Abstract
Background
Using resting-state functional magnetic resonance imaging (rsfMRI), we previously compared cohorts of bipolar I subjects in a manic state to those in a euthymic state to identify mood state-specific patterns of cortico-amygdala connectivity. Our results suggested that mania is reflected in the disruption of emotion regulation circuits. We sought to replicate this finding in a group of subjects with bipolar disorder imaged longitudinally across states of mania and euthymia
Methods
We divided our subjects into three groups: 26 subjects imaged in a manic state, 21 subjects imaged in a euthymic state, and 10 subjects imaged longitudinally across both mood states. We measured differences in amygdala connectivity between the mania and euthymia cohorts. We then used these regions of altered connectivity to examine connectivity in the longitudinal bipolar group using a within-subjects design.
Results
Our findings in the mania vs euthymia cohort comparison were replicated in the longitudinal analysis. Bipolar mania was differentiated from euthymia by decreased connectivity between the amygdala and pre-genual anterior cingulate cortex. Mania was also characterized by increased connectivity between amygdala and the supplemental motor area, a region normally anti-correlated to the amygdala in emotion regulation tasks.
Limitations
Stringent controls for movement effects limited the number of subjects in the longitudinal sample.
Conclusions
In this first report of rsfMRI conducted longitudinally across mood states, we find that previously observed between-group differences in amygdala connectivity are also found longitudinally within subjects. These results suggest resting state cortico-amygdala connectivity is a biomarker of mood state in bipolar disorder.
Keywords: bipolar, imaging, longitudinal, fMRI, mania, connectivity
Introduction
Bipolar disorders are defined by the appearance of pathological states of depression or mania. A growing body of neuroimaging studies has expanded our understanding of bipolar disorders as disease entities (reviewed in (Strakowski, 2012)). Despite the striking differences in cognition, emotion and behavior between the manic and euthymic states, our understanding of how neurophysiology differs between these mood states is more limited (reviewed in (Salvadore et al., 2010)). To better elucidate the physiology underlying differences in bipolar mood state, we recently utilized resting-state functional magnetic resonance imaging (rsfMRI) to compare differences in functional connectivity between bipolar mania and bipolar euthymia (Brady et al., 2016). In that study we compared two cohorts of subjects, one manic and the other euthymic, and examined whole-brain functional connectivity to several brain regions. We discovered significant differences in functional connectivity between the amygdala and cortical regions between the two populations. Specifically, the manic state was defined by a loss of functional connectivity between the amygdala and the pre-genual anterior cingulate cortex (pgACC). This finding extends existing models of bipolar disorder that implicate pathophysiology in the ACC (Phillips et al., 2008; Strakowski et al., 2012). We also observed increased connectivity between the amygdala and the supplemental motor area (SMA) in mania. This latter finding suggested an inversion of the activity observed in these structures during emotion regulation in healthy control subjects (Etkin et al., 2015; Kanske et al., 2011). Taken together, our results suggest that clinical states of dysregulated emotion may be reflected in state-related dysfunction in circuits of emotion regulation.
Additionally, we observed evidence of lateralization with the right amygdala showing more significant differences than the left.
These findings extend our understanding of the functional neuroanatomy of bipolar disorder by implicating specific structures in state related differences. One caveat of our analysis was that the experiment was a cross-sectional comparison of two cohorts of subjects rather than a longitudinal, within-subjects design. Few studies in bipolar disorder have employed a longitudinal imaging design across mood states (reviewed in (Brady et al., 2014)). Differences observed cross-sectionally between cohorts selected for mood state could be influenced by other differences between cohorts. To address this, we sought to examine state related amygdala circuit function utilizing a within-subjects design conducted longitudinally across mood states of mania and euthymia.
In the interim since the publication of our initial report we have continued to recruit subjects with bipolar disorder type I in a manic state, bipolar I subjects in a euthymic state, and bipolar I subjects imaged longitudinally in both mood states. Some subjects imaged in a manic state in our initial report subsequently returned for euthymic scans. To avoid the hazard of circular analysis i.e. deriving ROIs from a study sample then re-analyzing a partially overlapping data set with those ROIs (Kriegeskorte et al., 2009), we divided our bipolar subjects into three groups: A cohort of subjects imaged while manic, a cohort of subjects imaged while euthymic, and a third group imaged longitudinally across both mood states. We then conducted two analyses: First we analyzed whole-brain amygdala connectivity in mania and euthymia in a cross-sectional design by comparing the manic cohort to the euthymic cohort. We then used the regions of altered connectivity discovered in this comparison to examine functional connectivity in the group of bipolar subjects imaged longitudinally across mood states of mania and euthymia in a within-subjects design. As an additional control, we examined functional connectivity in these same regions in a group of healthy comparison (HC) subjects imaged longitudinally at a similar interval to the longitudinal bipolar subjects.
Methods
Participants
Study recruitment was performed as in our previous study (Brady et al., 2016). All participants gave written informed consent before participating. To ensure that participants understood the study, we conducted an informed consent survey, including simple questions about risks and benefits and the ability to withdraw consent. If the participants did not answer all questions correctly, the informed consent document was re-reviewed and understanding retested to ensure comprehension. Bipolar subjects in the mania cohort were typically recruited from McLean Hospital inpatient units during hospitalization for a manic episode.
Euthymic bipolar subjects were typically recruited from patients who had previously been hospitalized on these inpatient units. Longitudinal subjects were all recruited, characterized, and imaged while hospitalized for mania and were then contacted post-hospitalization for imaging and clinical characterization while euthymic. There was no overlap in subjects between the manic, euthymic, and longitudinal cohorts.
Diagnosis was determined using the Structured Clinical Interview for the DSM-IV (SCID)(First and New York State Psychiatric Institute. Biometrics Research, 2007)). All subjects were assessed using the Young Mania Rating Scale (YMRS), Montgomery–Asberg Depression Rating Scale (MADRS), and Positive And Negative Syndrome Scale (PANSS) at the time of imaging. All subjects met criteria for the diagnosis of bipolar disorder type I. Subjects imaged in a manic state all had a YMRS score of 20 or greater. Subjects in a euthymic state all had a YMRS score of 12 or less. In addition, all longitudinal subjects did not meet DSM criteria for any mood episode for at least one month prior to imaging in the euthymic state. All longitudinal HC comparison subjects had no history of psychiatric illness nor any family history of first degree relatives with psychiatric illness.
Exclusion criteria included age outside the range of 18–65, neurological illness, pregnancy or lactation, electroconvulsive therapy in the last three months, history of head trauma with a loss of consciousness lasting more than a few minutes, and contraindications to magnetic resonance imaging.
MRI data acquisition
All data were acquired on a 3T Siemens Trio-TIM scanner using a standard 12-channel head coil. No scanner upgrade occurred during the duration of data collection. Each scan consisted of two 6.2 minute rsfMRI runs with imaging parameters as follows: repetition time = 3000 milliseconds; echo time = 30 milliseconds; flip angle = 85°; 47 axial 3mm sections collected with interleaved acquisition. Structural data included a high-resolution, multiecho, T1-weighted, magnetization-prepared, gradient-echo image. All participants were told ‘remain still, stay awake, and keep your eyes open’. Video recording of the eyes was used to confirm the awake, eye-open state.
MRI data processing
Imaging data were preprocessed using the DPABI (Yan et al., 2016). rsfMRI runs with head motion exceeding 3mm in any dimension or 30 of maximum rotation about three axes were discarded from further analysis. After realigning, slice timing correction, and co-registration, framewise displacement (FD) was calculated for all volumes (Power et al., 2012). Volumes with a FD greater than 0.2mm were regressed out during nuisance covariate regression. Any rsfMRI run with 50% or more of volumes regressed out was discarded from further analysis. Structural images from each subject were then normalized and segmented into gray, white and CSF partitions using the DARTEL technique(Ashburner, 2007). A Friston 24-parameter model was used to regress out head motion effects (Friston et al., 1996). The CSF and white matter signals, global mean signal as well as the linear trend were also regressed as nuisance covariates. The resultant data were band pass filtered to select low frequency (0.01–0.08Hz) signals. Functional images were brought into MNI (Montreal Neurological Institute) space and then smoothed by a Gaussian kernel of 8mm3 full-width at half maximum. Voxels within a gray matter mask were used for further analyses.
Functional Connectivity Analysis
Bipolar mania cohort vs Bipolar euthymia cohort
In our comparison of the mania cohort to the euthymia cohort we measured whole-brain functional connectivity (FC) to the right amygdala using a ROI generated using Wake Forest University Pickatlas software (version 3.0.5)(Maldjian et al., 2003). The time course of voxels in this ROI was extracted and Pearson correlation coefficients between this timecourse and those of all other voxels were calculated. These values were transformed to Fisher’s z scores to generate FC maps. Two –sample T- contrasts were then performed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) to examine differences in FC between the mania and euthymia groups. Age, sex, and prescribed antipsychotic dosage in chlorpromazine equivalents (CPZE) covariates were regressed from the T-Tests as nuisance variables. The resulting contrast maps were thresholded for voxels with a P value < .005. The threshold for cluster level significance was determined using Monte Carlo simulation using AlphaSim as implemented in REST(Song et al., 2011). This resulted in a threshold of k=46 voxels for p<.05 clusterwise significance.
Longitudinal Cohort
We then used the regions of altered functional connectivity derived from this cross-sectional study as regions of interest (ROIs) to measure connectivity to the amygdala in the independent cohort of subjects imaged longitudinally across mood states of mania and euthymia. The time course of these regions and the amygdala ROI were extracted and we calculated Pearson correlation coefficients between them. These values were transformed to Fisher’s z scores.
Statistical Analyses
Subject characteristics
For the cross-sectional subject comparisons, comparisons of sex and the presence or absence of prescribed medications were made using a chi-square test of association. For continuous variables such as age, duration of illness, number of hospitalizations, symptom scales, and antipsychotic dosage, comparisons were made using a t-test or Mann-Whitney U test for non-normally distributed variables.
For the longitudinal study cohort, comparisons of the presence or absence of medications in subject’s regimens at both mood states used McNemar’s test for significant differences. Comparisons of symptom scales and the prescribed dosage of antipsychotic medications (chlorpromazine equivalents, CPZE) used a paired t-test.
For functional connectivity measures in the longitudinal group, the Fisher’s z-transformed correlation coefficients between ROIs in the manic and euthymic states were compared using a paired t-test. Repeated measures testing with inter-scan interval as a covariate was performed in SPSS (SPSS Inc., Chicago, IL, USA) using the GLM repeated measures function. The threshold for statistically significant differences was set to p < .025 (alpha <.05 corrected for two comparisons).
As an additional exploratory analysis, we examined whole-brain functional connectivity to the right amygdala ROI in the longitudinal cohort using a paired t-test in SPM12.
Results
Table 1 lists demographic and clinical characteristics of the study populations. In the cross-sectional comparison, the bipolar mania and euthymia cohorts were comparable in terms of age, gender, duration of illness, and number of prior hospitalizations. Subjects in the mania cohort were more symptomatic on all symptom scales and were more likely to be prescribed antipsychotics at a higher dose than the euthymia cohort (Table 1A).
Table 1.
Demographics and clinical characteristics of the subject groups
| 1A Cohort Comparison
| |||
|---|---|---|---|
| Demographics
|
Statistic | ||
| Bipolar-Manic Subjects (n=26) | Bipolar-Euthymic Subjects (n=21) | Mania vs Euthymia | |
| Sex | 20 Male, 6 Female | 13 Male, 8 Female | p=.263 |
| Age in years, mean (SD) | 27.7 (10.5) | 33 (12.9) | p=.094 |
| # of hospitalizations, mean (SD) | 3.79 (4.19) | 4.61 (3.8) | p=.423 |
| Duration of illness in years, mean (SD) | 11.6 (12.1) | 7.32 (7) | p=.308 |
| YMRS, mean (SD) | 28.6 (6.71) | 3.62 (3.84) | p= 4.75 × 10-9 |
| MADRS, mean (SD) | 14.3 (8.03) | 6.09 (5.48) | p = 2.54×10-4 |
| PANSS, mean (SD) | 56 (16.2) | 39 (8.85) | p=2.21 × 10-4 |
| PANSS positive subscale, mean (SD) | 22.7 (5.35) | 9.9 (3.51) | p=2×10-8 |
| Anticonvulsants | 8/26 | 10/21 | ns |
| Antipsychotics | 25/26 | 16/21 | p=.041 |
| CPZ equivalents, mean (SD) | 396 (349) | 203 (239) | p=0.014 |
| Benzodiazepines | 8/26 | 4/21 | ns |
| Lithium | 18/26 | 8/21 | p=.033 |
| Antidepressants | 1/26 | 3/21 | ns |
| 1B Longitudinal Comparison
| ||||
|---|---|---|---|---|
| Bipolar Subjects (n=10) | HC subjects (n=10) | |||
| Sex | 6 Male, 4 Female | 5 Male, 5 Female | ||
| Age in years at first scan, mean (SD) | 25.6 (8.90) | 27.8 (13.2) | ||
| # of hospitalizations, mean (SD) | 3.2 (2.3) | n/a | ||
| Duration of illness in years, mean (SD) | 6.1 (5.61) | n/a | ||
| Time in months Between Scans, mean (SD) | 15.2 (14.7) | 19.7 (18.9) | ||
| State | Statistic | |||
| Mania | Euthymia | Mania vs Euthymia | ||
| YMRS, mean (SD) | 26.7 (3.68) | 2 (2.31) | p= 9.93×10-9 | |
| MADRS, mean (SD) | 14.5 (11.9) | 3.2 (3.19) | p= 0.01 | |
| PANSS, mean (SD) | 58.9 (11.05) | 33.2 (6.01) | p= 7.81×10-6 | |
| PANSS positive subscale, mean (SD) | 23.2 (5.53) | 7.7 (1.06) | p= 8.48×10-6 | |
| Anticonvulsants | 6/10 | 5/10 | ns | |
| Antipsychotics | 9/10 | 7/10 | ns | |
| CPZ equivalents, mean (SD) | 364(227) | 262(382) | ns | |
| Benzodiazepines | 3/10 | 1/10 | ns | |
| Lithium | 8/10 | 8/10 | ns | |
| Antidepressants | 0/10 | 1/10 | ns | |
Abbreviations: CPZE: Chlorpromazine equivalents, MADRS: Montgomery-Asberg Depression Rating Scale, ns: non-significant, PANSS: Positive And Negative Symptom Scale, SD: Standard Deviation, YMRS: Young Mania Rating Scale
In the longitudinal bipolar group, bipolar subjects were more symptomatic during the manic state on all symptom scales. We did not observe significant differences in medication regimens between mood states (Table 1B).
Increased MADRS scores in manic subjects typically came from scores on the reduced sleep, concentration difficulties, and inner tension items.
We examined whole-brain functional connectivity to the right amygdala in the mania and euthymia cohorts. When compared to euthymia subjects, bipolar mania subjects demonstrated significantly less functional connectivity between the amygdala and the pre-genual ACC (pgACC) (Figure 1A). The bipolar mania subjects also demonstrated a significantly increased functional connectivity between the amygdala and the bilateral SMA (Figure 1B). These results are consistent with our prior results and not surprising as this data set partially overlaps with the data analyzed in our original study.
Figure 1. Whole-brain changes in functional connectivity to the right amygdala in bipolar mania compared to euthymia.
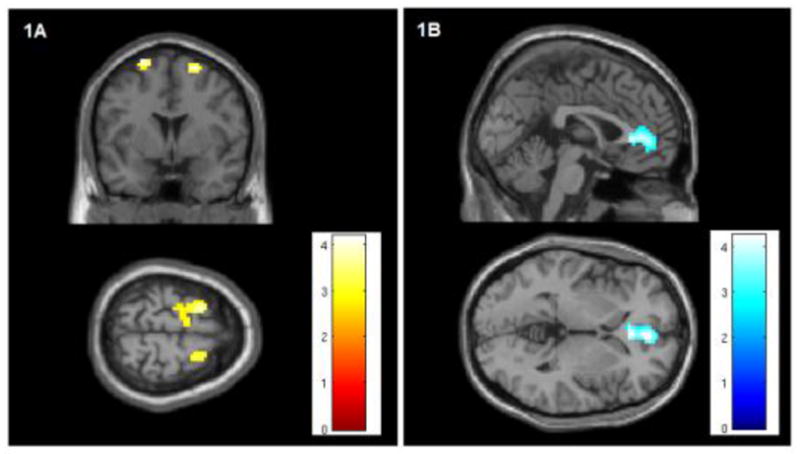
1.A The right amygdala demonstrates increased functional connectivity to the bilateral SMA in mania compared to euthymia (R: peak coordinates x21, y3, z66, T-stat 4.11, extent k=47 voxels. L: coordinates x-21, y6, z69, T-stat 4.12, extent =78 voxels). The color bar indicates T-statistic magnitude. Images are thresholded at voxel-wise P<.005. This region of significantly increased connectivity is shown projected onto a MNI152 template brain at the y=3 and z=69 levels.
1.B The right amygdala demonstrates decreased functional connectivity to the pre-genual ACC in bipolar mania compared to bipolar euthymia (peak coordinates x3, y45, z0, T-stat 4.25, extent = 201 voxels). The color bar indicates T-statistic magnitude. Images are thresholded at voxel-wise P<.005. This region of significantly decreased connectivity is shown projected onto a MNI152 template brain at the x=0 and z=0 levels.
We then examined functional connectivity in the independent group of subjects imaged longitudinally and observed that within-subjects, the manic state demonstrates significantly decreased amygdala- pgACC connectivity (p = .019) and significantly increased amygdala- SMA connectivity (p=.018) when compared to the euthymic state. Incorporating inter-scan interval as a covariate in a repeated measures ANCOVA of the effect of mood state continued to demonstrate a significant decrease in amygdala-ACC connectivity in mania (p =.041) and increase in amygdala-SMA connectivity in mania (p = .003).
To control for non-specific scanner effects, we examined functional connectivity between these ROIs in a cohort of healthy controls scanned at a comparable interval. We did not observe significant differences in amygdala-pgACC (p= .402) or amygdala-SMA connectivity (p=.162).
Although our bipolar longitudinal cohort is small for whole-brain analyses, we conducted an exploratory within-subject analysis of whole-brain functional connectivity to the right amygdala. Again, the most significant difference in FC between mood states was increased amygdala connectivity to the SMA in mania compared to euthymia (peak coordinates x12, y0, z69, T-stat 5.67, extent k=65 voxels, cluster-wise p-FWE=.034). No other brain regions demonstrated as significant a change in FC. Ongoing recruitment for a larger longitudinal cohort should allow for a better understanding of how mania is reflected in global changes in amygdala connectivity.
Discussion
To our knowledge this is the first study of resting-state functional connectivity longitudinally across mood states in bipolar disorder. In our prior analysis, we identified patterns of whole-brain connectivity to the amygdala that differentiated cohorts of manic and euthymic bipolar subjects. The current study re-examines these cortico-amygdala interactions in bipolar subjects followed longitudinally across mood states of mania and euthymia. Our current findings demonstrate that, within subjects, mania is marked by decreased amygdala-pgACC connectivity and increased amygdala-SMA connectivity compared to euthymia. The discovery of these relationships in a within-subjects design suggests that these interactions may be true biomarkers of mood state.
Limitations
A methodological limitation of the current study is that the number of longitudinal subjects included in the analysis was limited by controlling for movement effects. Obtaining high-quality MRI data from manic subjects is a formidable challenge. While the total number of subjects in this study is small, the number is larger than almost all of the published longitudinal imaging studies of bipolar mood states (reviewed in (Brady et al., 2014)).
Despite this limitation, these results support a neural circuit model of the pathophysiology of bipolar disorder in which the intrinsic connectivity of neural circuits involved in emotion regulation are, in fact, biomarkers of mood state. In this they may prove targets for engagement using therapeutic neuromodulatory interventions. They may also be potentially clinically valuable as biomarkers for early identification and intervention for manic episodes.
Highlights.
Resting state fMRI reveals bipolar mood specific patterns of functional connectivity
Mania and euthymia showed distinct patterns of cortico-amygdala connectivity
We examined amygdala connectivity in manic and euthymic bipolar cohorts
We replicated these results in subjects imaged longitudinally in mania and euthymia
Mania is reflected in circuit level dysfunction in regions of emotion regulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Brady R, Jr, Ongur D, Keshavan M. Neurobiology of mood-state shifts in bipolar disorder: a selective review and a hypothesis. Harv Rev Psychiatry. 2014;22:23–30. doi: 10.1097/HRP.0000000000000004. [DOI] [PubMed] [Google Scholar]
- Brady RO, Masters GA, Mathew IT, Margolis A, Cohen BM, Öngür DMK. State Dependent Cortico-Amygdala Circuit Dysfunction in Bipolar Disorder. Journal of Affective Disorders. 2016;201:79–87. doi: 10.1016/j.jad.2016.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nature reviews. Neuroscience. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- First MB New York State Psychiatric Institute. Biometrics Research, D. Structured clinical interview for DSM-IV-TR axis I disorders : SCID-I. Biometrics Research Dept., New York State Psychiatric Institutute; New York, N.Y: 2007. [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, Zarate CA., Jr The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010;71:1488–1501. doi: 10.4088/JCP.09r05259gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM. The Bipolar Brain: Integrating Neuroimaging and Genetics. Oxford; New York: 2012. [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, Delbello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]


