposed to fit with many of the symptoms of ‘thought disorder’ in schizophrenia [69]. The DMN is active in patients with schizophrenia, but both functional and structural connectivity is altered [1,70]. The normal, delta rhythm-related cortico-cortical connectivity between DMN regions is significantly reduced [71], as is task-related deactivation in favour of sensory and executive areas [72]. This ‘persistence’ of DMN activity is also seen in connectivity between frontal DMN loci and the hippocampus [73], but, interestingly, it is negatively correlated with hippocampal theta power in at-risk subjects.
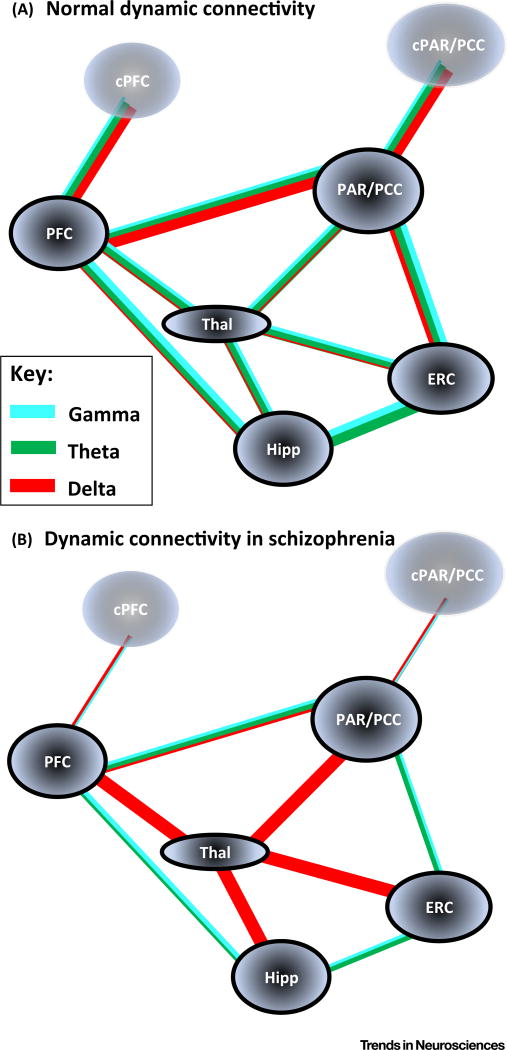
Figure 4. Frequency Mismatches in Core Default Mode Network (DMN) Regions and Related Sub- and Temporal Cortical Areas May Disrupt Internal Memory Recall/’Mental Time Travel’ Networks.
(A) Cartoon suggesting the core large-scale dynamic connectivity pathways and the relative contributions of locally generated delta (1–4 Hz, red lines), theta (5–8 Hz, green lines), and gamma (30–80 Hz, blue lines) rhythms to their functional interactions. The main neocortical regions of note form the core anterior and posterior components of the DMN (bilaterally represented), coupled predominantly with delta rhythms (see main text). In addition, the connectivity of these nodes with archi- and periallocortical areas (hippocampus and entorhinal cortex), and relevant thalamic nuclei (anterior and midline) is shown. Note the overall balance between gamma/theta-mediated interactions and the dominance of ipsilateral cortico-cortical delta-mediated interactions. (B) Cartoon summarising the precedented functional connectivity changes and local network oscillation changes in the network summarised in (A). Note the overall deficit in direct cortico-cortical interaction frequencies in favour of indirect interactions mediated by excessive thalamic delta rhythm generation. Interhemispheric interactions are shown losing their faster frequency components in line with the deficits in NR2A receptors in schizophrenia, the higher temporal precision and selective role of this subunit in inter- versus intrahemispheric connectivity [28], and the observation of interhemispheric delta phase-modulated coupling during hallucinations [85]. Reported changes in primary sensory and attentional network are not illustrated here for clarity and brevity. Abbreviations: ERC, entorhinal cortex; Hipp, hippocampus; PFC, prefrontal cortex (cPFC, contralateral); Par/PCC, parietal cortex/posterior cingulate cortex (cPAR/PCC, contralateral); Thal, thalamus.
Functional connectivity between DMN- and hippocampus-associated thalamic nuclei is also enhanced in patients [2,43]. This enhanced, and persistent connectivity correlates with enhanced thalamic delta rhythm generation via a predominantly NR2C receptor subunit-dependent mechanism (reviewed in [74]). Thus, the balance between the two key prefrontal-hippocampal pathways may be biased away from the theta rhythm-coordinated direct pathway vital for sequential memory, which is reduced in animal models [75], and towards the indirect pathway via thalamic nucleus reuniens [76], coordinated at the slower delta frequency. Working memory maintenance is dependent on theta-frequency fluctuations in hippocampal activity; thus, replacement of this temporal signature with the slower delta rhythm may underlie the prolonged temporal discrimination window seen in patients [77]. This situation would also create a temporal disconnect between prefrontal and thalamic inputs to hippocampus and the strong gamma/theta frequency-dependent connectivity with entorhinal cortex also vital for memory formation and retrieval in humans [78].
In considering how the enhanced delta frequency-mediated functional connectivity between hippocampus and frontal neocortical regions fits with reduced DMN cortico-cortical connectivity, we need to consider regional differences in delta rhythm generation (see above; Figure 4). In models of symptoms of schizophrenia, the intrinsic thalamocortical neuronal delta rhythm is exposed by reduced NMDA receptor function [79]. By stark contrast, reduced NMDA receptor function in local association neocortical circuits almost abolishes delta rhythms and their nested theta oscillation [46]. Therefore, this single experimental manipulation would be expected to generate two of the main connectivity changes in patients discussed above: (i) enhanced prefrontal-thalamic-hippocampal circuit function at delta frequency; and (ii) reduced cortico-cortical DMN connectivity at delta frequency. This brain-wide bias may, in part, also explain three of the other connectivity disruptions associated with schizophrenia: (i) reduced cortico-cortical, long-range coupling along the frontoparietal attention axis [67]; (ii) reduced sensory cortical activity spread generated by ascending thalamic delta rhythms [42]; and (iii) disrupted interhemispheric connectivity [80]. This latter facet of disrupted brain function in patients is one of the oldest connectivity theories and is supported by the link between schizophrenia pathology and expression of the NR2A NMDA receptor subunit (see above) and the selective dominance of this subunit in transcallosal excitatory synaptic connectivity [28]. However, care is needed here in distinguishing cause and effect, given that NR2A expression levels are highly dependent on prior sensory experience and early life stress [81].
Concluding Remarks
Evidence for specific, brain-wide disruptions in individual network oscillation frequencies is not robust in patients with schizophrenia. This perhaps is not surprising given that the same frequency of oscillation may arise through different combinations of mechanisms in different brain regions. Furthermore, individual oscillations are differentially interrelated in different brain regions. Therefore, attempting to uncover an electrophysiological signature for network oscillation as solid as, for example, basic mismatch negativity deficits, may appear a thankless task (see Outstanding Questions). However, by considering the underlying mechanisms and inter-relationships of just three cardinal rhythms (gamma, theta, and delta), and their role in coordinating activity within and between neocortex, thalamus, and hippocampus, some progress can perhaps be made. Using this focus, the altered temporal communication of DMN activity and the enhanced strength and persistence of its functional connectivity to hippocampus can be seen as a putative substrate for linking cognitive deficits with dysfunction of network rhythms and, ultimately, with primary pathology.
Outstanding Questions.
Given the large number of genetic mutations associated with symptoms of schizophrenia, it is unlikely that a single locus for pathology exists at the cellular or synaptic level. However, how complex a substrate do we have to consider? Or does this syndrome represent genuinely ‘brain-wide’ aberrant function?
Many of the cognitive deficits seen in patients can be directly related to altered spatiotemporal activity patterns, but are not exclusively associated with the syndrome, nor are they particularly labile to effective antipsychotic drug therapies. Is a focus on readily quantifiable primary sensory and memory dysfunction then likely to tell us anything useful about specific pathology at the neural dynamics level?
Dynamic connectivity motifs associated with altered affect, and deranged ‘theory of mind’ aspects of the syndrome may provide a more specific means to understand the link between molecular disease processes and symptoms more directly related to schizophrenia. However, do we have the tools to study this? Many brain regions involved are ‘deep’ and, thus, while amenable to MRI techniques, are hard to access in terms of spatiotemporal data sets using current non-invasive electrophysiological techniques.
A better understanding is needed of the relationship between EEG-frequency neuronal network oscillations and fMRI measures of activity. Is a more direct association between region-specific, cortical lamina-specific brain rhythms and accompanying BOLD signals in multiple neuromodulatory states likely quantifiable in the near future?
Finally, differences in the dominance of the DMN, particularly in the degree of coupling to hippocampus and medial temporal cortex, are seen in healthy individuals correlated with aspects of mind-wandering termed ‘mental time travel’ (MTT [82]). Mind wandering is defined as a disconnect between brain function causally related to current external sensory input in favour of recall of episodic and semantic memories. The DMN is active in this mental state, serving as a hub for wider cortical networks involving temporal cortical structures, including hippocampus. From this, it is tempting to suggest that mechanisms underlying the well-documented deficits in cognitive task performance involving working memory in schizophrenia are also, in part, responsible for the more numinous aspects of disordered thought that more specifically characterise this syndrome.
Figure I.
Trends.
Evidence for a brain-wide, syndrome-wide deficit in higher frequency [particularly gamma (30–80 Hz)] network oscillations in schizophrenia is equivocal.
Evidence for changes in lower frequency (delta/theta) network oscillations is stronger, but also plagued by inconsistencies.
Different oscillations are generated by different underlying mechanisms in different brain areas and can even emerge through different mechanisms in the same brain area.
Region-specific differences in the interdependence of, and dynamic relationship between, different frequency bands complicate interpretation of non-invasive recordings in schizophrenia.
The variability in clinical electrophysiological findings likely represents a move away from spatiotemporally balanced network oscillations to a state where bias exists through regional deficits (e.g., for delta rhythms in neocortex) and enhancements (e.g., delta rhythms in thalamic nuclei).
Acknowledgments
The authors thank the Wellcome Trust and NIH/NINDS (RO1NS044133).
References
- 1.Garrity AG. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 2.Skudlarski P. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol. Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopell NJ. Beyond the connectome: the dynome. Neuron. 2014;83:1319–1328. doi: 10.1016/j.neuron.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palva JM, et al. Phase synchrony among neuronal oscillations in the human cortex. J. Neurosci. 2005;25:3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittington MA, et al. Multiple origins of the cortical gamma rhythm. Dev. Neurobiol. 2011;71:92–106. doi: 10.1002/dneu.20814. [DOI] [PubMed] [Google Scholar]
- 7.Hughes SW. Novel modes of rhythmic burst firing at cognitively-relevant frequencies in thalamocortical neurons. Brain Res. 2008;1235:12–20. doi: 10.1016/j.brainres.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon JS, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch. Gen. Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haig AR, et al. Gamma activity in schizophrenia: evidence of impaired network binding? Clin. Neurophysiol. 2000;111:1461–1468. doi: 10.1016/s1388-2457(00)00347-3. [DOI] [PubMed] [Google Scholar]
- 10.Spencer KM, et al. Abnormal neural synchrony in schizophrenia. J. Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer KM, et al. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol. Psychiatry. 2008;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhlhaas PJ, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J. Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L, et al. Evidence for dysregulated high-frequency oscillations during sensory processing in medication-naïve, first episode schizophrenia. Schizophr. Res. 2013;150:519–525. doi: 10.1016/j.schres.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 14.de la Salle S, et al. Effects of ketamine on resting-state EEG activity and their relationship to perceptual/dissociative symptoms in healthy humans. Front. Pharmacol. 2016;7:348. doi: 10.3389/fphar.2016.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn G, et al. Increased absolute magnitude of gamma synchrony in first-episode psychosis. Schizophr. Res. 2008;105:262–271. doi: 10.1016/j.schres.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Barr MS, et al. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr. Res. 2010;121:146–152. doi: 10.1016/j.schres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Demiralp T, et al. DRD4 and DAT1 polymorphisms modulate human gamma band responses. Cereb. Cortex. 2007;17:1007–1019. doi: 10.1093/cercor/bhl011. [DOI] [PubMed] [Google Scholar]
- 18.Andreou C, et al. Increased resting-state gamma-band connectivity in first-episode schizophrenia. Schizophr. Bull. 2015;41:930–939. doi: 10.1093/schbul/sbu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutter L, et al. Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Hum. Brain Mapp. 2009;30:3254–3264. doi: 10.1002/hbm.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Pino I, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Fisahn A, et al. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb. Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh J, et al. Impaired hippocampal ripple-associated replay in a mouse model of schizophrenia. Neuron. 2013;80:484–493. doi: 10.1016/j.neuron.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham MO, et al. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J. Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 25.Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol. Psychiatry. 2012;71:987–995. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen SM, et al. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr. Res. 2015;167:98–107. doi: 10.1016/j.schres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamamika N, et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 28.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci. STKE. 2004;255:16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 29.Volk DW, Lewis DA. Effects of a mediodorsal thalamus lesion on prefrontal inhibitory circuitry: implications for schizophrenia. Biol. Psychiatry. 2003;53:385–389. doi: 10.1016/s0006-3223(02)01547-0. [DOI] [PubMed] [Google Scholar]
- 30.Sieckmeier PJ, Stufflebeam SM. Patterns of spontaneous magnetoencephalographic activity in patients with schizophrenia. J. Clin. Neurophysiol. 2010;27:179–190. doi: 10.1097/WNP.0b013e3181e0b20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keshavan MS, et al. Electroencephalographic sleep in schizophrenia: a critical review. Compr. Psychiatry. 1990;31:34–47. doi: 10.1016/0010-440x(90)90052-t. [DOI] [PubMed] [Google Scholar]
- 32.Simmonite M, et al. Reduced event-related low frequency EEG activity in patients with early onset schizophrenia and their unaffected siblings. Psychiatry Res. 2015;232:51–57. doi: 10.1016/j.pscychresns.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Donkers FC, et al. Reduced delta power and synchrony and increased gamma power during the P3 time window in schizophrenia. Schizophr. Res. 2013;150:266–268. doi: 10.1016/j.schres.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Kim JW, et al. Diagnostic utility of quantitative EEG in unmedicated schizophrenia. Neurosci. Lett. 2015;589:126–131. doi: 10.1016/j.neulet.2014.12.064. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann D, et al. Functionally aberrant electrophysiological cortical connectivities in first episode medication-naive schizophrenics from three psychiatry centers. Front. Hum. Neurosci. 2014;8:635. doi: 10.3389/fnhum.2014.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayanan B, et al. Multivariate genetic determinants of EEG oscillations in schizophrenia and psychotic bipolar disorder from the BSNIP study. Transl. Psychiatry. 2015;5:e588. doi: 10.1038/tp.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frantseva M, et al. Disrupted cortical conductivity in schizophrenia: TMS-EEG study. Cereb. Cortex. 2014;24:211–221. doi: 10.1093/cercor/bhs304. [DOI] [PubMed] [Google Scholar]
- 38.Garakh Z, et al. EEG correlates of a mental arithmetic task in patients with first episode schizophrenia and schizoaffective disorder. Clin. Neurophysiol. 2015;126:2090–2098. doi: 10.1016/j.clinph.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Hall TM, et al. A common structure underlies low-frequency cortical dynamics in movement, sleep, and sedation. Neuron. 2014;83:1185–1199. doi: 10.1016/j.neuron.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nacher V, et al. Coherent delta-band oscillations between cortical areas correlate with decision making. Proc. Natl. Acad. Sci. U.S.A. 2013;110:15085–15090. doi: 10.1073/pnas.1314681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riecke L, et al. Endogenous delta/theta sound-brain phase entrainment accelerates the buildup of auditory streaming. Curr. Biol. 2015;25:3196–3201. doi: 10.1016/j.cub.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 42.Massimini M, et al. Triggering sleep slow waves by transcranial magnetic stimulation. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8496–8501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damaraju E, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes SW, et al. Dynamic clamp study of Ih modulation of burst firing and delta oscillations in thalamocortical neurons in vitro. Neuroscience. 1998;87:541–550. doi: 10.1016/s0306-4522(98)00170-5. [DOI] [PubMed] [Google Scholar]
- 45.Fatemi SH, et al. Deficits in GABA(B) receptor system in schizophrenia and mood disorders: a postmortem study. Schizophr. Res. 2011;128:37–43. doi: 10.1016/j.schres.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carracedo LM, et al. A neocortical delta rhythm facilitates reciprocal interlaminar interactions via nested theta rhythms. J. Neurosci. 2013;33:10750–10761. doi: 10.1523/JNEUROSCI.0735-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyasaka M, Domino EF. Neural mechanisms of ketamine-induced anesthesia. Int. J. Neuropharmacol. 1968;7:557–573. doi: 10.1016/0028-3908(68)90067-1. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, et al. Inhibition of NMDARs in the nucleus reticularis of the thalamus produces delta frequency bursting. Front. Neural Circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Troyano-Rodrigueza E, et al. Phencyclidine inhibits the activity of thalamic reticular gamma–aminobutyric acidergic neurons in rat brain. Biol. Psychiatry. 2014;76:937–945. doi: 10.1016/j.biopsych.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 50.Aguayo LG, et al. Voltage- and time-dependent effects of phencyclidines on the endplate current arise from open and closed channel blockade. Proc. Natl. Acad. Sci. 1986;83:3523–3527. doi: 10.1073/pnas.83.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi T, Su TP. The sigma receptor: evolution of the concept in neuropsychopharmacology. Curr. Neuropharmacol. 2005;3:267–280. doi: 10.2174/157015905774322516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu JY, Frank LM. Hippocampal-cortical interaction in decision making. Neurobiol. Learn. Mem. 2015;117:34–41. doi: 10.1016/j.nlm.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gillies MJ, et al. A model of atropine-resistant theta oscillations in rat hippocampal area CA1. J. Physiol. 2002;543:779–793. doi: 10.1113/jphysiol.2002.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fung SJ, et al. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am. J. Psychiatry. 2010;1167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 56.Roopun AK, et al. Temporal interactions between cortical rhythms. Front. Neurosci. 2008;2:145–154. doi: 10.3389/neuro.01.034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colgin LL, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 59.Palva S, Palva M. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front. Psychol. 2011;2:204. doi: 10.3389/fpsyg.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rotstein HG, et al. Slow and fast inhibition and an H-current interact to create a theta rhythm in a model of CA1 interneuron network. J. Neurophysiol. 2005;94:1509–1518. doi: 10.1152/jn.00957.2004. [DOI] [PubMed] [Google Scholar]
- 61.Middleton S, et al. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc. Natl. Acad. Sci. U.S.A. 2008;105:18572–18577. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melzer S, et al. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- 63.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeuchi D, et al. Reversal of interlaminar signal between sensory and memory processing in monkey temporal cortex. Science. 2011;331:1443–1447. doi: 10.1126/science.1199967. [DOI] [PubMed] [Google Scholar]
- 65.Hall S, et al. Unbalanced peptidergic inhibition in superficial neocortex underlies spike and wave seizure activity. J. Neurosci. 2015;35:9302–9310. doi: 10.1523/JNEUROSCI.4245-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fornito A, Bullmore ET. Reconciling abnormalities of brain network structure and function in schizophrenia. Curr. Opin. Neurobiol. 2015;30:44–50. doi: 10.1016/j.conb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Deserno L, et al. Reduced prefrontal-parietal effective connectivity and working memory deficits in schizophrenia. J. Neurosci. 2012;32:12–20. doi: 10.1523/JNEUROSCI.3405-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Littow H, et al. Aberrant functional connectivity in the default mode and central executive networks in subjects with schizophrenia - a whole-brain resting-state ICA study. Front. Psychiatry. 2015;6:26. doi: 10.3389/fpsyt.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, et al. Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci. Rep. 2015;5:14655. doi: 10.1038/srep14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baenninger A, et al. Abnormal coupling between default mode network and delta and beta band brain electric activity in psychotic patients. Brain Connect. 2017;7:34–44. doi: 10.1089/brain.2016.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pomarol-Clotet E. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol. Med. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 73.Meyer-Lindenberg AS. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch. Gen. Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 74.Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr. Opin. Neurobiol. 2012;22:537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sigurdsson T, et al. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cassel JC, et al. Importance of the ventral midline thalamus in driving hippocampal functions. Prog. Brain Res. 2015;219:145–161. doi: 10.1016/bs.pbr.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Northoff G, Duncan NW. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Prog. Neurobiol. 2016;146:26–45. doi: 10.1016/j.pneurobio.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Fell J, et al. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat. Neurosci. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, et al. NMDAR antagonist action in thalamus imposes d oscillations on the hippocampus. J. Neurophysiol. 2012;107:3181–3189. doi: 10.1152/jn.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frith C. The neural basis of hallucinations and delusions. C. R. Biol. 2005;328:169–175. doi: 10.1016/j.crvi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 81.Matta JA, et al. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron. 2011;70:339–351. doi: 10.1016/j.neuron.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karapanagiotidis T. Tracking thoughts: exploring the neural architecture of mental time travel during mind-wandering. Neuroimage. 2017;147:272–281. doi: 10.1016/j.neuroimage.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 83.Roopun AK, et al. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr. Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Traub RD, et al. Fast rhythmic bursting can be induced in layer 2/3 cortical neurons by enhancing persistent Na+ conductance or by blocking BK channels. J. Neurophysiol. 2003;89:909–921. doi: 10.1152/jn.00573.2002. [DOI] [PubMed] [Google Scholar]
- 85.Spencer KM, et al. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 87.Kruskal PB, et al. Circuit reactivation dynamically regulates synaptic plasticity in neocortex. Nat. Commun. 2013;4:2574. doi: 10.1038/ncomms3574. [DOI] [PubMed] [Google Scholar]
- 88.Ainsworth M. Rates and rhythms: a synergistic view of frequency and temporal coding in neuronal networks. Neuron. 2012;75:572–582. doi: 10.1016/j.neuron.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Borgers C, Kopell NJ. Gamma oscillations and stimulus selection. Neural Comput. 2008;20:383–414. doi: 10.1162/neco.2007.07-06-289. [DOI] [PubMed] [Google Scholar]
- 90.Akam T, Kullmann DM. Oscillations and filtering networks support flexible routing of information. Neuron. 2010;67:308–320. doi: 10.1016/j.neuron.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kopell N, et al. Are different rhythms good for different functions? Front. Hum. Neurosci. 2010;4:187. doi: 10.3389/fnhum.2010.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lakatos P, et al. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]