Abstract
Background:
The placement of an external ventricular drain (EVD) for monitoring and treatment of increased intracranial pressure is not without risk, particularly for the development of associated ventriculitis. The goal of this study was to investigate whether changes in cerebrospinal fluid (CSF), serum, or clinical parameters are correlated with the development of ventriculitis before it occurs, allowing for the determination of optimal timing of CSF collection.
Methods:
An observational retrospective study was conducted between January 2006 and May 2012. A total of 466 patients were identified as having an in-situ EVD placed. Inclusion criteria were age >18 years, glasgow coma scale (GCS) 4-15, and placement of EVD for any indication. Exclusion criteria included recent history of meningitis, cerebral abscess, cranial surgery or open skull fracture within the previous 30 days. A broad definition of ventriculitis was used to separate patients into three initial categories, two of which had sufficient patients to proceed with analysis: suspected ventriculitis and confirmed ventriculitis. CSF sampling was conducted on alternating weekdays.
Results:
A total of 466 patients were identified as having an EVD and 123 patients were included in the final analysis. The incidence of ventriculitis was 8.8%. Only the ratio of glucose CSF: serum <0.5 was found to be of statistical significance, though not correlated to developing a ventriculitis.
Conclusions:
This study demonstrates no reliable tested CSF, serum, or clinical parameters that are effectively correlated with the development of ventriculitis in an EVD patient. Thus, we recommend and will continue to draw CSF samples from patients with in-situ EVDs on our current schedule for as long as the EVD remains in place.
Keywords: Cerebrospinal fluid, external ventricular drain, infection, surveillance, ventriculits
INTRODUCTION
The use of external ventricular drain (EVD) serves a dual function in neurosurgical patients. It not only serves as a diagnostic monitor to detect elevated intracranial pressure (ICP), but also serves a therapeutic role in its ability to drain cerebrospinal fluid (CSF) in the setting of elevated ICP. While the ICP device is used in many neurosurgical patients, including but not limited to, those with subarachnoid hemorrhage (SAH), intracranial hemorrhage (ICH), or acute hydrocephalus; it is not without complications. The most commonly noted complication is ventriculitis. Incidence rates vary depending on the institution, and range from 0–22%, but more commonly tend to lie within the 10–17% range.[2,5,10,16,18,21,24,26] The rate of ventriculitis tends to be associated with a higher morbidity and mortality for the neurosurgical patient.[16,26] Recent improvements in sterility techniques, antibiotic-impregnated catheters, and appropriate antibiotic administration have helped to decrease the rate of ventriculitis.[16,26]
A literature review by R. Beer et al., spanning 18 years (1990–2008) confirmed an infection rate within the range commonly cited (5–20%), but found that it varies considerably from center to center as well as from different studies.[3,4] Their analysis further indicated that gender, age, duration of EVD, as well as number of CSF samples taken from the EVD have all been identified as risk factors for the development of ventriculitis in previous studies. These studies have shown serious consequences of ventriculitis, which can include impaired intellectual capacity, prolonged hospital stay, as well as death.[11] The overall mortality rate among patients with ventriculitis has been reported to be in the 8–70% range, with the highest rates reported before the introduction of third-generation cephalosporins.[9]
In a prospective epidemiologic study, Mayhall et al.[14] analyzed the risk factors for ventriculitis in 172 consecutive neurosurgical patients. Their proposal stated that infection rates may be lowered by prophylactic exchange of catheters at 5-day intervals. However, a decade later, Holloway et al.[10] showed that replacement of catheters prior to 5 days did not show a lower infection rate as compared to catheters exchanged at intervals of 5 days or greater. Over time, several approaches have been suggested to prevent ventriculitis, such as use of prophylactic antibiotics at time of insertion, during the first 24 hours post-insertion, and in a prolonged systemic fashion while the EVD is in place.[27] However, there is no consistent practice regarding the use of prolonged systemic antibiotics with EVD and the preference is largely related to tradition and training.[15,27]
Early detection of ventriculitis is imperative for successful treatment and for minimizing the possibility of future infections.[24] However, the clinical detection of ventriculitis can be difficult. For example, a study by Muttaiyah et al.[18] determined that there was no associated temperature spike with ventriculitis. However, CSF glucose levels were notably lower in these patients.
An important consideration in the control of ventriculitis is the surveillance of CSF and monitoring for clinical symptoms of ventriculitis.[17] Currently, the issues of when and how often CSF sampling should be performed remains controversial.[17] Routine CSF sampling has been reported as daily,[5,17,20,22] every 3–5 days[1,6,25,26] only at EVD insertion, or only as clinically indicated.[10,13,28] In a study by Williams et al.,[24] investigators sought to examine how the incidence of ventriculitis was affected by CSF sampling frequency, by decreasing surveillance from daily to every 3 days. Their results demonstrated that the incidence of ventriculitis decreased from 17% to 10.8% after changing CSF surveillance to every 3 days.[24]
Naturally, one would assume that a breach of the EVD drainage system for sampling the CSF would increase the risk of contamination and subsequent infection. However, this risk must be balanced by the morbidity and mortality of ventriculitis, if it remains undetected. This is especially important in light of the established literature showing the difficulty in clinical detection of ventriculitis. Current standard practice at our institution is to sample CSF upon insertion of EVD, followed by sampling every Monday, Wednesday, and Friday for as long as the EVD remains in place. As a result of these challenges, we decided to examine whether changes in any CSF, serum, or clinical parameters are correlated with the development of ventriculitis before it occurs, and if the current collection schedule of CSF is necessary.
MATERIALS AND METHODS
The patients included in this study were identified during the course of the study using their medical record numbers. We performed a retrospective observational study of the Neurosurgical Census, for patients with in situ EVD from January 2006 to May 2012. A total of 466 patients were identified. Medical records were then used to classify patients by age and gender; the duration of EVD, CSF and serum laboratory data, daily temperatures, changes in GCS, CSF culture, and the presence of other infections. To be included in the study, patients had to be 18 years or older, have an EVD (regardless of indication), and GCS 4-15. Patients with any recent (defined as within the last 30 days) history of meningitis, cerebral abscess, craniotomy, or open skull fracture were excluded from the study [Figure 1]. Our institution is a Level II trauma center. Thus, traumatic brain injury was the etiology for the majority of the patients in our study. However, there were various other indications for EVD placement, including but not limited to: subarachnoid hemorrhage, interventricular hemorrhage, cerebral edema, and hydrocephalus. In our opinion and based on our experience, once the aforementioned exclusion criteria were applied (i.e., history of meningitis, cerebral abscess, craniotomy or open skull fracture), the indication for EVD placement should not usually correlate with the development of ventriculitis.
Figure 1.
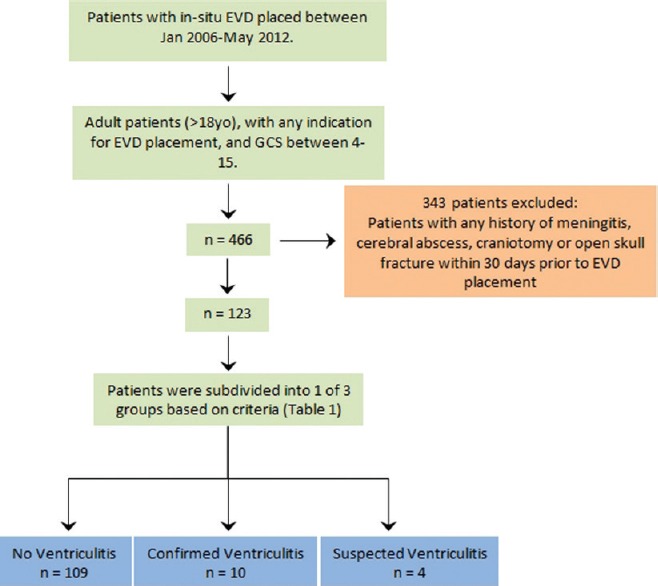
The criteria for patient selection, as well as the number of patients that were included in the study
At our institution, EVDs are placed by standard neurosurgical sterile technique in either the operating room (OR), the emergency department (ED), or in the intensive care unit (ICU), and are then connected to a sterile closed circuit system. Since the indication for most of these EVDs was emergent, the setting could have been anywhere in OR, ED, or ICU. However, since an institutional protocol is established, the best effort is made to follow the standard sterile technique regardless of location. Therefore, in our opinion and given the standard placement protocol that is followed, the patient's physical location in the hospital should not significantly affect the risk for the development of ventriculitis.
Antibiotic-impregnated catheters were not available at our institution during the study time period. However, based on recent literature, antibiotic-impregnated catheters do appear to decrease the rate of ventriculitis.[8] For this study, uniformity of collected data was paramount, and thus all ventriculostomy catheters included here were non-antibiotic impregnated Integra trauma catheters.[7,15,16,23] After the EVD has been sutured into place, a BioPatch is placed where the catheter exits the skin and a Tegaderm is placed to cover the BioPatch and catheter. As per standard neurosurgical protocol, a non-contrast Computerized Tomography (CT) scan was obtained after placement to rule out hemorrhage caused by placement and to confirm satisfactory placement of the catheter.
In regards to patients with an EVD, per current institutional protocol for the Department of Neurosurgery, CSF samples are drawn every other day during the week (i.e., Monday, Wednesday, Friday) for as long as the EVD remains in place. CSF samples are drawn solely by the neurosurgery residents or attending physicians. Our collection procedure was performed per institutional protocol developed by the neurosurgery department. Significant increase in the cytology number has been observed at our institution when CSF is collected more distally or from the drainage burette. Therefore, collection is performed in the following manner and in a sterile fashion in order to minimize infection risk to the patient. The stopcock just distal to the catheter insertion site is turned off from the drainage system and the proximal port is cleaned with chlorahexidine twice. A sterile syringe is connected to the port and 3–5 ml of CSF is slowly withdrawn and then discarded. The port is then cleaned a second time and another 3–5 ml of CSF is withdrawn, and sent for culture and analysis using standard methods.
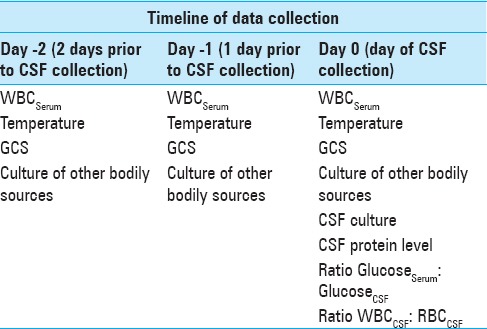
For patients in our study, the aforementioned laboratory and clinical parameters were collected on the day of EVD placement (“day 0”). Additionally, the following data was collected on the day of EVD placement (“day 0”), as well the day prior (“day 1”) and 2 days prior (“day 2): serum white blood cells (WBC), temperature, GCS, and cultures from other bodily sources (including sputum, nasal, urine, blood, neck, or tracheostomy). The schema for the time-based collection of all laboratory and clinical data can be found in Table 1.
Table 1.
Schema showing the timeline of data collection on the day of CSF draw (Day 0), and previous two days
To define ventriculitis, we used the criteria proposed by Lozier et al.,[12] including fever (≥38.5°C, with/without clinical signs of meningitis), associated with at least one positive CSF culture and at least one element of CSF abnormality, including low CSF glucose levels (<40 mg/dl, or <50% of serum glucose in patients with hyperglycemia), high CSF protein (>100 mg/dl), or CSF pleocytosis (>100/mm3). In addition, we also looked at the ratio of CSF glucose to serum glucose (<0.5) and the ratio of WBC in the CSF to red blood cells (RBC) in the CSF (>1:250).
Patients were broadly divided into three categories: No ventriculitis, suspected ventriculitis, and confirmed ventriculitis. A CSF culture with the growth of any organisms denoted confirmed ventriculitis. In the absence of any organisms, the previously mentioned criteria from Lozier et al.[12] were used to denote suspected ventriculitis: CSF glucose to serum glucose ratio <0.5, CSF WBC to RBC ratio >1:250, and CSF protein >100 mg/dL [Figure 2]. Patients who did not meet all of these criteria, or did not have any organisms present on CSF culture were placed into the no ventriculitis group.
Figure 2.
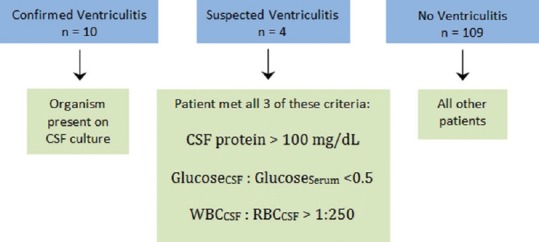
Illustrates the criteria that were used to place patients into one of three categories
Statistical analysis
All statistical analyses were conducted using the SAS software for Windows version 9.3 (Cary, NC). Descriptive statistics were presented as means and standard deviations for continuous variables (for example, age), and frequencies and percentages for categorical variables. Crosstab analyses were conducted to identify the association between two categorical variables using the Chi-square test or Fisher's exact test if the expected cell count does not meet the assumption. Given the small count in the suspected ventriculitis (N = 4), this category was excluded from the analysis. This was done to prevent any crossover contamination of the data by the suspected ventriculitis group into the other two groups (confirmed & no ventriculitis). These two groups are essentially positive and negative for ventriculitis, respectively, and the intermediate suspected ventriculitis group does not clearly belong in either one based on established criteria in the literature.[12] Independent t-test was adopted to analyze whether there is a statistically significant difference between patients with and without ventriculitis. All statistical tests were two-sided. P value <0.05 was considered to be statistically significant.
RESULTS
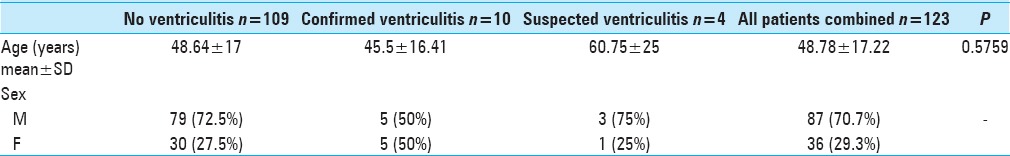
A total of 123 were included in the final analysis. The majority of these patients were males (N = 87, 70.7%) with the average age being 48.8 ± 17.2 years. A total of 10 (8.1%) patients diagnosed with ventriculitis, 4 (3.3%) with suspected ventriculitis, and 109 (88.6%) without ventriculitis were identified in this sample [Table 2].
Table 2.
Demographic breakdown of all patients with respect to their ventriculitis status
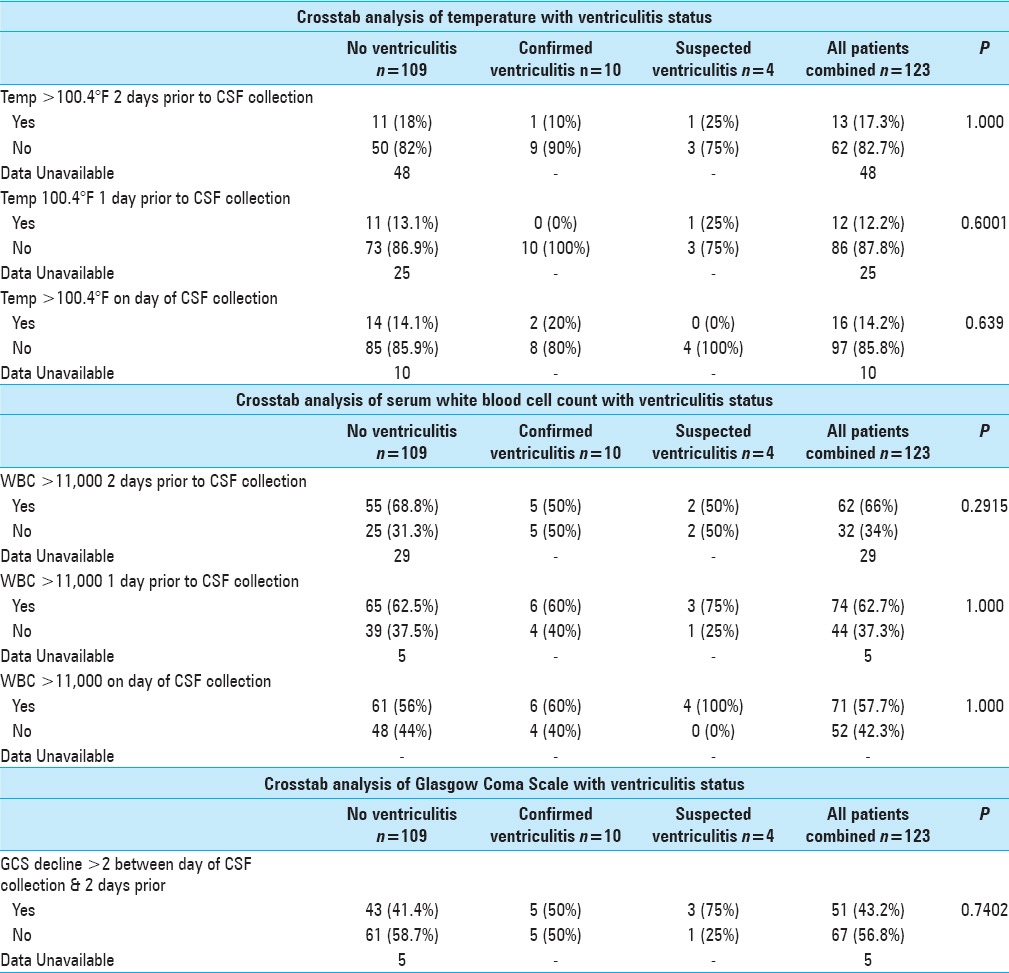
Crosstab analyses were conducted using the Fisher's exact test [Table 3]. The suspected ventriculitis patients were excluded due to the extremely small sample size (N = 4). These patients were not included in either of the other two categories, since they did not clearly belong in either the confirmed group or the no ventriculitis group based on well-established criteria in the literature.[12] Regardless of the sampling time frame (2 days ago, 1 day ago, or 0 day ago), temperature >100.4F or WBC >11,000 or decline in GCS by two or more points, were not associated with the ventriculitis status.
Table 3.
Summary of crosstab analysis results with respect to temperature, white blood cell count, and GCS
However, despite the lack of statistically significance (P = 0.0866), patients who were diagnosed with ventriculitis were twice as more likely to have a WBC to RBC ratio >1:250 (40% vs 16.5% for ventriculitis and no ventriculitis cohort, separately). Similarly, patients who were diagnosed with ventriculitis were twice as likely to have CSF Glucose <0.5 serum (50% vs 21.2% for ventriculitis and no ventriculitis cohort, separately). Lastly, using glucose as a continuous variable, patients with ventriculitis had statistically significant lower concentration of glucose than the counterpart (0.48 and 0.62 for ventriculitis and no ventriculitis cohort, separately, P = 0.0298) [Table 4].
Table 4.
Summary of crosstab analysis results of CSF parameters with respect to ventriculitis status
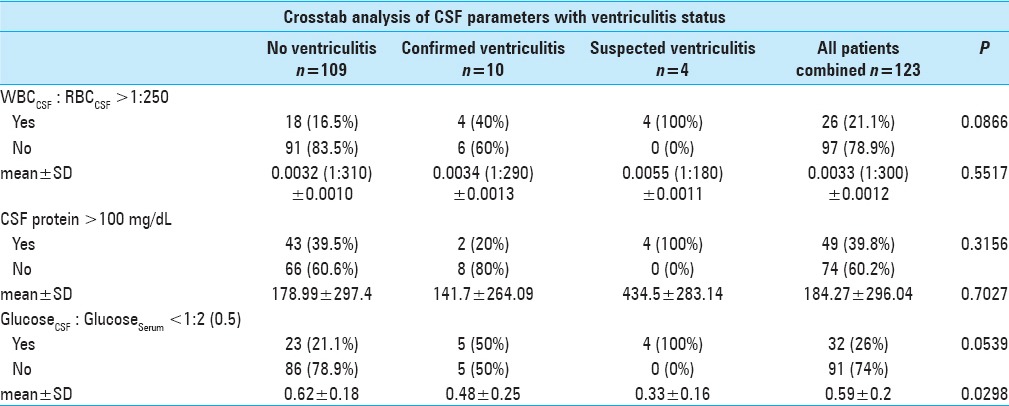
Crosstab analysis of ventriculitis status with respect to fever greater than 100.4°F did not show statistical significance, regardless of day of collection. A temperature greater than 100.4°F 2 days prior to CSF collection, one day prior to collection, and on the day of collection resulted in respective P values of 1.000, 0.600, and 0.639 [Table 3]. Leukocytosis was also not statistically significant, regardless of day of collection. In fact, WBC greater than 11,000/μL that occurred 2 days prior to CSF collection, one day prior to collection, or even on the day of collection resulted in respective P values of 0.292, 1.000, and 1.000 [Table 3]. In addition, a GCS decline of 2 points or greater, between the day of CSF collection and 2 days prior to collection failed to show statistical significance [Table 3]. Lastly, CSF protein levels greater than 100 mg/dL also did not show statistical significance with respect to ventriculitis status [Table 4].
With respect to microbiology in our patient population, Coagulase-negative staphylococci (including Staphylococcus epidermidis) was found to be the most common organism isolated from positive CSF cultures, occurring approximately 53% of the time. Additional organisms isolated included Corynebacterium species, methicillin-resistant Staphylococcus aureus, Propionibacterium acnes, Enterobacter aerogenes, and Cryptococcus neoformans.
Only one of our criteria for the diagnosis of ventriculitis, the ratio of glucose in the CSF: serum proved to be statistically significant when this ratio is less than 0.5 or 1:2 (P = 0.0298). Approximately 21% of patients without ventriculitis had a glucose CSF: serum ratio <0.5, as compared to 50% of patients with confirmed ventriculitis. While statistically significant, this was not found to be a correlated with the development of ventriculitis (P = 0.0539). It was noted that patients who were diagnosed with ventriculitis were twice as likely to have a glucose CSF: serum ratio of less than half.
DISCUSSION
Our study sought to investigate the presence of the aforementioned CSF, serum, and clinical parameters that may be correlated with the development of ventriculitis before it occurs; thereby allowing for the determination of an optimal CSF surveillance schedule. Our study population consisted mostly of intubated and sedated ICU patients who presented a challenge for evaluation of clinical symptoms and signs of ventriculitis. In addition, evaluation of any new focal neurological symptoms or change in mental status in our patient population proved difficult, thus necessitating the use of additional parameters for detection of ventriculitis. In our study, the incidence of ventriculitis was determined to be 8.8% overall. This was found to be slightly lower than the established literature values.[2,5,10,16,18,21,24,26] The rates of ventriculitis have a wide range in the established literature, which is likely due to the variety of definitions used for ventriculitis along with different CSF collection techniques. The importance of being able to diagnose ventriculitis as early as possible is critical for the treatment of the infection as well as mitigating the morbidity and mortality associated with it.
Upon further investigating additional criteria for suspected ventriculitis, the ratio of CSF WBC: RBC had inconsistent findings. As noted in Table 4, our patients with confirmed ventriculitis were over twice as likely to have a ratio above 1:250, when compared to patients without ventriculitis. Pfausler et al.[19] reported an ability to diagnose ventriculitis up to 3 days prior to conventional diagnosis (positive CSF culture) by calculating the ratio of leucocytes to erythrocytes in CSF and leucocytes to erythrocytes in the peripheral blood, which they defined as the cell index (CI). However, Pfisterer et al.[20] suggest that an upward trend in CSF leukocytosis should lead the physician to be suspicious of a contamination. As shown in Table 4, our study found a marginally significant association between the CSF, WBC: RBC ratio, and diagnosis of ventriculitis (P = 0.0866).
While the levels of protein in the CSF have classically been a valuable indicator, care must be taken when looking at mean values for this parameter. As Table 4 indicates, our mean CSF protein value for all patients combined is 184.27 mg/dL with a standard deviation of 296.04. The mean protein value for all 3 categories is similarly high (all above 100 mg/dL), with large standard deviations. This is a result of several outlying values, which have skewed the mean value for this parameter. Out of 123 patients combined, 12 patients had CSF protein levels of >500 mg/dL and five of these were greater than 1000 mg/dL, which causes a misleading elevation in our mean values for this parameter (as reflected in the large standard deviation). Although we feel most of these outliers were likely due to contamination of the CSF sample with tissue debris, we have shown the mean and standard deviation nonetheless for statistical completeness.
We acknowledge that this was a retrospective observational study, and with that comes certain limitations. The exact timing of temperature and of GCS documentation to match with one and 2 days prior to onset of ventriculitis was difficult to verify and sometimes unavailable. In addition, even though a standard sterile neurosurgical technique was mandated for CSF collection, human error is always a factor. We feel this may have been reflected in the outlying CSF protein values >500 mg/dL. We also recognize that the patient population with ventriculitis was small and therefore limited our statistical analysis. We propose a continuation of this study in a prospective manner that will allow for more meticulous documentation and collection of necessary data points. We aim to look at additional parameters at various intervals to see if they would be more reliably correlated with the onset of ventriculitis in patients with in-situ EVDs, thus allowing for the optimal timing of CSF collection in these patients.
CONCLUSION
We feel confident that this study demonstrates no reliable CSF, serum, or clinical parameters that are effectively correlated with the development of ventriculitis in an EVD patient. As shown in Table 1, we examined a variety of CSF parameters, including the presence of organisms, protein, glucose, and ratios of WBC to RBC. We also included other factors such as the presence of organisms at other sites (non-CSF), temperature, a decline in GCS as well as WBC in the serum. These parameters were collected and studied for up to two days prior to the day of CSF collection. While the mean ratio of glucose in the CSF was shown to be statistically significant, at a value of less than 1:2, this parameter failed to show a statistical difference between ventriculitis vs. non-ventriculitis patients (P = 0.0539).
In conclusion, we found that the various CSF, serum, and clinical variables that we studied were not reliably correlated with the development of ventriculitis. In the future, we plan on carrying this study forward in a prospective manner to help us determine the optimal timing of CSF collection for patients with in-situ EVDs. At this time, the results from our study do not invalidate our departmental protocol of CSF collection in monitoring of ventriculitis. Thus, we will continue to draw CSF samples on patients with in-situ EVDs on our previously established schedule of Monday/Wednesday/Friday for as long as the EVD remains in place.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Omid Hariri, Email: ohaririUCLA@gmail.com.
Saman Farr, Email: SamanFarr@gmail.com.
Shokry Lawandy, Email: LawandyDO@gmail.com.
Bailey Zampella, Email: b.zampella@ruhealth.org.
Dan Miulli, Email: MiulliD@armc.sbcounty.gov.
Javed Siddiqi, Email: SiddiqiJ@armc.sbcounty.gov.
REFERENCES
- 1.Alleyne CH, Hassan M, Zabramski JM, et al. The efficacy and Cost of Prophylactic and Perioprocedural Antibiotics in Patients with External Ventricular Drains. Neurosurgery. 2000;47:1124–7. doi: 10.1097/00006123-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Aucoin PJ, Kotilainen HR, Gantz NM, Davidson R, Kellogg P, Stone B, et al. Intracranial Pressure Monitors: Epidemiologic Study of Risk Factors and Infection. Am J Med. 1986;80:369–76. doi: 10.1016/0002-9343(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 3.Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol. 2008;255:1617–24. doi: 10.1007/s00415-008-0059-8. [DOI] [PubMed] [Google Scholar]
- 4.Bogdahn U, Lau W, Hassel W, Gunreben G, Mertens HG, Brawanski A, et al. Continuous-Pressure Controlled, External Ventricular Drainage for Treatment of Acute Hydrocephalus – Evaluation of Risk Factors. Neurosurgery. 1992;31:898–903. doi: 10.1227/00006123-199211000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Bota DP, Lefranc F, Vilallobos HR, Brimioulle S, Vincent JL, et al. Ventriculostomy-Related Infections in Critically Ill Patients: A 6-year Experience. J Neurosurg. 2005;103:468–72. doi: 10.3171/jns.2005.103.3.0468. [DOI] [PubMed] [Google Scholar]
- 6.Brown EM. The Management of Neurosurgical Patients with Postoperative Bacterial or Aseptic Meningitis or External Ventricular Drain-Associated Ventriculitis. Br J Neurosurg. 2000;14:7–12. doi: 10.1080/02688690042834. [DOI] [PubMed] [Google Scholar]
- 7.Clark WC, Muhlbauer MS, Lowrey R, Hartman M, Ray MW, Watridge CB, et al. Complications of Intracranial Pressure Monitoring in Trauma Patients. Neurosurgery. 1989;25:20–4. doi: 10.1097/00006123-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Cui Z, Muhlbauer MS, Lowrey R, Hartman M, Ray MW, Watridge CB, et al. Impact of antibiotic- and silver-impregnated external ventricular drains on the risk of infections: A systematic review and meta-analysis. Am J Infect Control. 2015;43:23–32. doi: 10.1016/j.ajic.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Remeš F, Tomáš R, Jindrák V, Vaniš V, Setlík M. Intraventricular and lumbar intrathecal administration of antibiotics in postneurosurgical patients with meningitis and/or ventriculitis in a serious clinical state. J Neurosurg. 2013;119:1596–602. doi: 10.3171/2013.6.JNS122126. [DOI] [PubMed] [Google Scholar]
- 10.Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM, et al. Ventriculostomy Infections: The Effect of Monitoring Duration and Catheter Exchange in 584 Patients. J Neurosurg. 1996;85:419–26. doi: 10.3171/jns.1996.85.3.0419. [DOI] [PubMed] [Google Scholar]
- 11.Humphreys H, Jenks PJ, et al. Surveillance and management of ventriculitis following neurosurgery. J Hosp Infect. 2015;89:281–6. doi: 10.1016/j.jhin.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES. Ventriculostomy-Related Infections: A Critical Review of the Literature. Neurosurgery. 2002;51:170–82. doi: 10.1097/00006123-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM, et al. Ventriculitis Complicating Use of Intraventricular Catheters in Adult Neurosurgical Patients. Clin Infect Dis. 2001;33:2028–33. doi: 10.1086/324492. [DOI] [PubMed] [Google Scholar]
- 14.Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-Related Infections. N Engl J Med. 1984;310:553–9. doi: 10.1056/NEJM198403013100903. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy PJ, Patil S, Conrad SA, Scott LK. International and Specialty Trends in the Use of Prophylactic Antibiotics to Prevent Infectious Complications After Insertion of Extraventricular Drainage Devices. Neurocrit Care. 2010;12:220–4. doi: 10.1007/s12028-009-9284-y. [DOI] [PubMed] [Google Scholar]
- 16.Mikhaylov Y, Wilson TJ, Rajajee V, Thompson BG, Maher CO, Sullivan SE, et al. Efficacy of Antibiotic-Impregnated External Ventricular Drains in Reducing Ventriculostomy-Associated Infections. J Clin Neurosci. 2014;21:765–8. doi: 10.1016/j.jocn.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Mounier R, Lobo D, Cook F, Martin M, Attias A, Aït-Mamar B, et al. From the Skin to the Brain: Pathophysiology of Colonization and Infection of External Ventricular Drain, a Prospective Observational Study. PLoS One. 2015;10:e0142320. doi: 10.1371/journal.pone.0142320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muttaiyyah S, Ritchie S, Upton A, Roberts S. Clinical Parameters Do Not Predict Infection in Patients with External Ventricular Drains: A Retrospective Observational Study of Daily Cerebrospinal Fluid Analysis. J Med Microbiol. 2008;57:207–9. doi: 10.1099/jmm.0.47518-0. [DOI] [PubMed] [Google Scholar]
- 19.Pfausler B, Beer R, Engelhardt K, Kemmler G, Mohsenipour I, Schmutzhard E, et al. Cell Index-a New Parameter for the Early Diagnosis of Ventriculostomy (External Ventricular Drainage)-Related Ventriculitis in Patients with Intraventricular Hemorrhage. Acta Neurochir (Wein) 2004;146:477–81. doi: 10.1007/s00701-004-0258-8. [DOI] [PubMed] [Google Scholar]
- 20.Pfisterer W, Mühlbauer M, Czech T, Reinprecht A. Early Diagnosis of External Ventricular Drainage Infection: Results of a Prospective Study. J Neurol Neurosurg Psychiatry. 2003;74:929–32. doi: 10.1136/jnnp.74.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raman M, Lipman J, Shorr A, Shankar A. A Meta-Analysis of Ventriculostomy-Associated Cerebrospinal Fluid Infections. BMC Infect Dis. 2015;15:3. doi: 10.1186/s12879-014-0712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roitberg BZ, Khan N, Alp MS, Hersonskey T, Charbel FT, Ausman JI, et al. Bedside External Ventricular Drain Placement for the Treatment of Acute Hydrocephalus. Br J Neurosurg. 2001;15:324–7. doi: 10.1080/02688690120072478. [DOI] [PubMed] [Google Scholar]
- 23.Root BK, Barrena BG, Mackenzie TA, Bauer DF, et al. Antibiotic Impregnated External Ventricular Drains: Meta and Cost Analysis. World Neurosurg. 2016;86:306–15. doi: 10.1016/j.wneu.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Williams TA, Leslie GD, Dobb GJ, Roberts B, van Heerden PV. Decrease in Proven Ventriculitis by Reducing the Frequency of Cerebrospinal Fluid Sampling from Extraventricular Drains. J Neurosurg. 2011;115:1040–6. doi: 10.3171/2011.6.JNS11167. [DOI] [PubMed] [Google Scholar]
- 25.Wong GK, Poon WS, Wai S, Yu LM, Lyon D, Lam JM, et al. Failure of Regular External Ventricular Drain Exchange to Reduce Cerebrospinal Fluid Infection: Result of a Randomized Controlled Trial. J Neurol Neurosurg Psychiatry. 2002;73:759–61. doi: 10.1136/jnnp.73.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worley E, Astle S, Watson JC. Prospective Evaluation of Ventriculostomy Infections. Cureus. 2015;7:e312. doi: 10.7759/cureus.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright K, Young P, Brickman C, Sam T, Badjatia N, Pereira M, et al. Rates and Determinants of Ventriculostomy-Related Infections During a Hospital Transition to Use of Antibiotic-Coated External Ventricular Drains. Neurosurg Focus. 2013;34:1–5. doi: 10.3171/2013.2.FOCUS12271. [DOI] [PubMed] [Google Scholar]
- 28.Zingale A, Ippolito S, Pappalardo P, Chibbaro S, Amoroso R. Infections and Re-Infections in Long-term External Ventricular Drainage: A Variation Upon a Theme. J Neurosurg Sci. 1999;43:125–33. [PubMed] [Google Scholar]


