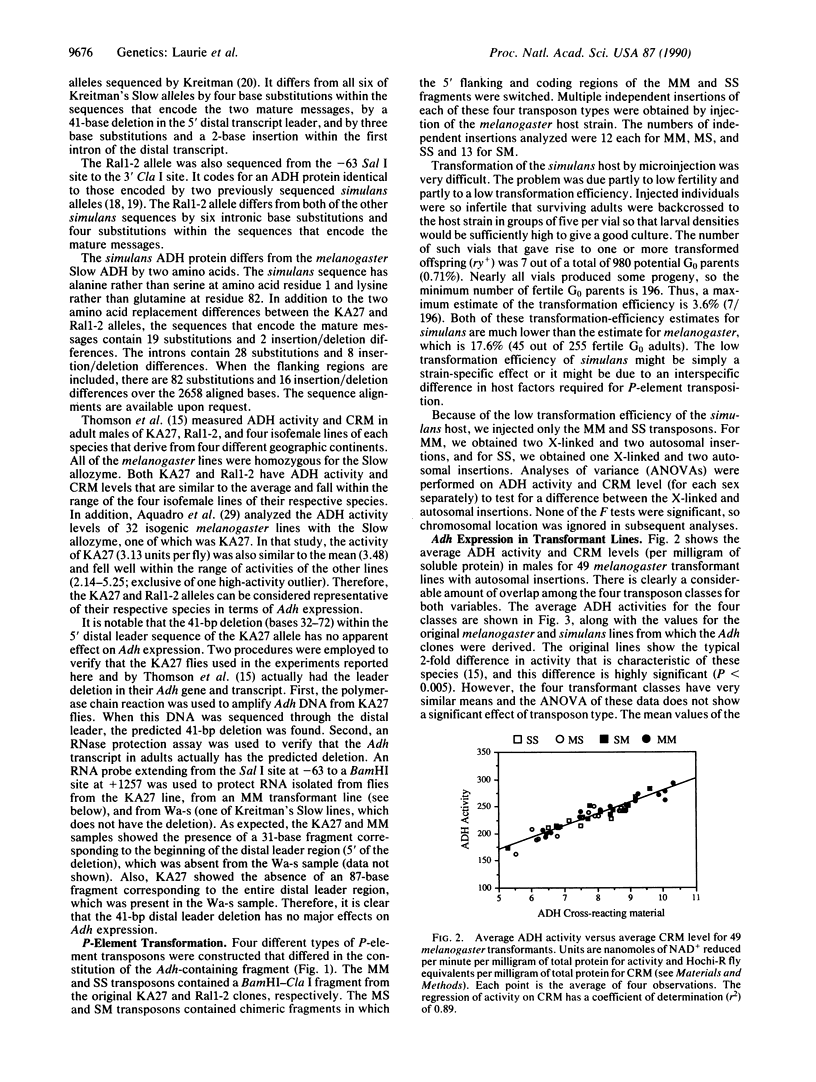
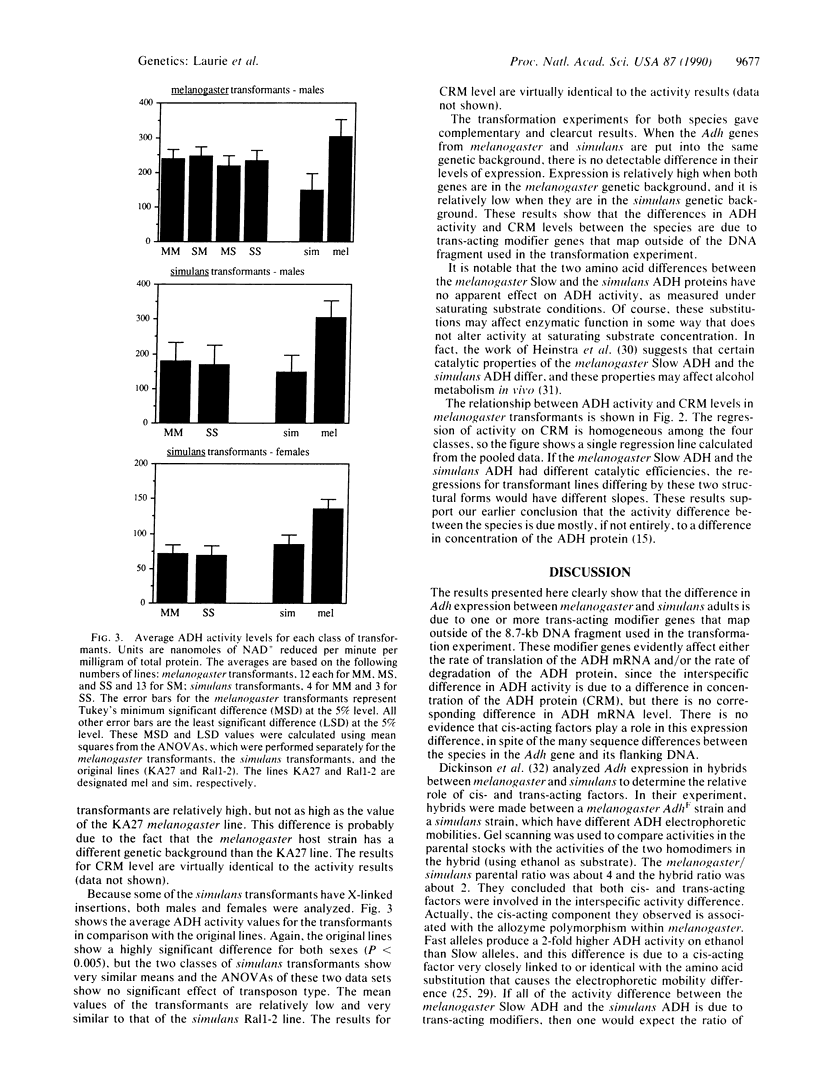
Abstract
Drosophila melanogaster and its sibling species, Drosophila simulans, differ in expression of the enzyme alcohol dehydrogenase (ADH). Adult melanogaster flies that are homozygous for the Slow allozyme have approximately twice the level of ADH activity and crossreacting material as simulans adults. There is no corresponding difference in ADH mRNA, however, so this difference in ADH protein level is evidently due to a difference in the rate of translation of the two RNAs and/or to a difference in protein stability. Here we report an interspecific gene-transfer experiment, using P-element transformation, to determine whether this expression difference is due to genetic background differences between the species (trans-acting modifiers) or to cis-acting factors within the Adh gene. When the Adh genes from D. melanogaster and D. simulans are put into the same genetic background, there is no detectable difference in their level of expression. The level is relatively high in the melanogaster background and relatively low in the simulans background. Therefore, the interspecific difference in Adh expression is due entirely to trans-acting modifiers, in spite of the many sequence differences between the Adh genes of the two species, which include two amino acid substitutions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquadro C. F., Desse S. F., Bland M. M., Langley C. H., Laurie-Ahlberg C. C. Molecular population genetics of the alcohol dehydrogenase gene region of Drosophila melanogaster. Genetics. 1986 Dec;114(4):1165–1190. doi: 10.1093/genetics/114.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bodmer M., Ashburner M. Conservation and change in the DNA sequences coding for alcohol dehydrogenase in sibling species of Drosophila. 1984 May 31-Jun 6Nature. 309(5967):425–430. doi: 10.1038/309425a0. [DOI] [PubMed] [Google Scholar]
- Brennan M. D., Dickinson W. J. Complex developmental regulation of the Drosophila affinidisjuncta alcohol dehydrogenase gene in Drosophila melanogaster. Dev Biol. 1988 Jan;125(1):64–74. doi: 10.1016/0012-1606(88)90059-0. [DOI] [PubMed] [Google Scholar]
- Brennan M. D., Wu C. Y., Berry A. J. Tissue-specific regulatory differences for the alcohol dehydrogenase genes of Hawaiian Drosophila are conserved in Drosophila melanogaster transformants. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6866–6869. doi: 10.1073/pnas.85.18.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn V. H., Moore G. P. Organization and evolution of the alcohol dehydrogenase gene in Drosophila. Mol Biol Evol. 1988 Mar;5(2):154–166. doi: 10.1093/oxfordjournals.molbev.a040487. [DOI] [PubMed] [Google Scholar]
- Coté B., Bender W., Curtis D., Chovnick A. Molecular mapping of the rosy locus in Drosophila melanogaster. Genetics. 1986 Apr;112(4):769–783. doi: 10.1093/genetics/112.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R., Bocquet C., Arens M. F., Fouillet P. Biological role of alcohol dehydrogenase in the tolerance of Drosophila melanogaster to aliphatic alochols: utilization of an ADH-null mutant. Biochem Genet. 1976 Dec;14(11-12):989–997. doi: 10.1007/BF00485131. [DOI] [PubMed] [Google Scholar]
- David J., Bocquet C. Compared toxicities of different alcohols for two Drosophila sibling species: D. melanogaster and D. simulans. Comp Biochem Physiol C. 1976;54(2):71–74. doi: 10.1016/0306-4492(76)90066-6. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson W. J., Rowan R. G., Brennan M. D. Regulatory gene evolution: adaptive differences in expression of alcohol dehydrogenase in Drosophila melanogaster and Drosophila simulans. Heredity (Edinb) 1984 Apr;52(Pt 2):215–225. doi: 10.1038/hdy.1984.23. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Regulatory elements involved in Drosophila Adh gene expression are conserved in divergent species and separate elements mediate expression in different tissues. EMBO J. 1986 Jun;5(6):1275–1289. doi: 10.1002/j.1460-2075.1986.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Murray N., Lehrach H. Lambda phage vectors--EMBL series. Methods Enzymol. 1987;153:103–115. doi: 10.1016/0076-6879(87)53051-8. [DOI] [PubMed] [Google Scholar]
- Geer B. W., Langevin M. L., McKechnie S. W. Dietary ethanol and lipid synthesis in Drosophila melanogaster. Biochem Genet. 1985 Aug;23(7-8):607–622. doi: 10.1007/BF00504295. [DOI] [PubMed] [Google Scholar]
- Gibson J. B., Wilks A. V. The alcohol dehydrogenase polymorphism of Drosophila melanogaster in relation to environmental ethanol, ethanol tolerance and alcohol dehydrogenase activity. Heredity (Edinb) 1988 Jun;60(Pt 3):403–414. doi: 10.1038/hdy.1988.58. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A., Posakony J. W., Maniatis T. Correct developmental expression of a cloned alcohol dehydrogenase gene transduced into the Drosophila germ line. Cell. 1983 Aug;34(1):59–73. doi: 10.1016/0092-8674(83)90136-8. [DOI] [PubMed] [Google Scholar]
- Heinstra P. W., Scharloo W., Thörig G. E. Physiological significance of the alcohol dehydrogenase polymorphism in larvae of Drosophila. Genetics. 1987 Sep;117(1):75–84. doi: 10.1093/genetics/117.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinstra P. W., Thörig G. E., Scharloo W., Drenth W., Nolte R. J. Kinetics and thermodynamics of ethanol oxidation catalyzed by genetic variants of the alcohol dehydrogenase from Drosophila melanogaster and D. simulans. Biochim Biophys Acta. 1988 Nov 17;967(2):224–233. doi: 10.1016/0304-4165(88)90013-x. [DOI] [PubMed] [Google Scholar]
- Higuchi R. G., Ochman H. Production of single-stranded DNA templates by exonuclease digestion following the polymerase chain reaction. Nucleic Acids Res. 1989 Jul 25;17(14):5865–5865. doi: 10.1093/nar/17.14.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Kreitman M. Nucleotide polymorphism at the alcohol dehydrogenase locus of Drosophila melanogaster. Nature. 1983 Aug 4;304(5925):412–417. doi: 10.1038/304412a0. [DOI] [PubMed] [Google Scholar]
- Laurie-Ahlberg C. C., Maroni G., Bewley G. C., Lucchesi J. C., Weir B. S. Quantitative genetic variation of enzyme activities in natural populations of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1073–1077. doi: 10.1073/pnas.77.2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie-Ahlberg C. C., Stam L. F. Use of P-element-mediated transformation to identify the molecular basis of naturally occurring variants affecting Adh expression in Drosophila melanogaster. Genetics. 1987 Jan;115(1):129–140. doi: 10.1093/genetics/115.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton A. N., Laurie-Ahlberg C. C., Emigh T. H., Curtsinger J. W. Naturally occurring enzyme activity variation in Drosophila melanogaster. II. Relationships among enzymes. Genetics. 1982 Oct;102(2):207–221. doi: 10.1093/genetics/102.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]



