Abstract
Background
Cardiac infiltration is an important cause of death in sarcoidosis. Tran-sthoracic echocardiography (TTE) has limited sensitivity for the detection of cardiac sarcoidosis (CS). Late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) is used to diagnose CS but has limitations of cost and availability. We sought to determine whether TTE- derived global longitudinal strain (GLS) may be used to identify individuals with CS, despite preserved left ventricular ejection fraction (LVEF), and whether abnormal GLS is associated with major cardiovascular events (MCE).
Methods
We studied 31 patients with biopsy- proven extra- cardiac sarcoidosis, LVEF>50% and LGE on CMR (CS+ group), and 31 patients without LGE (CS−group), matched by age, sex, and severity of lung disease. GLS was measured using vendor- independent speckle tracking software. Parameters of left and right ventricular systolic and diastolic function were also studied. Receiver- operating characteristic curves were used to identify GLS cutoff for CS detection, and Kaplan–Meier plots to determine the ability of GLS to predict MCE.
Results
LGE was associated with reduced GLS (−19.6±1.9% in CS− vs −14.7±2.4% in CS+, P<.01) and with reduced E/A ratio (1.1±0.3 vs 0.9±0.3, respectively, P =.01). No differences were noted in other TTE parameters. GLS magnitude inversely correlated with LGE burden (r=−.59). GLS cutoff of −17% showed sensitivity and specificity 94% for detecting CS. Patients who experienced MCE had worse GLS than those who did not (−13.4±0.9% vs −17.7±0.4%, P=.0003).
Conclusions
CS is associated with significantly reduced GLS in the presence of preserved LVEF. GLS measurements may become part of the TTE study performed to screen for CS.
Keywords: cardiac sarcoidosis, longitudinal strain, myocardial deformation, speckle tracking
1 | INTRODUCTION
Sarcoidosis is a systemic disorder characterized by noncaseating gran-ulomatous infiltration of multiple organs, including the heart. Cardiac involvement can be difficult to detect and is clinically apparent in only 5% of patients with sarcoidosis. However, autopsy series reveal evidence of cardiac sarcoidosis or its associated myocardial damage (CS) in 20–30% of patients with systemic sarcoidosis1–4 and is a significant cause of mortality even in the absence of obvious clinical symptoms.5,6 In multiple centers, myocardial late gadolinium enhancement (LGE) imaging by cardiovascular magnetic resonance (CMR) has become the favored modality for the evaluation of patients with suspected CS. In fact, the presence of LGE in patients with extra- cardiac sarcoidosis indicates myocardial damage, even in the absence of left ventricular (LV) systolic dysfunction, and is used to identify individuals at increased risk for death and significant ventricular arrhythmia. This methodology has been shown to be superior to the current clinical approach based on the Japanese Ministry of Health and Welfare criteria for the evaluation of CS.7–9 However, LGE CMR has limitations in terms of cost and availability, as well as feasibility in patients with renal dysfunction, claustrophobia, or certain ferromagnetic implants.
Conversely, transthoracic echocardiography (TTE) is widely available and recommended as the first- line screening tool for the detection of CS by the recent expert consensus statement published by the Heart Rhythm Society, in which TTE was assigned a class IIA recommendation.10 However, it is well recognized that conventional 2D echocardiography has limited sensitivity for the diagnosis of CS—which may be as low as 25%,10,11 and probably even lower in individuals with preserved LV ejection fraction (EF). Importantly, significant adverse outcomes may occur despite the absence of a detectable abnormality on TTE, such as a reduced LVEF or abnormal regional wall motion.10–12 Recent studies have suggested that newer indices of myocardial deformation derived from TTE, such as global longitudinal strain (GLS), may help detect the presence of CS before a reduction in LVEF or regional wall- motion abnormalities become apparent.13,14 However, the role of GLS in detection and risk stratification of CS has not been fully elucidated. This study was motivated by our belief that earlier detection of CS could result in earlier treatment with immunosuppressive therapy ultimately improving patient outcomes.15–18
Accordingly, we aimed to (1) determine whether GLS measured using TTE could be used to identify patients at risk of CS, as defined by presence of LV LGE on CMR, despite preserved LV function, (2) identify a GLS cutoff that should prompt referral for further testing, and (3) determine whether abnormal GLS is associated with increased risk of death or significant ventricular arrhythmia.
2 | METHODS
2.1 | Study population
We retrospectively identified patients with biopsy- proven extra- cardiac sarcoidosis referred for CMR and TTE to evaluate for CS who had preserved LVEF (>50%). Coronary artery disease or paroxysmal atrial fibrillation was not used as exclusion criteria, as long as the patients were imaged while in sinus rhythm. Patients with LVEF <50% were excluded since they have an increased risk of CS and would also be expected to have abnormalities in GLS thereby skewing the results. Forty- one were found to have LGE on CMR; after excluding 5 individuals whose CMR and TTE had been performed more than 12 months apart and 5 with inadequate TTE image quality, 31 patients with LGE remained (CS+ group). Of the patients without LGE, 31 additional patients were then matched to the CS+ group, based on age, sex and severity of pulmonary sarcoidosis, using Scadding stage and pulmonary function test (PFT) parameters (% of predicted total lung capacity, forced expiratory volume [FEV1], forced vital capacity [FVC], FEV1/FVC ratio, and diffusing capacity for carbon monoxide). These additional 31 patients constituted the control CS−group. Patient outcomes were determined for a minimum of 1 year. Major cardiovascular events (MCE) were defined prior to data analysis as all- cause death, ventricular fibrillation, sustained ventricular tachycardia, or occurrence of appropriate defibrillator shock therapy, as determined by chart review, implantable device interrogation when available, telephone interview, and review of social security death index. Institutional Review Board approval was obtained for this study. Some of the patients included in this cohort were also part of our previously published CMR study.9
2.2 | Transthoracic echocardiography and strain
Comprehensive TTE, including two-dimensional imaging, Doppler echocardiography and tissue Doppler, was performed using a commercial imaging system (iE33, Philips Healthcare, Andover, MA, USA) with an S5-1 transducer. Diastolic function and LV filling pressures were assessed using pulsed-wave Doppler of mitral inflow, tissue Doppler of the septal and lateral mitral annulus and left atrial volumes (using the biplane area-length method), according to American Society of Echocardiography guidelines.19 All the Doppler measurements were performed on 3 beats and the resultant values were averaged. LVEF was calculated using the modified Simpson’s biplane method.20 Right ventricular (RV) dysfunction was qualitatively categorized as mild, moderate, or severe by an experienced echocardiographer. Semi-automated vendor-independent software (EchoInsight Cardio-Oncology, Epsilon Imaging, Ann Arbor, MI, USA) was used for strain analysis. The endocardial border was traced manually on the end-systolic frame and then tracked throughout the cardiac cycle using an automated speckle tracking algorithm to measure GLS in the apical two-, three-, and four-chamber views. Radial and circumferential strains were not included in our analysis, because the inter-and intra-observer variability was determined to be suboptimal in the study cohort. All echocardiographic measurements used in this study were obtained by image analysis specifically by the study investigators, who were blinded to existing information from clinical reports, including CS status.
2.3 | Cardiovascular magnetic resonance
Cardiovascular magnetic resonance was performed using a 1.5 T scanner (Achieva, Philips Healthcare, Best, The Netherlands) using a five-channel flexible surface coil. Steady-state free precession cines of the left ventricle in 3 long-axis planes (two-, three-, and four-chamber views) were acquired, along with a contiguous stack of short-axis slices spanning the entire left ventricle (retrospectively gated, usual repetition time 2.9 ms, echo time 1.5 ms, flip angle 60°, temporal resolution 25–40 ms). LGE images of the same views were obtained 10 minutes after infusion of gadodiamide or gadobenate dimeglumine (0.1–0.2 mmol/kg) using a T1-weighted gradient echo pulse sequence with a phase-sensitive inversion recovery reconstruction (typical repetition time 4.5 ms, echo time 2.2 ms, inversion time 200–300 ms, flip angle 30°, phase-sensitive inversion recovery flip angle 5°, voxel size 2 × 2 × 10 mm, sense factor 1–2). Philips Extended MR Workspace software was used to measure LV end- diastolic and end- systolic volume indices (EDVi, ESVi), LV mass index (LVMi) and EF, RV EDVi, ESVi, and EF. CS was defined as the presence of any myocardial LGE, as first visually detected by an experienced CMR reader, and then confirmed quantitatively using the Virtue software (Diagnosoft, Morrisville, NC, USA). LGE was considered to be present if any LV myocardial region had a signal intensity >5 standard deviations above the mean signal intensity of normal remote myocardium, as determined by the reader. The LGE burden (%LGE) was calculated as the total amount of LV LGE as a percentage of LV mass.
2.4 | Reproducibility analysis
Longitudinal strain measurements were repeated in a randomly selected group of 15 patients by two readers for purposes of reproducibility analysis. This included repeated measurements by the same observer, at least one month later, as well as measurements by a second independent observer, both blinded to all prior measurements. Inter-observer variability and intra-observer variability were calculated as an absolute difference between the corresponding pair of repeated measurements as a percentage of their mean in each patient and then averaged over the entire group.
2.5 | Statistical methods
Continuous variables were summarized as mean±standard deviation, while categorical variables were presented as absolute numbers and percentages, unless otherwise stated. Area under the curve (AUC) was determined from receiver-operating characteristic (ROC) analysis to determine the ability of various systolic (GLS, LVEF) and diastolic (E/A, medial and lateral E/e′, average e′, E/average e′, left atrial volume) parameters obtained from TTE to detect the presence of LGE on CMR, as well as to determine whether GLS could predict MCE. An optimal cutoff value for the detection of LGE by GLS was also determined from the ROC analysis. For each parameter, unpaired t tests were used to evaluate the significance of the difference between the two groups (CS+ and CS−). Fisher’s exact test or chi-square tests were employed as appropriate to test significance of the differences in ratios. P-values <.05 were considered significant. Linear regression was performed to delineate the relationship between GLS and burden of LGE. Kaplan–Meier curves were generated to detect differences in MCE between groups divided by the above-determined GLS cutoff value over a period of 12 months, and log-rank test was used to test the significance of the differences in the survival time between these two groups. Univariate Cox proportional hazard model was used to assess the relationship between GLS magnitude and survival. Statistical calculations were performed using SPSS (Version 22.0, Armonk, NY, USA) and R statistical software (The R Foundation for Statistical Computing, Vienna, Austria).
3 | RESULTS
3.1 | Population demographics and LGE
Characteristics of the study group are summarized in Table 1. Our study group consisted of middle-aged (57±7 years) predominantly female (69%) African American (58%) patients diagnosed with sarcoidosis an average of 15±7.2 years earlier. Almost all (90%) had pulmonary or thoracic lymph node involvement. There were two patients with 3 or more cardiovascular risk factors. In terms of LGE, there appeared to be a significant difference between patients with and without smoking history, but this was likely skewed by the fact that only four subjects actively smoked. Patients with a history of prior or current steroid use were significantly more likely to have LGE on CMR. By definition of the CS− and CS+ groups, there was no significant difference in age, gender, race, prevalence of pulmonary sarcoidosis, time since diagnosis, or severity of pulmonary disease (Table 1).
TABLE 1.
Patient characteristics
| Total population N=62 | LGE - N=31 | LGE+N=31 | P-value | |
|---|---|---|---|---|
| Age | 57.2±10.9 | 58.4±10.3 | 58.8±11.3 | .89 |
| Female | 43 (69%) | 22 (71%) | 21 (68%) | .78 |
| Hypertension | 27 (44%) | 11 (35%) | 16 (52%) | .21 |
| Diabetes mellitus | 12 (19%) | 4 (13%) | 9 (29%) | .07 |
| Dyslipidemia | 17 (27%) | 7 (23%) | 10 (32%) | .4 |
| Coronary artery disease | 9 (15%) | 4 (13%) | 5 (16%) | .72 |
| Smoking | 4 (7%) | 0 (0%) | 4 (13%) | .04* |
| Atrial fibrillation | 13 (21%) | 9 (29%) | 4 (13%) | .12 |
| Steroids current | 33 (53%) | 12 (39%) | 21 (68%) | .02* |
| Steroids ever | 34 (55%) | 12 (39%) | 22 (71%) | .01* |
| History of immuno-modulator therapy | 31 (50%) | 13 (42%) | 18 (58%) | .21 |
| TLC (%) | 82±18 | 83±18 | 82±18 | .92 |
| FVC (%) | 74±20 | 75±21 | 73±20 | .75 |
| FEV1 (%) | 78±24 | 79±25 | 76±24 | .58 |
| DLCO (%) | 76±27 | 78±26 | 73±27 | .42 |
| Left bundle branch block | 0 | 0 | 0 | NS |
| Right bundle branch block | 2 (3%) | 0 | 2 (6%) | .16 |
| 1st/2nd/3rd degree AV block | 5 (8%)/0/0 | 2 (6%)/0/0 | 3 (10%)/0/0 | .67 |
| Holter monitor (N=10) SVT/NSVT | 3/2 | 2/1 | 1/1 | NS |
TLC, total lung capacity; FVC, forced vital capacity; FEV1, forced expiratory volume; DLCO, diffusing capacity for carbon monoxide; LBBB, left bundle branch block; RBBB, right bundle branch block; AV, atrioventricular; SVT, supraventricular tachycardia; NSVT, nonsustained ventricular tachycardia.
Statistically significant, P<0.05.
3.2 | Association between LGE and transthoracic echocardiography findings
Fig. 1 shows examples of CMR LGE images and 2D speckle tracking–derived strain images obtained in two patients, one from each group (CS− and CS+). There was no significant difference in LVEF between the two groups of patients with and without LGE; no patient was found to have a regional wall-motion abnormality based on visual inspection. There was a significant difference in E/A ratio (P=.01, Table 2) between the two groups, but no difference in any other diastolic function parameters (septal and lateral E/e′, average e′, E/average e′, deceleration time, or left atrial volume). There was a significant difference in RV systolic pressure between the two groups (P=.005, Table 2) and a trend toward increased RV dysfunction in the CS+ group (9.7% of CS− patients had RV dysfunction vs 25.8% of the CS+), which did not reach statistical significance (P=.1). CMR parameters of LV and RV size and function are summarized in Table 3, showing that with the exception of RV EF, all values were normal on the average and no significant differences were noted between the two groups.
FIGURE 1.
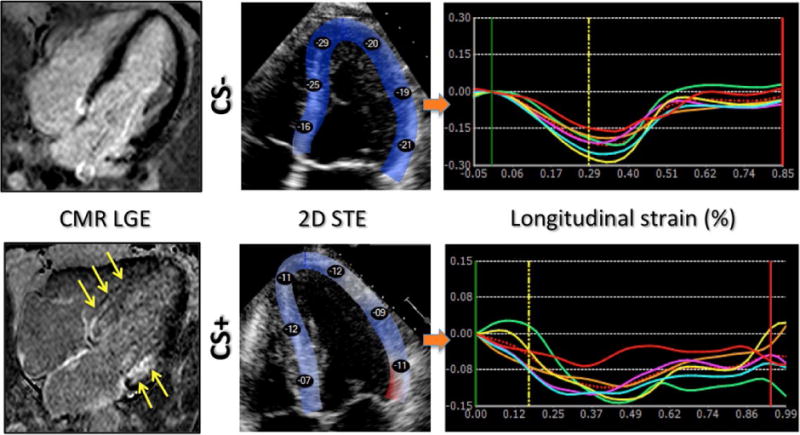
Example of CMR LGE images (left) and 2D echocardiographic images with color-encoded myocardial longitudinal strain (middle; peak segmental strains in % are shown in the color overlay), obtained in two patients: one without cardiac involvement (top, CS−) and the other with (bottom, CS+). Regional strain abnormalities are noted in the patient with LGE in the same myocardial area (yellow arrows). In this patient, segmental longitudinal strain time curves (bottom right) depict reduced peak strain values in all segments, compared to the patient with no LGE above (see scale differences of the y-axes)
TABLE 2.
Comparison of echocardiographic parameters in individuals with and without cardiac sarcoidosis or its associated myocardial damage (CS+ and CS− groups)
| Total population N=62 | LGE - N=31 | LGE+N=31 | P-value | |
|---|---|---|---|---|
| GLS | 17.2±3.3 | 19.6±1.9 | 14.7±2.4 | <.01* |
| LVEF(%) | 61.2±6 | 61±6 | 61±6 | .97 |
| E/A | 1±0.3 | 1.1±0.3 | 0.91±0.26 | .01* |
| E/E′ septal | 11.3±4.3 | 11.1±3 | 11.5±5.4 | .71 |
| E/E′ lateral | 8.2±3.6 | 8.5±3.1 | 8±4.1 | .65 |
| Average E′ | 7.9±2.3 | 8.3±2.3 | 7.5±2.2 | .25 |
| E/Average E′ | 9.5±3.7 | 9.6±2.9 | 9.3±4.5 | .79 |
| Deceleration time (ms) | 215.9±38.7 | 218±39 | 214±39 | .76 |
| LAVi (mL/m2) | 30±11 | 31±10 | 29±12 | .48 |
| >Mild RV dysfunction | 11 (18%) | 3 (9.7%) | 8 (25.8%) | .1 |
| RVSP (mm Hg) | 38±18 (N=28) | 28±5 (N=10) | 43±20 (N=18) | 0.005* |
LGS, global longitudinal strain; LVEF, left ventricular ejection fraction; LAVi, left atrial volume index; RV, right ventricular; RVSP, right ventricular systolic pressure.
P<.05.
TABLE 3.
Comparison of CMR parameters in individuals with and without cardiac sarcoidosis or its associated myocardial damage (CS+ and CS− groups)
| Total population N=62 |
LGE − N=31 |
LGE + N=31 |
P-value | |
|---|---|---|---|---|
| LGE burden (%) |
4.2±7.5 | 0±0 | 8.4±8.7 | <.00001 |
| LV EDVi (mL/m2) |
72±16 | 74±18 | 70±14 | .35 |
| LV ESVi (mL/m2) |
29±9 | 30±11 | 28±7 | .52 |
| LV EF (%) |
60±6 | 60±7 | 60±4 | .65 |
| LV mass index (g/m2) |
48±13 | 46±12 | 50±14 | .21 |
| RV EDVi (mL/m2) |
78±18 | 78±18 | 78±19 | .87 |
| RV ESVi (mL/m2) |
38±14 | 36±11 | 40±16 | .20 |
| RV EF (%) |
52±8 | 54±6 | 50±9 | .03 |
LGE, late gadolinium enhancement; LV/RV, left/right ventricular; EF, ejection fraction; EDVi/ESVi, end- diastolic/end- systolic volume index.
In contrast, GLS measured using TTE was significantly lower in individuals with LGE on CMR (GLS −14.7±2.4% in those with LGE and −19.6±1.9% in those without LGE; P<.01), with almost no overlap between the two groups (Fig. 2). Reduced GLS was associated with the presence of any amount of LGE. Linear regression between GLS and LGE burden resulted in a moderate correlation (r=.59). Based on ROC analysis, AUC for detection of any LGE on CMR using GLS was 0.94 (Fig. 3). The parameters with next highest AUC values were E/A ratio (0.69) and RV dysfunction (0.58). Optimal GLS cutoff for separating the two groups and thus detecting the presence of LGE was ≥−17% (Fig. 2, right), resulting in sensitivity, specificity, NPV, and PPV of 94% for the detection of any LGE in this patient cohort.
FIGURE 2.
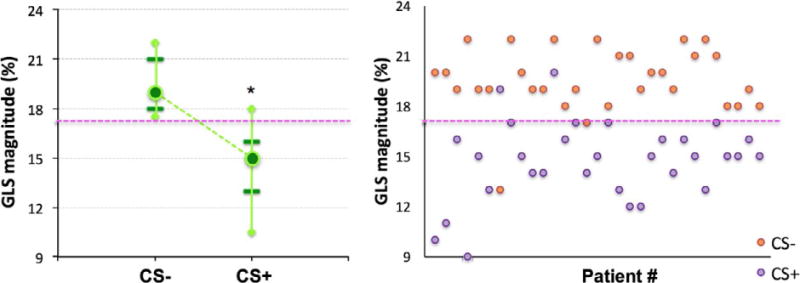
Whisker plot summary (left) and individual patients’ data (right) of LV global longitudinal strain (GLS) magnitude obtained separately for the patients with and without cardiac sarcoidosis (CS+ and CS−, respectively). Median values (circles), 5th and 95th percentiles (diamonds), as well as 20th and 80th percentiles (horizontal lines) are shown. The 17% cutoff value that best separated the two groups is shown as a dashed horizontal line. *Statistically significant, P<0.05, between the two groups. See text for details
FIGURE 3.
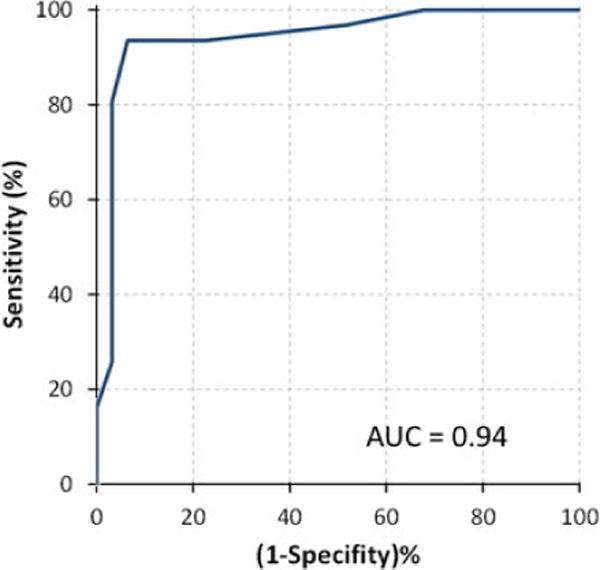
Receiver-operating characteristic curve for detection of late gadolinium enhancement using global longitudinal strain
The eight patients who experienced MCE (including five deaths, three cases of ventricular tachycardia) had worse GLS than the 54 patients who did not have MCE (−13.4±2.5% vs −17.7±3.0%, P=.0003). Kaplan–Meier curves (Fig. 4) showed that there was a significant difference (log-rank test P<.008) in survival between the two groups of patients defined using the above-determined GLS cutoff value of ≥−17%. None of the patients with GLS magnitude ≥17% had experienced MCE. Cox analysis showed that the hazard ratio for GLS was 0.80 (CI 0.635–0.996), P=.046.
FIGURE 4.
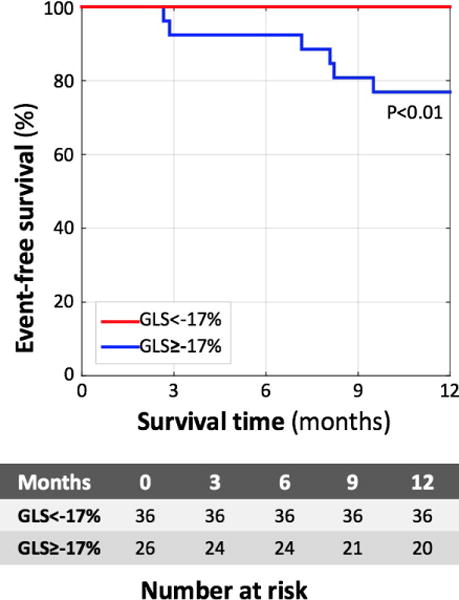
Prediction of major cardiovascular events in individuals with sarcoidosis using global longitudinal strain. Kaplan–Meier curves demonstrating that event-free survival of individuals with GLS >−17 (blue) is worse than those with GLS<−17% (red)
Intra-observer variability and inter-observer variability of the GLS measurements were 9.0±12.9% and 11.1±13.3%, respectively.
4 | DISCUSSION
With growing utilization of CMR, it is increasingly recognized that sarcoidosis affects the heart, even when LV EF is preserved. Although the diagnostic accuracy of CMR LGE in patients with sarcoidosis is not 100% at early stages of the disease,21 it is correlated with histology and patient outcomes, making this methodology a primary noninvasive tool to confirm the diagnosis of cardiac sarcoidosis.10 Sudden cardiac death and progressive heart failure are well-known complications of sarcoidosis patients with myocardial involvement,5,6 even in those without significant abnormality on echocardiography.10 TTE is widely used to screen for cardiac sarcoidosis, but is well known to have limited diagnostic sensitivity.10,12 Hence, efforts to improve the sensitivity of TTE for detecting CS are needed. In this study, despite the presence of preserved LVEF on TTE, we found that GLS was significantly worse in patients with CS compared to those without CS. In our cohort, a GLS cutoff value of −17% had a high sensitivity and specificity for identifying patients with CS, as determined by the presence of LGE on CMR, and the reduction in GLS magnitude was inversely related to the burden of LGE. Additionally, in our cohort, sarcoidosis patients with a GLS>−17% were at increased risk of death and/or significant ventricular arrhythmias, whereas those with a GLS<−17% were not.
Cardiac involvement in sarcoidosis is clinically evident in only 5% of patients, but is detectable in 20–30% at autopsy1–4 and perhaps in as many as 35% using newer imaging techniques such as LGE CMR.7,12,22 The importance of diagnosing myocardial damage in these patients lies in the fact that sudden cardiac death is an important complication of sarcoidosis.5,6 The most frequently used diagnostic criteria to detect CS are those of the Japanese Ministry of Health and Welfare (JMHW), which require either a histological diagnosis, or a significant abnormality on ECG, nuclear imaging, cardiac catheterization, or TTE. However, several studies have suggested that JMHW criteria have poor sensitivity for detection of CS, and may even be inadequate for identifying those individuals at risk of death or life-threatening arrhythmia.10 This is not surprising when the diagnostic modalities used are considered. Apart from being invasive, endomyocardial biopsy frequently misses CS due to its patchy nature,23,24 while ECG and TTE are often inadequate for the detection of CS, with sensitivities as low as 8 and 25%, respectively.10,12
Newer imaging modalities such as LGE CMR and 18F-fluoro-2-deoxyglucose positron emission tomography (FDG-PET) can diagnose CS with greater sensitivity. Although FDG-PET has proven useful in the detection of CS, especially in patients at higher risk of adverse events,25–28 CMR marginally outperforms FDG-PET in terms of specificity.29,30 Several recent studies have suggested that LGE CMR is a powerful noninvasive tool for the detection of CS, capable of detecting small areas of scar that predate abnormalities in global or regional LV function,9 and more sensitive than ECG, conventional echocardiography, or the JMHW criteria in predicting adverse events.7,8,10–12,22,31,32 However, CMR has limitations in terms of cost and availability and may not be suitable for patients who are obese, have metal implants, or advanced renal dysfunction. Despite the advantages offered by CMR and FDG-PET, these modalities do not possess all the qualities required for an optimal screening test33 in terms of accessible facilities, universal acceptability, and cost. Indeed, the most recent expert consensus document published by the Heart Rhythm Society (HRS) advises that CMR and FDG-PET be performed only if an abnormality is found on the initial screening.10
Conversely, TTE is widely available, broadly accepted, and has lower costs than either CMR or FDG-PET. Despite the poor diagnostic sensitivity of conventional TTE, it remains a first-line screening tool along with ECG and clinical history for the detection of cardiac involvement in patients with biopsy-proven extra-cardiac sarcoidosis, carrying a class IIA recommendation for screening in the HRS consensus document.10 There is clearly a need for identifying other TTE parameters that may increase its sensitivity for the detection of CS. Our study expands on recent publications that have suggested that indices of myocardial deformation, such as GLS, are abnormal in the presence of CS,34 even when other TTE parameters are within normal limits. Similarly, others have reported reduced radial 3D strain in CS patients when compared to individuals with dilated cardiomyopathy with matched LV EF.35
The use of GLS during a TTE examination to screen for CS is attractive due to its quick, noninvasive nature, which requires minimal training to perform. In addition, images can be analyzed off line, resulting in minimal disturbance of workflow. GLS is the most reproducible of the myocardial deformation indices and has shown promise in detecting early damage to the LV myocardium in the context of other conditions, such as chemotherapy-related LV dysfunction.36 In terms of CS, one TTE-based study of 41 patients with extra-cardiac sarcoidosis and 20 matched controls found that despite having similar systolic function, sarcoidosis patients had lower GLS.37 Joyce et al.14 confirmed these findings in a larger cohort and further showed that patients with reduced GLS had an increased probability of attaining a composite endpoint which included all-cause mortality, heart failure hospitalization, device implantation, new arrhythmias, or future development of CS. We found that reduced GLS was also associated with an increased risk of hard endpoints such as all-cause death and significant ventricular arrhythmias, even after accounting for the severity of lung disease. Based on the work of Orii et al.,13 we understand that in patients with extra-cardiac sarcoidosis, reduction in regional longitudinal strain is related to the presence of LGE in myocardial segments. We also found that the abnormalities in GLS are not only associated with the presence of LGE, but also with the burden of LGE seen on CMR.
The role of GLS in guiding treatment strategy remains to be determined, but case reports have documented improvement in GLS after corticosteroids.38 Thus, earlier detection of CS could have an impact in this cohort. Despite the absence of robust clinical trials and questions over aneurysm formation, corticosteroids remain the cornerstone of sarcoidosis therapy.15 Steroid-sparing agents are employed if the disease is refractory or progressive, again without irrefutable substantiation.16 Evidence is now emerging that corticosteroids are potentially more effective in patients with normal LV systolic function in the early stages of the disease.17,18 Increased utilization of GLS during screening TTE to identify individuals at increased risk of having CS may lead to early referral to advanced imaging tests and thus help identify those who might benefit the most from treatment initiation with either corticosteroids or steroid-sparing agents.
Furthermore, we found not only that individuals with CS have significantly lower GLS than those without CS, but also that there was minimal overlap in GLS values between the two groups. In fact, we propose that a GLS cutoff value −17% would not only accurately identify CS, but would also identify specific patients at increased risk of MCE. If confirmed in other cohorts, the use of GLS may become an important screening strategy for the detection of CS.
There was an association between E/A ratio and the presence of LGE in our population and a trend toward more RV dysfunction in the CS+ group, consistent with a previous study by our group.9 However, neither parameter appeared to be robust enough to use as a screening tool for CS, while GLS was significantly more promising. Other more conventional parameters (medial and lateral E/e′, average e′, E/average e′, left atrial volume) obtained during TTE proved to be of little value in screening for CS in our cohort.
5 | LIMITATIONS
The retrospective, single-center nature of our study entails several inherent limitations. We could not rule out referral bias on the part of physicians who sent their patients for CMR, nor could the clinical reasoning behind test selection be reliably determined. Moreover, image quality was not sufficiently consistent to allow reproducible analysis of circumferential and radial strain components. Also, we did not include RV LGE assessment in this study, because identifying LGE in the RV myocardium is quite challenging, likely secondary to the fact that the RV free wall is thin and due to the limited in-plane and through-plane resolution of the LGE images. Furthermore, the size of our cohort and event rates were small, and accordingly our findings need to be validated in larger cohorts. Also, because CS is a rare condition, we were not able to test the GLS cutoff value in an independent group of patients. Accordingly, the reported sensitivity, specificity, NPV, and PPV represent the best-case scenario, and the true diagnostic accuracy of this index remains to be determined in future studies. Additionally, patients in the CS+ group were more likely to have been treated by steroids, to have smoked cigarettes, diabetes, and hypertension, and thus we cannot rule out the effect of these factors on GLS and outcomes.
6 | CONCLUSIONS
Global longitudinal strain is significantly impaired in patients with sarcoidosis who have LGE evidence of myocardial damage, despite the presence of normal LVEF. In this cohort, reduction in GLS magnitude correlated with the extent of myocardial damage and was associated with increased risk of all-cause mortality and/or significant ventricular arrhythmias. GLS measurements appear to provide incremental diagnostic and prognostic information over conventional echocardiographic parameters in the evaluation of suspected CS.
Footnotes
DISCLOSURES
Research support from Philips Healthcare and Epsilon Imaging. Dr. Gillian Murtagh is currently an employee of Abbott Diagnostics (since 7/6/15), however she was employed by the University of Chicago at the time of the study.
References
- 1.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952;31:1–132. [PubMed] [Google Scholar]
- 2.Johns CJ, Michele TM. The clinical management of sarcoidosis. A 50-year experience at the Johns Hopkins Hospital. Medicine (Baltimore) 1999;78:65–111. doi: 10.1097/00005792-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol. 2009;104:571–577. doi: 10.1016/j.amjcard.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 4.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 5.Roberts WC, McAllister HA, Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11) Am J Med. 1977;63:86–108. doi: 10.1016/0002-9343(77)90121-8. [DOI] [PubMed] [Google Scholar]
- 6.Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119:167–172. [PubMed] [Google Scholar]
- 7.Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–511. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Patel AR, Klein MR, Chandra S, et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. Eur J Heart Fail. 2011;13:1231–1237. doi: 10.1093/eurjhf/hfr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Burstow DJ, Tajik AJ, Bailey KR, DeRemee RA, Taliercio CP. Two- dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol. 1989;63:478–482. doi: 10.1016/0002-9149(89)90323-8. [DOI] [PubMed] [Google Scholar]
- 12.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–1435. doi: 10.1378/chest.07-2784. [DOI] [PubMed] [Google Scholar]
- 13.Orii M, Hirata K, Tanimoto T, et al. Myocardial damage detected by two- dimensional speckle-tracking echocardiography in patients with extracardiac sarcoidosis: comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2015;28:683–691. doi: 10.1016/j.echo.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Joyce E, Ninaber MK, Katsanos S, et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur J Heart Fail. 2015;17:51–62. doi: 10.1002/ejhf.205. [DOI] [PubMed] [Google Scholar]
- 15.Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–1041. doi: 10.1016/j.cjca.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Korsten P, Mirsaeidi M, Sweiss NJ. Nonsteroidal therapy of sarcoidosis. Curr Opin Pulm Med. 2013;19:516–523. doi: 10.1097/MCP.0b013e3283642ad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato Y, Morimoto S, Uemura A, Hiramitsu S, Ito T, Hishida H. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:133–137. [PubMed] [Google Scholar]
- 18.Yodogawa K, Seino Y, Ohara T, Takayama H, Katoh T, Mizuno K. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16:140–147. doi: 10.1111/j.1542-474X.2011.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med. 2014;189:109–112. doi: 10.1164/rccm.201309-1668LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smedema JP, Snoep G, van Kroonenburgh MP, et al. The additional value of gadolinium-enhanced MRI to standard assessment for cardiac involvement in patients with pulmonary sarcoidosis. Chest. 2005;128:1629–1637. doi: 10.1378/chest.128.3.1629. [DOI] [PubMed] [Google Scholar]
- 23.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 24.Ardehali H, Howard DL, Hariri A, et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 2005;150:459–463. doi: 10.1016/j.ahj.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–1998. [PubMed] [Google Scholar]
- 26.Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–248. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 27.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborne MT, Hulten EA, Singh A, et al. Reduction in (1)(8)F-fluorode-oxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–174. doi: 10.1007/s12350-013-9828-6. [DOI] [PubMed] [Google Scholar]
- 29.Freeman AM, Curran-Everett D, Weinberger HD, et al. Predictors of cardiac sarcoidosis using commonly available cardiac studies. Am J Cardiol. 2013;112:280–285. doi: 10.1016/j.amjcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F- fluoro- 2- deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. doi: 10.1007/s00259-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 31.Schuller JL, Zipse M, Crawford T, et al. Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2012;23:925–929. doi: 10.1111/j.1540-8167.2012.02350.x. [DOI] [PubMed] [Google Scholar]
- 32.Betensky BP, Tschabrunn CM, Zado ES, et al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm. 2012;9:884–891. doi: 10.1016/j.hrthm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Wilson JM. Jungner YG [Principles and practice of mass screening for disease] Bol Oficina Sanit Panam. 1968;65:281–393. [PubMed] [Google Scholar]
- 34.Tigen K, Sunbul M, Karaahmet T, et al. Early detection of bi-ventricular and atrial mechanical dysfunction using two-dimensional speckle tracking echocardiography in patients with sarcoidosis. Lung. 2015;193:669–675. doi: 10.1007/s00408-015-9748-0. [DOI] [PubMed] [Google Scholar]
- 35.Tsuji T, Tanaka H, Matsumoto K, et al. Capability of three-dimensional speckle tracking radial strain for identification of patients with cardiac sarcoidosis. Int J Cardiovasc Imaging. 2013;29:317–324. doi: 10.1007/s10554-012-0104-7. [DOI] [PubMed] [Google Scholar]
- 36.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 37.Aggeli C, Felekos I, Tousoulis D, Gialafos E, Rapti A, Stefanadis C. Myocardial mechanics for the early detection of cardiac sarcoidosis. Int J Cardiol. 2013;168:4820–4821. doi: 10.1016/j.ijcard.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Nakano S, Kimura F, Osman N, et al. Improved myocardial strain measured by strain-encoded magnetic resonance imaging in a patient with cardiac sarcoidosis. Can J Cardiol. 2013;29:1531 e9–11. doi: 10.1016/j.cjca.2013.02.023. [DOI] [PubMed] [Google Scholar]


