Abstract Abstract
An octocoral survey conducted in the mesophotic coral ecosystem (MCE) of Eilat (Gulf of Aqaba, northern Red Sea) yielded a new species of the speciose reef-dwelling genus Sinularia. It features encrusting colony morphology with a thin, funnel-shaped polypary. Sinularia mesophotica sp. n. (family Alcyoniidae) is described and compared to the other congeners with similar morphology. Both the morphological and molecular examination justified the establishment of the new species, also assigning it to a new genetic clade within Sinularia. The results highlight its unique phylogenetic position within the genus, and this is the first described species of a mesophotic zooxanthellate octocoral.
Keywords: Octocorallia, taxonomy, new species, mesophotic coral ecosystem, Eilat, Red Sea
Introduction
The taxonomy of the northern Red Sea octocorals has been quite extensively studied, albeit mostly confined to the reefs above 30 m (references in Shoham and Benayahu 2017). Studies have demonstrated a high octocoral richness in the Red Sea, revealing new taxa and new zoogeographical records (e.g., Verseveldt and Benayahu 1978, 1983; Haverkort-Yeh et al. 2013; Ofwegen et al. 2013, 2016). Octocorals of the mesophotic coral ecosystems (MCEs), in contrast, have remained little studied (Shoham and Benayahu 2017). To date, the only newly-described mesophotic Red Sea octocoral is the azooxanthellate Scleronephthya lewinsohni Verseveldt and Benayahu, 1978 of the family Nephtheidae, discovered at a depth of 55–82 m. Recently, Shoham and Benayahu (2017) recorded a higher species richness and higher number of species in Eilat’s upper MCEs (30-45 m) compared to the shallower reefs there. The latter study also revealed an almost exclusive dominance of zooxanthellate octocorals in the upper MCE. Following an octocoral survey conducted in Eilat’s MCEs, we describe here a new species of the genus Sinularia.
Materials and methods
Samples were collected by ROV (ECA H800) operated by the Sam Rothberg R/V of the Interuniversity Institute for Marine Sciences in Eilat. In-situ photography was carried out using a low light black and white camera VS300 (Eca Robotics) and 1CAM Alpha HD camera (SubCimaging). Samples were obtained using the ROV arm. Colony fragments were removed and preserved in 100% ethanol for molecular work. The original samples were placed in 70% ethanol for taxonomic identification, for which sclerites from different parts of the colonies (polyp, polypary surface and interior, base surface and interior) were obtained by dissolving the tissues in 10% sodium hypochlorite, followed by rinsing in fresh water. Sclerites were then prepared for scanning electron microscopy as follows: rinsed with double-distilled water; dried at room temperature, coated with gold-palladium; and examined with a SEM Jeol 6480LV electron microscope and at high vacuum under an environmental scanning electron microscope (ESEM, JSM-6700 Field Emission Scanning Electron Microscope, operated at 10 kV). Wet preparations of tissue smears were examined under a light microscope (X 200) in order to verify presence of symbiotic algae (zooxanthellae). Material studied is deposited at the Steinhardt Museum of Natural History, National Center for Biodiversity Studies, Tel Aviv University, Israel (ZMTAU) and Naturalis Biodiversity Center, formerly Rijksmuseum van Natuurlijke Historie, Leiden, the Netherlands (RMNH).
Molecular phylogenetic analyses
DNA was extracted from the EtOH-preserved samples, and two mitochondrial gene regions (mtMutS, igr1 + COI) were sequenced using previously published primers and protocols (McFadden et al. 2011). Sequences were aligned to a dataset that included published sequences from 143 specimens representing >85 nominal species of Sinularia (McFadden et al. 2009, 2014; Benayahu et al. 2013). Maximum likelihood analyses of each gene region separately as well as the concatenated sequence were conducted using PhyML (Guindon et al. 2003) with 100 bootstrap replicates. A GTR+I+G model of evolution was substituted for the best-fit TVM+I+G model selected using ModelTest 3.0 (Posada and Crandall 1998) but not available in PhyML. MEGA v. 5 (Tamura et al. 2011) was used to calculate pairwise genetic distance values (Kimura 2-parameter) between sequences. New sequences have been deposited in GenBank (KY971524–KY971525), and alignments and treefiles in TreeBase (http://purl.org/phylo/treebase/phylows/study/TB2:S20934).
Results
Systematic description
Order Alcyonacea Lamouroux, 1912
Family Alcyoniidae Lamouroux, 1912
Genus Sinularia May, 1898
Sinularia mesophotica sp. n.
http://zoobank.org/658B7592-DE2D-4929-AF61-C11FABCF2879
Figure 1.
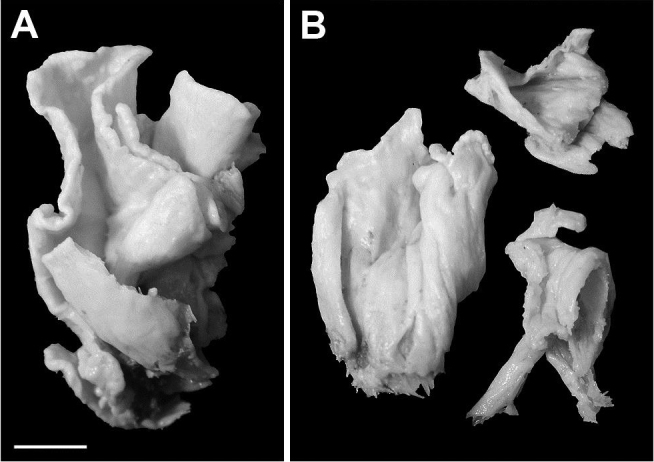
Sinularia mesophotica sp. n.; A Holotype ZMTAU Co 37425 B paratypes ZMTAU Co 37492. Scale bar: 1 cm (A also applies to B).
Figure 2.
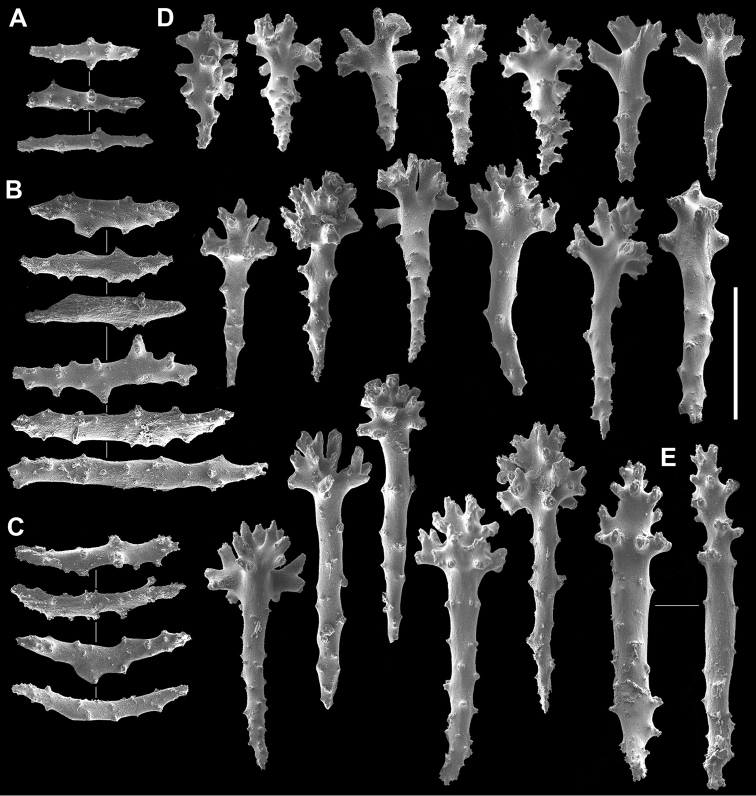
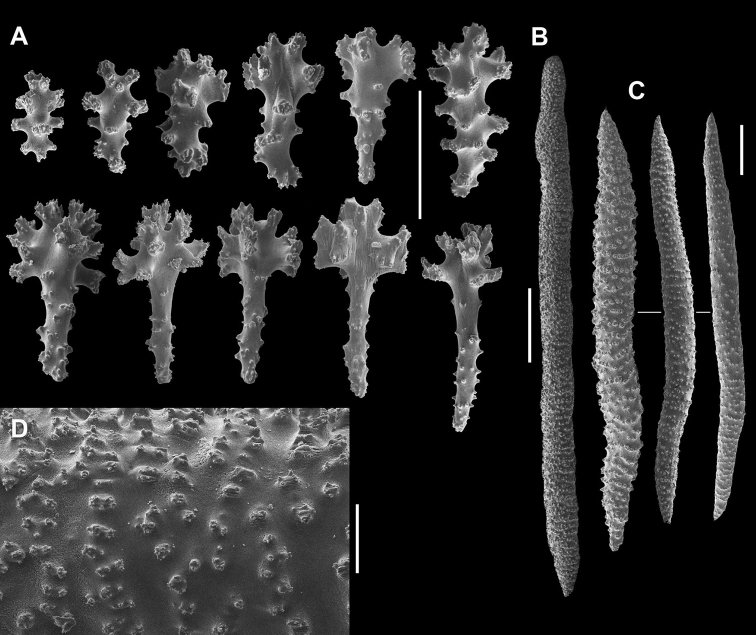
Sinularia mesophotica sp. n., holotype ZMTAU Co 37425. Sclerites from the polypary. A tentacle rods B straight collaret spindles C bent collaret spindles D clubs E larger clubs. Scale bar: 0.10 mm.
Figure 3.
Sinularia mesophotica sp. n., holotype ZMTAU Co 37425. Sclerites of the base of colony. A clubs of the surface layer B spindles of interior C tuberculation of the spindles. Scale bars: 0.10 mm (A, C), 1 mm (B).
Figure 4.
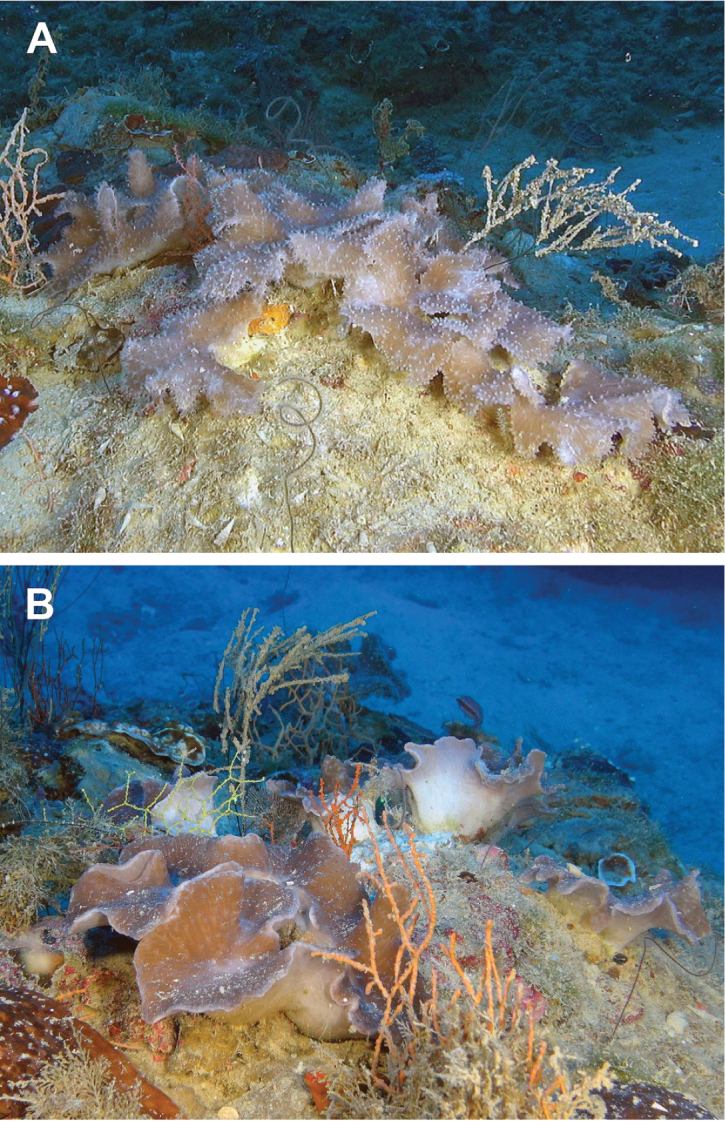
Underwater photographs of Sinularia mesophotica sp. n. A patch of colonies B funnel-shaped morphology of colonies.
Type material examined.
Holotype: ZMTAU Co 37425, Israel, northern Red Sea, Gulf of Aqaba, Eilat, Dekel Beach (29°32'2.49"N, 34°57'44.56"E), 62 m, 31 May 2016, coll. M. Weis; three paratypes: ZMTAU Co 37492 same collection details.
Diagnosis.
The holotype is part of an encrusting colony with a thin, funnel-shaped polypary, also featuring a curly margin (Fig. 1A). In a side-view its maximum dimensions are 5 × 2.5 cm. Polyps with tentacle rods and collaret sclerites (Fig. 2A–C). Tentacle rods up to 0.10 mm long (Fig. 2A). Collaret consists of almost straight spindles, up to 0.20 mm long (Fig. 2B), and shorter bent ones, up to 0.14 mm long (Fig. 2C). Surface layer of the polypary with clubs (Fig. 2D), some featuring a central wart, while in others it is less discernible, or even absent. Clubs vary from 0.10 mm long to 0.25 mm long, and a few with poorly developed heads attain 0.27 mm (Fig. 2E). Surface layer of the colony base contains clubs up to 0.22 mm; some similar to those of polypary, and others have wide heads (Fig. 3A). Polypary and base interior bear spindles, some branched, up to 3.2 mm long (Fig. 3B), with well-spaced simple tubercles (Fig. 3C).
Color.
The ethanol-preserved holotype is beige.
Etymology.
The new species name reflects its mesophotic habitat.
Living features.
Colonies grow as dense patches over reefal-calcareous substrate. Their polypary is flat and horizontally oriented (Fig. 4A) or funnel-shaped (Fig. 4B), with upper part dark-brown, due to dense zooxanthellae as verified by light microscopy, and lower part brighter, almost white. The flexibility and softness of the living colonies was recognized by their movement in the water currents generated by the ROV arm.
Variability.
ZMTAU Co 37492 comprises three paratypes, each of them represented by fragments of colonies (Fig. 1B). Their general morphology and sclerites are identical to those of the holotype ZMTAU Co 37425.
Molecular phylogenetic analyses of Sinularia mesophotica
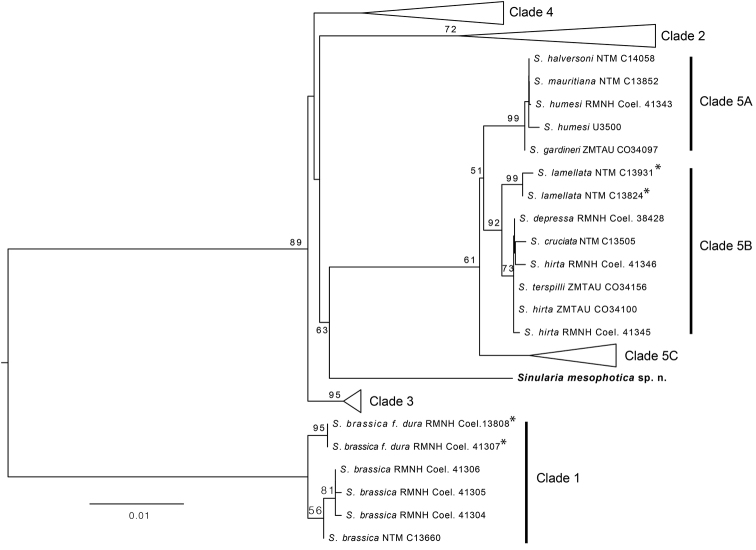
Phylogenetic analyses of mtMutS, igr1 + COI (not shown) and the concatenated sequence (Fig. 5) all placed Sinularia mesophotica n. sp. in a unique position outside the five previously recognized clades of Sinularia (McFadden et al. 2009). The mean genetic distance (Kimura 2-parameter) between S. mesophotica n. sp. and all other species was 5.7% (s.d. ± 1.0%), comparable to the mean distances between different clades (2.8–7.2%), and much greater than what is typically observed between species within each clade (0.2–2.9%) (McFadden et al. 2009). The mtMutS tree (not shown) suggested that S. mesophotica n. sp. is a sister taxon to clade 5 (bootstrap support = 78%), but neither igr1+COI (not shown) nor the concatenated gene tree resolved the basal relationships among S. mesophotica sp. n. and clades 2–5 (Fig. 5).
Figure 5.
Maximum likelihood tree of concatenated mtMutS and igr1+COI mitochondrial gene sequences. Clade numbering system follows McFadden et al. (2009); some clades have been collapsed to facilitate readability. Asterisks indicate species with a funnel-shaped morphology similar to that of Sinularia mesophotica sp. n. Bootstrap values >50% are indicated adjacent to nodes.
Discussion
Prior to the present study two Sinularia species have been described as possessing a distinct funnel-shaped polypary. Certain morphologies of S. brassica May, 1898, originally assigned to S. dura (Pratt 1903), share such morphology (see Benayahu et al. 1998: figs 6, 7, 9, 13, 25). Notably, S. brassica features club-sclerites with heads consisting of two or three diverging, wide-toothed prominences, markedly different from those of S. mesophotica.
The holotype of Sinularia lamellata Verseveldt and Tursch, 1979 features a “thin, plate-like funnel-wall” (see p. 143, plate 6), thus also resembling the colonies of S. mesophotica (this study: Figs 1, 4). For comparison, in the current study we re-examined the sclerites of the holotype of S. lamellata (RMNH Coel no. 12864), and SEM images are provided (Figs 6, 7). The surface layer of the polypary contains clubs, up to 0.16 mm long, some featuring a central wart comprised of a markedly spiny head (Fig. 6A). The polyps have point sclerites with head either poorly developed or distinct, up to 0.12 mm long (Fig. 6B), along with longer ones, up to 0.23 mm (Fig. 6C). There are no collaret or tentacle sclerites. The lack of collaret and tentacle sclerites in this specimen was observed previously (McFadden et al. 2009), and is probably caused by storing the specimen in formalin as many sclerites showed damage. A specimen from Palau identified as S. lamellata was also examined by McFadden et al. (2009), and that specimen showed collaret, point and tentacle sclerites (in situ photograph of that specimen available in WoRMS). The surface of the polypary contains some pointed spindles, up to 0.40 mm long (Fig. 6D). The longer ones might be transitional forms to the internal spindles of the polypary (Fig. 6E). Neither the point sclerites nor the spindles of the polypary were mentioned in the original description (see Verseveldt and Tursch 1979). The surface of the colony base contains clubs similar to those of the polypary, measuring up to 0.18 mm long, but some feature a wider head (Fig. 7A).
Figure 6.
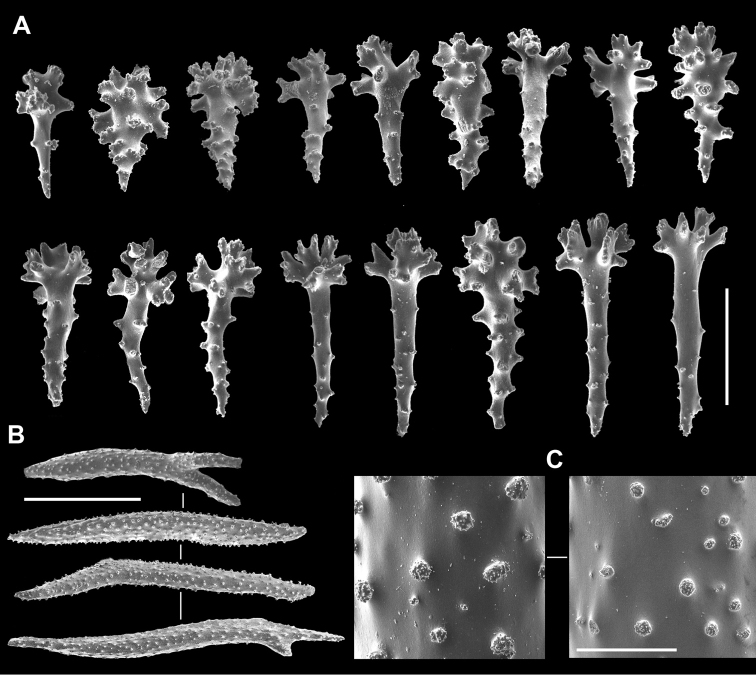
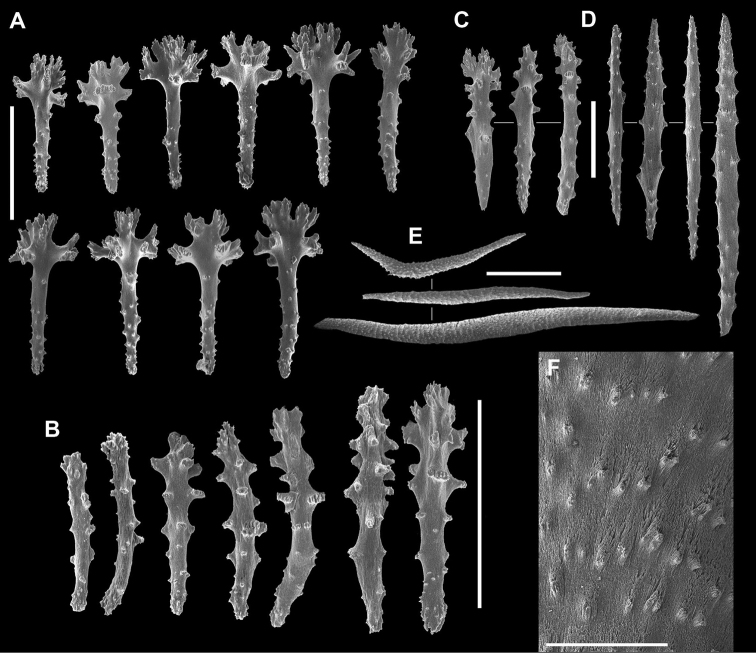
Sinularia lamellata RMNH Coel no. 12864. Sclerites of the polypary. A clubs of surface layer B–C point sclerites D spindles from surface E spindles from interior F tuberculation. Scale bars: 0.10 mm (A–D, F), 1 mm (E).
Figure 7.
Sinularia lamellata RMNH Coel no. 12864. Sclerites of the colony base; A clubs of surface layer B–C spindles from interior base D tuberculation of spindles. Scale bars: 0.10 mm (A, D), 1 mm (B, C).
The interior of the polypary has almost straight spindles or slightly bent ones, up to 4.8 mm long (Fig. 6E), ornamented with simple low conical warts (Fig. 6F). The interior of the colony base has slightly bent or almost straight spindles, up to 6.3 mm long (Fig. 7B, C), with simple warts (Fig. 7D).
A comparison of Sinularia lamellata with S. mesophotica reveals that the club heads of the two species are quite different, as the former feature terminal prominences consisting of closely-packed, pointed, thin spikes (see also Verseveldt and Tursch 1979), while in the latter they are mostly truncate. Furthermore, S. lamellata possesses spindles on the surface of the polypary, where none exist in S. mesophotica. The polyp sclerites of both species markedly differ, with S. mesophotica having only collaret and tentacle sclerites while S. lamellata has points as well as collaret and tentacle sclerites. Similarly, the coenenchymal spindles of S. lamellata are longer and their ornamentation differs from that of S. mesophotica. It is thus evident that S. mesophotica differs morphologically from both S. brassica and S. lamellata, the two previously described Sinularia species that possess a similar funnel-shaped polypary.
Sinularia frondosa Verseveldt, 1978 also has somewhat similar sclerites, but this species does not have the funnel-shaped polypary. Moreover, McFadden et al. (2009) re-examined the type of S. frondosa and found that it shares the morphological characters of clade 5B, like S. lamellata.
The phylogenetic analyses also support the genetic distinction of Sinularia mesophotica sp. n. from S. brassica, S. lamellata, and all other species of Sinularia for which molecular data are available (Fig. 5). Sinularia brassica, including the morphologically and genetically distinct dura form with a funnel shape, is phylogenetically distinct from all other Sinularia species, comprising the previously described clade 1 (McFadden et al. 2009), whereas reference specimens identified morphologically as S. lamellata belong to clade 5B. In contrast, S. mesophotica occupies a unique phylogenetic position outside of any of the five recognized clades to which all other known Sinularia species belong, and can be considered to represent a sixth, phylogenetically distinct clade (Fig. 5).
The five previously recognized clades of Sinularia, as well as distinct subclades within the two large clades 4 and 5, can be distinguished morphologically based on a suite of four primary morphological characters. These include the presence of sclerites in the (a) tentacles, (b) collaret, and (c) points regions of the polyp, as well as (d) the shape of the club sclerites in the colony surface tissues (McFadden et al. 2009). For example, clade 1 (S. brassica) has scales in the tentacles but lacks a collaret and points, and the surface club sclerites are characterized by very wide heads of unique form. In contrast, species belonging to clade 5B (which includes S. lamellata) have sclerites in the tentacles, a collaret and points, and surface clubs with a distinct central wart. Clade 6 (S. mesophotica sp. n.) can be distinguished from all other clades by the presence of rods in the tentacles, a collaret but no points, and surface clubs with a central wart that may be indistinct or absent. It is evident that S. mesophotica differs both morphologically and genetically from all other congeners, and is thus justified to be a new species.
Supplementary Material
Acknowledgements
We thank the Interuniversity Institute for Marine Sciences in Eilat (IUI) for the use of the Sam Rothberg R/V and the professional assistance of its crew members. We are indebted to EcoOcean staff members for operating the ROV. We acknowledge M. Weis and R. Liberman for help in the field, A. Gonzalez for laboratory assistance, A. Shlagman for curatorial skills, V. Wexler for digital editing and N. Paz for editorial assistance. This research was supported by the TASCMAR project (Tools and Strategies to access original bioactive compounds from cultivated marine invertebrates and associated symbionts) which received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 634674; and by the Israel Cohen Chair in Environmental Zoology to YB. Collection of animals complied with a permit issued by the Israel Nature and National Parks Protection Authority.
Citation
Benayahu Y, McFadden CS, Shoham E, van Ofwegen LP (2017) Search for mesophotic octocorals (Cnidaria, Anthozoa) and their phylogeny. II. A new zooxanthellate species from Eilat, northern Red Sea. ZooKeys 676: 1–12. 10.3897/zookeys.676.12751
References
- Benayahu Y, Ofwegen LP van, Alderslade PA. (1998) Case study of variation in two nominal species of Sinularia (Coelenterata: Octocorallia), S. brassica May, 1898, and S. dura (Pratt, 1903), with a proposal for their synonymy. Zoologische Verhandelingen 208: 277–309. [Google Scholar]
- Benayahu Y, Ofwegen LP van, Soong K, Dai C-F, Jeng M-S, Shlagman A, Hsieh HJ, McFadden CS. (2013) Diversity and distribution of Octocorals (Coelenterata: Anthozoa) on the coral reefs of Penghu, Taiwan. Zoological Studies 51: 1529–1548. [Google Scholar]
- Guindon S, Gascuel O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. https://doi.org/10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- Haverkort-Yeh RD, McFadden CS, Benayahu Y, Berumen M, Halàsz A, Toonen RJ. (2013) A taxonomic survey of Saudi Arabian Red Sea octocorals (Cnidaria: Alcyonacea). Marine Biodiversity 43: 279–291. https://doi.org/10.1007/s12526-013-0157-4 [Google Scholar]
- May W. (1898) Die von Dr. Stuhlmann im jahre 1889 gesammelten ostafrikantischen Alcyonaceen des Hamburger Museums. Mitteilungen aus dem Naturhistorischen Museum in Hamburg 15: 1–38. [Google Scholar]
- McFadden CS, Benayahu Y, Pante E, Thoma JN, Nevarez PA, France SC. (2011) Limitations of mitochondrial gene barcoding in Octocorallia. Molecular Ecology Resources 11: 19–31. https://doi.org/10.1111/j.1755-0998.2010.02875.x [DOI] [PubMed] [Google Scholar]
- McFadden CS, Ofwegen LP van, Beckman EJ, Benayahu Y, Alderslade P. (2009) Molecular systematics of the speciose Indo‐Pacific soft coral genus, Sinularia (Anthozoa: Octocorallia). Invertebrate Biology, 128: 303–323. https://doi.org/10.1111/j.1744-7410.2009.00179.x [Google Scholar]
- McFadden CS, Brown AS, Brayton C, Hunt CB, Ofwegen LP van. (2014) Application of DNA barcoding to biodiversity studies of shallow-water octocorals: molecular proxies agree with morphological estimates of species richness in Palau. Coral Reefs 33: 275–286. https://doi.org/10.1007/s00338-013-1123-0 [Google Scholar]
- Ofwegen LP van, Benayahu Y, McFadden CS. (2013) Sinularia leptoclados (Ehrenberg, 1834) (Cnidaria: Octocorallia) re-examined. ZooKeys 272: 29–59. https://doi.org/10.3897/zookeys.272.4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofwegen LP van, McFadden CS, Benayahu Y. (2016) Sinularia polydactyla (Ehrenberg, 1834) (Cnidaria, Octocorallia) re-examined, with the description of a new species. ZooKeys 581: 71–126. https://doi.org/10.3897/zookeys.581.7455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D, Crandall KA. (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. https://doi.org/10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Pratt EM. (1903) The Alcyonaria of the Maldives. II. The genera Sarcophytum, Lobophytum, Sclerophytum, and Alcyonium. Fauna and Geography of Maldives and Laccadive Archipelago 2: 503–539. [Google Scholar]
- Shoham E, Benayahu Y. (2017) Higher species richness of octocorals in the upper mesophotic zone in Eilat (Gulf of Aqaba) compared to shallower reef zones. Coral Reefs 36: 71–81. https://doi.org/10.1007/s00338-016-1528-7 [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. https://doi.org/10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verseveldt J. (1978) Alcyonacea (Coelenterata: Octocorallia) from some Micronesian Islands. Zoologische Mededelingen 53: 49–55. [Google Scholar]
- Verseveldt J, Benayahu Y. (1983) On two old and fourteen new species of Alcyonacea (Coelenterata, Octocorallia) from the Red Sea. Zoologische Verhandelingen 208: 1–33. [Google Scholar]
- Verseveldt J, Benayahu Y. (1978) Descriptions of one old and five new species of Alcyonacea (Coelenterata: Octocorallia) from the Red Sea. Zoologische Mededelingen 53: 57–74. [Google Scholar]
- Verseveldt J, Tursch A. (1979) Octocorallia from the Bismarck Sea, Part 1. Zoologische Mededelingen 11: 1–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.