Abstract
Objectives:
This study was undertaken to estimate the incidence and burden of cerebral microhemorrhage (CM) in patients with heart disease who underwent cardiopulmonary bypass (CPB), as detected on susceptibility-weighted imaging (SWI), a magnetic resonance (MR) sequence that is highly sensitive to hemorrhagic products.
Materials and Methods:
With Institutional Review Board waiver of consent, MR imaging (MRI) of a cohort of 86 consecutive pediatric patients with heart failure who underwent heart transplantation evaluation were retrospectively reviewed for CM. A nested case–control study was performed. The CPB group consisted of 23 pediatric patients with heart failure from various cardiac conditions who underwent CPB. The control group was comprised of 13 pediatric patients with similar cardiac conditions, but without CPB history. Ten patients in the CPB group were female (age: 5 days to 16 years at the time of the CPB and 6 days to 17 years at the time of the MRI). The time interval between the CPB and MRI ranged from 11 days to 4 years and 5 months. Six patients in the control group were female, age range of 2 days to 6 years old. The number of CM on SWI was counted by three radiologists (PK, EK and DK). The differences in number of CM between groups were tested for significance using Mann–Whitney U-test, α = 0.05. Using the univariate analysis of variance model, the differences in number of CM between groups were also tested with adjustment for age at MRI.
Results:
There are statistically significant differences in CM on SWI between the CPB group and control group with more CM were observed in the CPB group without and with adjustment for age at MRI (P < 0.001).
Conclusions:
Exposure of CPB is associated with increased prevalence and burden of CM among pediatric patients with heart failure.
KEYWORDS: Cardiopulmonary bypass, cerebral microhaemorrhage, paediatric, susceptibility-weighted-imaging

INTRODUCTION
Many children with heart failure from congenital heart disease (CHD) and other acute heart conditions need to undergo cardiac surgery with cardiopulmonary bypass (CPB) to survive.[1,2] One-third of the babies born with major congenital anomalies have CHD worldwide.[3] The first cardiac surgery performed with CPB was in 1953,[4] and the first successful cardiac transplantation in a newborn baby was performed at our institution on November 20, 1985. The survival rate of CHD patients has been increasing due to advances in cardiovascular diagnosis and surgery over the past decades.[3]
Neurological complications after CPB are a known cause of morbidity and mortality in this population.[5,6,7,8,9] The neurological complications include seizures, intracranial hemorrhage, cranial nerve abnormalities, disorders of the peripheral nervous system, and persistent irritability.[6] Contributing factors to these neurological complications include gaseous microemboli, reperfusion syndrome, hypothermic circulatory arrest, and low-flow CPB.[5,9]
A recent study found that the most common abnormal magnetic resonance imaging (MRI) finding in pediatric patients who underwent CPB was white matter injury, followed by focal infarction.[10] Intracranial hemorrhage was observed in only a few patients in both pre- and post-operative MRIs.[10]
Susceptibility-weighted imaging (SWI) is a magnetic resonance (MR) sequence that is highly sensitive to the presence of hemosiderin.[11,12,13] It has been shown to be 3–6 times more sensitive than conventional T2*-weighted gradient-echo (T2*GE) sequences to detect microhemorrhages.[14] SWI has demonstrated microhemorrhages in cerebral amyloid angiopathy, posttraumatic injuries, intra-arterial hemolytic disorders, and petechial hemorrhages after acute cerebral ischemia.[3,15,16,17,18] Although it has not yet been described in the literature, we have also observed cerebral microhemorrhage (CM) in pediatric patients who underwent CPB. The purpose of this study is to estimate the prevalence of CM in pediatric patients with heart failure, undergoing CPB.
MATERIALS AND METHODS
Patients
We retrospectively reviewed 86 pediatric patients who underwent cardiac transplantation evaluation at Loma Linda University Medical Centre for heart failure from 2001 to 2010 after receiving permission from our Institutional Review Board. The medical record for each patient was reviewed for demographics and CPB history. Inclusion criteria for the CPB group consisted of postoperative MRI with SWI. Inclusion criteria for the control group consisted of MRI with SWI without CPB. Exclusion criteria for both CPB and control groups include congenital brain anomaly, central nervous system infection, congenital metabolic disorder, anticoagulation disorders, large intracranial hemorrhage, acute infarction/ischemia, and poor quality SWI. Forty-one patients (41/86) who underwent CPB had preoperative MRI but not have a postoperative MRI, and nine patients (9/86) with poor quality SWI were all excluded from the study.
Cardiopulmonary bypass group
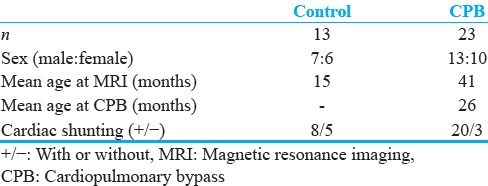
The 23 patients in the CPB group had a variety of heart conditions including hyperplastic left ventricle (9), double outlet right ventricle (4), Shone's complex (2), pulmonary atresia (1), tetralogy of Fallot (2), Ebstein's anomaly (1), double outlet left ventricle (1), atrioventricular septal defect (1), viral cardiomyopathy (1), and myocarditis (1). Ten patients were female. Age at CPB ranged from 5 days to 16 years at the time of surgery. The time interval between the CPB and MRI ranged from 11 days to 4 years [Table 1].
Table 1.
Patient demographics
Control group
Thirteen pediatric patients with similar cardiac conditions and SWI, but without CPB surgery, comprised the control group. The patients in this group had the following heart conditions: Hyperplastic left ventricle (3), dilated cardiomyopathy (3), double outlet right ventricle (1), Ebstein's anomaly (2), viral cardiomyopathy (1), restrictive cardiomyopathy (1), hypoplastic right ventricle (1), and dextro-transposition of great arteries (one). Six patients were female. Age at MRI ranged from 2 days to 6 years [Table 1].
Susceptibility weighted imaging
Each patient underwent a MRI using a conventional 1.5-T MRI system (Magnetom Vision, Siemens Medical Solutions, Iselin, NJ, USA). The SWI sequence consists of a strongly susceptibility-weighted, low bandwidth (78 Hz/pixel), three-dimensional fast low-angle shot sequence (TR/TE 1/4 57/40 ms, FA 1/4 20_), with first-order flow compensation in three orthogonal directions. Thirty-two partitions of 2 mm were acquired using a rectangular field of view (5/8 of 256 mm) and a matrix size of 160 × 512, resulting in 16 slices with an effective slice thickness of 4 mm. The acquisition time was 4.25 min. Images underwent additional postprocessing, using a phase mask that led to an enhancement of the phase differences between paramagnetic substances and surrounding tissue.[19]
Susceptibility-weighted imaging assessment
Each patient's SWI series was evaluated by three radiologists, blinded to CPB status. The number of CM was counted and agreed by consensus. Any nonvenous focal area of low-signal intensity on SWI measuring <5 mm in diameter was considered to be a microhemorrhage [Figures 1 and 2]. Total CM was recorded for each patient up to 50. Greater than one lesion was considered to be positive for the presence of CM.
Figure 1.
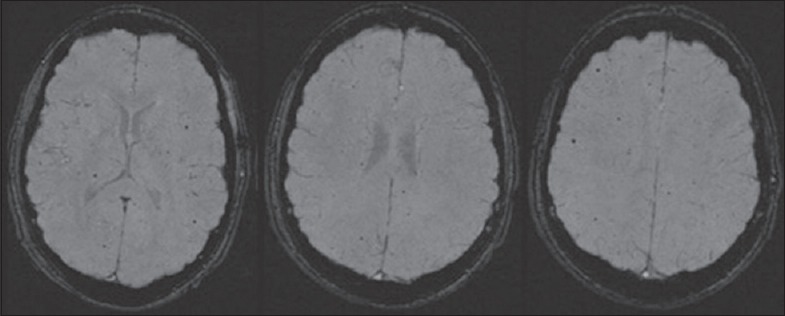
Axial susceptibility-weighted imaging images of a 16-year-old male with a history of teratology of Fallot and repair demonstrated multiple cerebral microhemorrhages.
Figure 2.
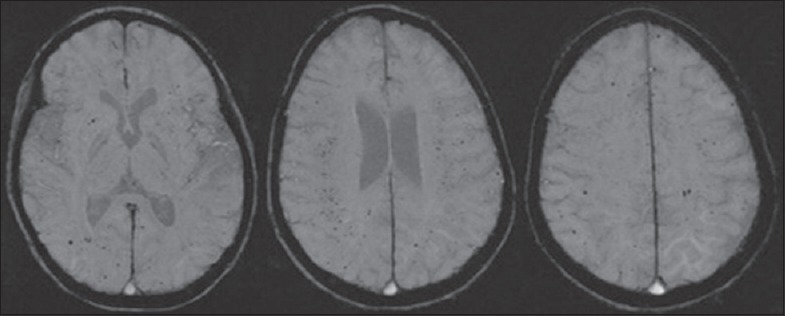
Axial susceptibility-weighted imaging images of a 6-year-old female with a history of unbalanced atrioventricular canal, double outlet right ventricle, and pulmonary atresia, and cardiac transplantation demonstrated multiple cerebral microhemorrhages.
Statistical analysis
The prevalence of CM was calculated for each group. The differences in median CM number between groups were tested for significance using Mann–Whitney U-test, α = 0.05. The differences of right to left shunting and sex were tested using Pearson Chi-square tests. Correlation between age at MRI and number of CMs was also tested using Spearman's rho. Using the univariate analysis of variance model, the differences in number of CM between groups were also tested with adjustment of age at MRI.
RESULTS
Twenty-three patients in our CPB group had 0–50+ intracranial microhemorrhages on SWI [Figures 1 and 2]. In the control group, there were 0–10 microhemorrhages on SWI. There was increased prevalence of cerebral hemorrhage in the CPB group compared to the control group (91% vs. 46.2%). There was a statistically significant difference in CM on SWI between the CPB group and control group with more CM observed in the CPB group (P < 0.001). Using the univariate analysis of variance model to adjust for age at MRI, the CPB group patients had statistically significant increase number of CMs compared with the control group (P < 0.001). Pearson Chi-square test showed no significant differences of right to left shunting and sex between the CPB group and control group (P = 0.08 and P = 0.88, respectively). Spearman's rho test showed a positive correlation between age at MRI and number of CMs (r = 0.5; P < 0.001).
DISCUSSION
In our study, compared to controls, the 23 patients who underwent CPB showed higher prevalence and greater burden of CMs compared with the control group both with and without the adjustment of age at MRI.
Intracranial microhemorrhages are seen in patients with thrombotic microangiopathy caused by disorders such as thrombotic thrombocytopenic purpura, disseminated intravascular coagulation, hemolytic uremic syndrome, and malignant hypertension.[20] In the setting of CPB, we suspected that the gaseous microemboli may be the cause of CM observed in this study. Gaseous microemboli are generated by the CPB circuit components or turbulent flow in the tubing,[9] and upon entering the cerebral circulation, they can cause brain hypoxia, ischemia, or necrosis by blocking capillaries and larger arterioles.[9] The immune system can also potentially react against these microemboli and cause more tissue damage,[9] similar to the cascade of events described in thrombotic microangiopathy.[20]
Congenital cardiac disease with right to left shunting is a known risk factor of cerebrovascular emboli. One study estimated that CHD patients have 10–100 times higher risk of development cerebrovascular accidents (CVAs) than the normal population.[12] The prevalence of CVA in patients with CHD with cyanotic lesions and shunting is elevated 10-fold.[12] Some of the CM we observed in our cohort may be related to right to left shunting. However, the difference of right to left shunting between the CPB group and control group is not statistical significant.
Previous studies have shown that solid and air emboli are introduced during CPB from the CPB circuit, manipulation of the heart and great vessels, administration of medication, and blood drawn from the circuit.[21,22] Neuromonitoring tools, such as transcranial Doppler ultrasonography and near-infrared spectroscopy, have been used to detect these microemboli.[22,23,24,25] Multiple studies have suggested that these emboli are the cause of acute neuropsychological dysfunction postoperatively in patients who underwent CPB.[8,22] Brown et al. studied the brain specimens of 36 patients who died within 3 weeks after CPB and found thousands of cerebral microemboli in patients soon after CPB.[26] These studies support our MR findings of increased prevalence of CM in patients with CPB compared with the control group.
Using conventional MRI but not SWI, Mahle et al., Miller et al., Dent et al., and Algra et al. found that the two most common abnormal postoperative brain MR findings in pediatric patients who underwent CPB were periventricular leukomalacia and focal ischemic lesions.[10,27,28,29] In our study, most of these microhemorrhages detected on SWI were not detectable on conventional sequences. This is likely because SWI is 6 times more sensitive to the detection of CMBs compared to conventional T2*GE [14] and may explain why Algra et al. observed intracranial hemorrhage in only a few CPB patients.[10]
There are some limitations in our study. First, our sample size is small and may not fully represent the general study populations. Second, right to left shunting is a confounder in this population. We propose to overcome these limitations by conducting a prospective study to study the MR brain findings of pediatric patients with heart failure who undergo heart transplantation evaluation and review the preoperative and postoperative brain MRI in patients who underwent CPB. In addition, the association of number of microhemorrhage and long-term neurological outcomes should also be evaluated.
Our study shows that there is increased frequency and burden of microhemorrhage in pediatric patients undergoing CPB and that CM may be the most common postoperative findings in this population. One quarter of the CHD patients underwent cardiac surgery with utilization of CPB.[1,2] Neurological complications ranging from behavioral disorder to seizures occur after CPB in 25% of the patients.[6] The cause of neurological compilations is not clearly known, but there are several contributing factors. Moreover, one of the contributing factors of neurological complications is gaseous microemboli.[9,22,30]
CONCLUSION
We propose that history of CPB should be considered in the differential diagnosis in pediatric cardiac patients with CMs on SWI, along with cardiac shunting and anticoagulation therapy. Although the exact mechanism on how these microhemorrhages occur is unknown, CMs on SWI may be a useful marker of the effect of pediatric CPB on neurological outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Sheri Harder for all her contributions on this study.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2017/7/1/27/209809
REFERENCES
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics-2008 update: A report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: Trends and racial disparities, 1979-1997. Circulation. 2001;103:2376–81. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 3.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–7. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Cohn LH. Fifty years of open-heart surgery. Circulation. 2003;107:2168–70. doi: 10.1161/01.CIR.0000071746.50876.E2. [DOI] [PubMed] [Google Scholar]
- 5.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–55. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 6.Menache CC, du Plessis AJ, Wessel DL, Jonas RA, Newburger JW. Current incidence of acute neurologic complications after open-heart operations in children. Ann Thorac Surg. 2002;73:1752–8. doi: 10.1016/s0003-4975(02)03534-8. [DOI] [PubMed] [Google Scholar]
- 7.Motallebzadeh R, Bland JM, Markus HS, Kaski JC, Jahangiri M. Neurocognitive function and cerebral emboli: Randomized study of on-pump versus off-pump coronary artery bypass surgery. Ann Thorac Surg. 2007;83:475–82. doi: 10.1016/j.athoracsur.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Stump DA, Kon NA, Rogers AT, Hammon JW. Emboli and neuropsychological outcome following cardiopulmonary bypass. Echocardiography. 1996;13:555–8. doi: 10.1111/j.1540-8175.1996.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 9.Win KN, Wang S, Undar A. Microemboli generation, detection and characterization during CPB procedures in neonates, infants, and small children. ASAIO J. 2008;54:486–90. doi: 10.1097/MAT.0b013e3181857e6a. [DOI] [PubMed] [Google Scholar]
- 10.Algra SO, Jansen NJ, van der Tweel I, Schouten AN, Groenendaal F, Toet M, et al. Neurological injury after neonatal cardiac surgery: A randomized, controlled trial of 2 perfusion techniques. Circulation. 2014;129:224–33. doi: 10.1161/CIRCULATIONAHA.113.003312. [DOI] [PubMed] [Google Scholar]
- 11.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–8. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann A, Chockalingam P, Balint OH, Dadashev A, Dimopoulos K, Engel R, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223–6. doi: 10.1136/hrt.2010.196147. [DOI] [PubMed] [Google Scholar]
- 13.Sehgal V, Delproposto Z, Haacke EM, Tong KA, Wycliffe N, Kido DK, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22:439–50. doi: 10.1002/jmri.20404. [DOI] [PubMed] [Google Scholar]
- 14.Tong KA, Ashwal S, Holshouser BA, Shutter LA, Herigault G, Haacke EM, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: Improved detection and initial results. Radiology. 2003;227:332–9. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 15.Barnes SR, Haacke EM, Ayaz M, Boikov AS, Kirsch W, Kido D. Semiautomated detection of cerebral microbleeds in magnetic resonance images. Magn Reson Imaging. 2011;29:844–52. doi: 10.1016/j.mri.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santhosh K, Kesavadas C, Thomas B, Gupta AK, Thamburaj K, Kapilamoorthy TR. Susceptibility weighted imaging: A new tool in magnetic resonance imaging of stroke. Clin Radiol. 2009;64:74–83. doi: 10.1016/j.crad.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Wycliffe ND, Choe J, Holshouser B, Oyoyo UE, Haacke EM, Kido DK. Reliability in detection of hemorrhage in acute stroke by a new three-dimensional gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography: A retrospective study. J Magn Reson Imaging. 2004;20:372–7. doi: 10.1002/jmri.20130. [DOI] [PubMed] [Google Scholar]
- 18.Ayaz M, Boikov AS, Haacke EM, Kido DK, Kirsch WM. Imaging cerebral microbleeds using susceptibility weighted imaging: One step toward detecting vascular dementia. J Magn Reson Imaging. 2010;31:142–8. doi: 10.1002/jmri.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitamura G, Kido D, Wycliffe N, Jacobson JP, Oyoyo U, Ashwal S. Hypoxic-ischemic injury: Utility of susceptibility-weighted imaging. Pediatr Neurol. 2011;45:220–4. doi: 10.1016/j.pediatrneurol.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Ellchuk TN, Shah LM, Hewlett RH, Osborn AG. Suspicious neuroimaging pattern of thrombotic microangiopathy. AJNR Am J Neuroradiol. 2011;32:734–8. doi: 10.3174/ajnr.A2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blauth CI. Macroemboli and microemboli during cardiopulmonary bypass. Ann Thorac Surg. 1995;59:1300–3. doi: 10.1016/0003-4975(95)00105-t. [DOI] [PubMed] [Google Scholar]
- 22.Groom RC, Quinn RD, Lennon P, Welch J, Kramer RS, Ross CS, et al. Microemboli from cardiopulmonary bypass are associated with a serum marker of brain injury. J Extra Corpor Technol. 2010;42:40–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Groom RC, Quinn RD, Lennon P, Donegan DJ, Braxton JH, Kramer RS, et al. Detection and elimination of microemboli related to cardiopulmonary bypass. Circ Cardiovasc Qual Outcomes. 2009;2:191–8. doi: 10.1161/CIRCOUTCOMES.108.803163. [DOI] [PubMed] [Google Scholar]
- 24.Guarracino F. Cerebral monitoring during cardiovascular surgery. Curr Opin Anaesthesiol. 2008;21:50–4. doi: 10.1097/ACO.0b013e3282f3f499. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez RA, Rubens FD, Wozny D, Nathan HJ. Cerebral emboli detected by transcranial Doppler during cardiopulmonary bypass are not correlated with postoperative cognitive deficits. Stroke. 2010;41:2229–35. doi: 10.1161/STROKEAHA.110.590513. [DOI] [PubMed] [Google Scholar]
- 26.Brown WR, Moody DM, Challa VR, Stump DA, Hammon JW. Longer duration of cardiopulmonary bypass is associated with greater numbers of cerebral microemboli. Stroke. 2000;31:707–13. doi: 10.1161/01.str.31.3.707. [DOI] [PubMed] [Google Scholar]
- 27.Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–7. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12 Suppl 1):I109–14. [PubMed] [Google Scholar]
- 29.Miller G, Mamourian AC, Tesman JR, Baylen BG, Myers JL. Long-term MRI changes in brain after pediatric open heart surgery. J Child Neurol. 1994;9:390–7. doi: 10.1177/088307389400900411. [DOI] [PubMed] [Google Scholar]
- 30.Stump DA. Embolic factors associated with cardiac surgery. Semin Cardiothorac Vasc Anesth. 2005;9:151–2. doi: 10.1177/108925320500900208. [DOI] [PubMed] [Google Scholar]


