Abstract
Background and Objective:
Acinetobacter baumannii has emerged as an important pathogen in hospital and environment that can acquire transport element and antibiotic-resistant genes. The aim of this study was to determine the resistances to different antibiotics, frequency of Class 1 integron in A. baumannii and then molecular typing for A. baumannii isolated from Intensive Care Unit (ICU).
Materials and Methods:
A total of 100 isolates of A. baumannii were collected from patients admitted to hospitals in Kermanshah from April 2014 to September 2015. The isolates were identified using biochemical test. Antimicrobial susceptibility test for 20 antibiotics was determined by Kirby–Bauer antibiotic testing (or disc diffusion). The prevalence rate of class integrons among the isolates was determined using polymerase chain reaction and finally 80 isolates of A. baumannii obtained from the Intensive Care Unit were selected for molecular typing by pulsed-field gel electrophoresis (PFGE).
Results:
The maximum drug resistance was observed against cefotaxime, ceftriaxone, mezlocillin, imipenem, and ceftazidime and piperacillin. Twenty-nine isolates were multidrug resistant (MDR); about 21 isolates were extensively-drug resistant and none were pandrug resistance and 42 isolates (42%) contained Class 1 integrons. The results did not show a significant correlation between the presence of Class 1 integrons and incidence of MDR A. baumannii. Five clusters were obtained by PFGE.
Conclusion:
This study did not show a significant correlation between the presence of Class 1 integrons and incidence of MDR A. baumannii. By PFGE analysis, the high level of similarity between some pulsotypes in A. baumannii strains showed genetic correlation between them.
Keywords: Acinetobacter baumannii, Class 1 integron, pulsed-field gel electrophoresis
INTRODUCTION
Acinetobacter baumannii is a coccobacilli, Gram-negative, nonmoving, oxidase – negative, Bacteria.[1] This human pathogenic bacterium is opportunistic, which will be causes spread hospital infection, including bacteremia, urinary, tract infection, and pneumonia.[2] This organism can be preserved in environment, condition and through pollution levels lead to the spread of infection in hospital.[3] The integrons in A. baumannii isolates, are a mobile genetic elements and they have ability to acquire and expand expressed of the antimicrobial resistance factors.[4] Integrons of Class 1 and 2 are the most recognized integrons that were frequently in A. baumannii and they had resistance with multiple categories to beta-lactams, aminoglycosides, fluoroquinolone and carbapenems.[5] The frequency of multidrug-resistant (MDR) A. baumannii isolates increased in last decade. MDR was defined resistant to three classes of antibiotics, including cephalosporins (cefepime and ceftazidime) carbapenems (imipenem and meropenem), and quimolones (ciprofloxacin) and also resistant to carbapenem, called extensively-drug resistant (XDR). Pandrug resistance (PDR) A. baumannii isolates shall be the XDR A. baumannii isolates that were resistant to tigecycline and polymyxins.[6] The aim of this study was to determine the resistances to different antibiotics, frequency of Class 1 integron in A. baumannii and then molecular typing for A. baumannii isolates that obtained from Intensive Care Unit (ICU) was carried out by pulsed-field gel electrophoresis (PFGE).
MATERIALS AND METHODS
Bacterial isolates
A total of 100 clinic isolates of A. baumannii were collected from patients admitted in Kermanshah during April 2014–September 2015 from four hospitals, which includes Taleghani (A), Imam Reza (B), Imam Khomeini (C), and Mohammad Kermanshahi (D). These isolates obtained from sputum, blood, urine, wounds, abdominal abscesses, synovial were identified using biochemical tests such as oxidase test, and environment TSI, API 20 NE kit and to ensure that these samples are A. baumannii use of growth in 42°C.
Antimicrobial susceptibility test
This isolates sensitivity to twenty antibiotics from most company that include: Ceftriaxone (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), meropenem (10 μg), cefepime (30 μg), amikacin (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), polymixin B (300 units), gentamicin (10 μg), imipenem (10 μg), mezlocycline (15 μg), minocycline (30 μg), tetracycline (30 μg), tigecycline (15 μg), colistin (10 μg), tobramycin (10 μg), gatifloxacin (5 μg), piperacillin (100 μg), co-trimoxazole (1.25 μg) using table of CLSI 2012, and disk diffusion determines their sensitivities.
Polymerase chain reaction assay
DNA genomic of all A. baumannii isolates were extracted by boiling method (5–10 min in 100°C). Detection of Class 1 integron in clinical isolates of A. baumannii was carried out by polymerase chain reaction (PCR) with primers illustrated in Table 1 as described previously by Mirnejad et al.[7]
Table 1.
Primers of polymerase chain reaction
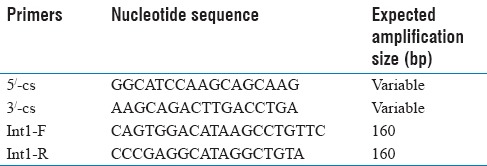
Primers Class 1 integron/F/R were used to amplify 160 bp fragments [Table 1], according to the following program: Initial denaturation at 95°C for 5 min, followed by 30 cycles at 49°C for 30 s, 55°C s 72°C for 3 s, and a final extension at 72°C for 5 min. All PCR amplification was performed in duplicate. The PCR product were analyzed using electrophoresis technique an 0.26 g/L agarose gel for 40 s at 80 VOH, strained by ethidium bromide, and visualized under transilluminator. Amplification products were further evaluated by sequencing.
Statistical analysis
Statistical analyses were performed using SPSS (version 20) (IBM, Chicago, IL, USA). Fisher's and Chi-square exact tests were used to the relationship between antibiotic resistant pattern and integron-positive genotype. P < 0.05 was considered statistically significant.
Pulsed-field gel electrophoresis
PFGE analysis was performed using methods (cell lysis, cell washing, and digestion by ApaI restriction enzyme) as described Mohajeri et al. without any change.[8]
RESULTS
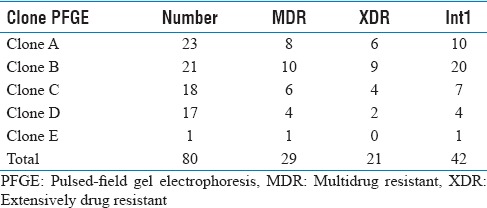
A total of 100 A. baumannii isolates were highly resistant to ceftriaxone, cefotaxime, mezlocillin (>90%) and imipenem, ceftazidime, piperacillin (>80%) [Tables 2 and 3] and were susceptible to colistin, polymyxin B, minocycline and tetracycline. MDR Bactria were mainly isolated from sputum (60%), abdominal abscesses (19%), blood (6%), urine (5%), wound (2%), synovial fluid (2%), from male (61%), age (mean: 40.3 ± 25.1, minimum = 1, maximum = 83), and female (39%) age (mean: 40.3 ± 25.7, minimum = 1, maximum = 83). MDR and XDR frequencies were 29% and 21%, respectively, and none were PDR. The results were analyzed by Chi-square Fisher (KI2) using SPSS software and P = 0.05. According to our result, there was not related to the incidence of MDR (P = 0.2) and XDR (P = 0.5) with Class 1 integron. 5/-cs-3/-cs was the area with variable region and there were the genes cassette intgrates and performed for detection of complete Class 1 (42, 76%) [Figure 1]. According to the [Table 4], resistance to cefepime, tobramycin, polymyxin B and ceftazidime, with Class 1 integron was significant. Results of this study showed that resistance to Levofloxacin, cefepime, mezlocillin, ciprofloxacin, piperacillin, cefotaxime, ceftazidime, ceftriaxone, and gentamicin are high in ICU ward [Table 3]. Five clusters were obtained by PFGE including: A (n = 23), B (n = 21), C (n = 18), D (n = 17) and E (n = 1) [Figure 2]. Eighty (80%) strains were isolated from Hospital A, B and C. All were isolated from ICU and the most from respiratory tract secretions sample. Clone A was dominant and widespread in ICU ward Taleghani Hospital, other clones especially Clone B were in the next dominant clones after Clone A. Also Clone A and B (16%) had the most frequency in the male. Pulsotype E consisted of single isolate and isolated from the female patient. All isolates belonging to the same genotype had similar genotype and phenotypes, such as distribution gene (Class 1 integron), resistance pattern, and source of isolation [Table 5].
Table 2.
Resistance in different antibiotic classes
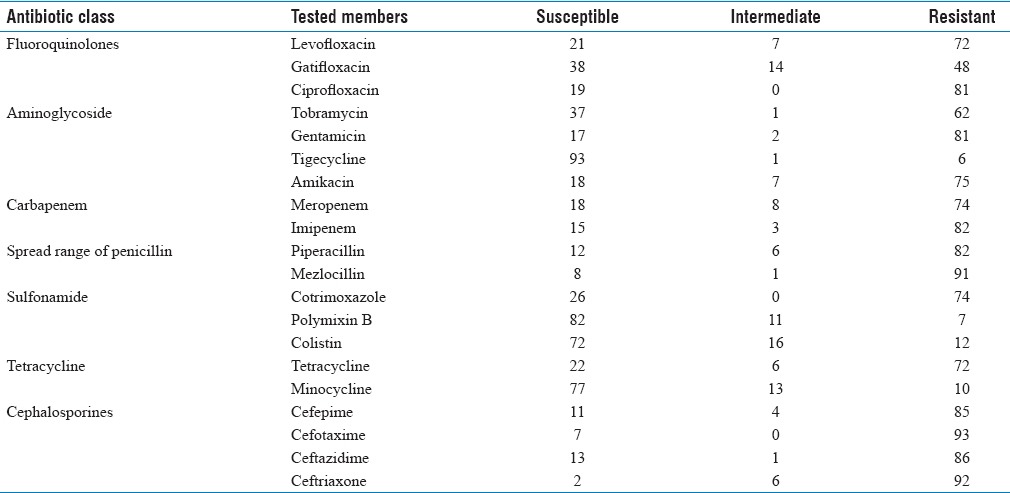
Table 3.
The table of the resistance of antibiotics

Figure 1.
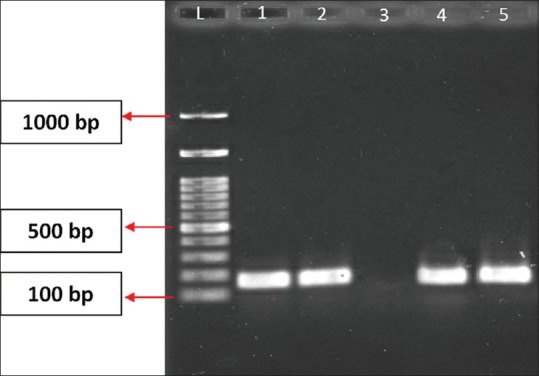
Polymerase chain reaction image of Class 1 integron. L = Ladder 100 bp, 1, 2 and 5 = sample, 3 = negative control, 4 = positive control
Table 4.
The table of relation between ward and Class 1 integron
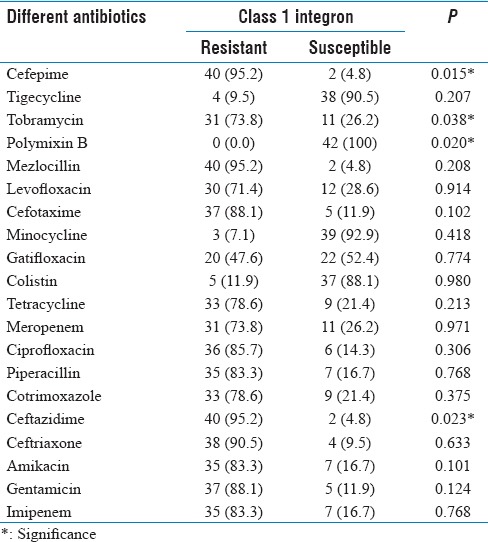
Figure 2.
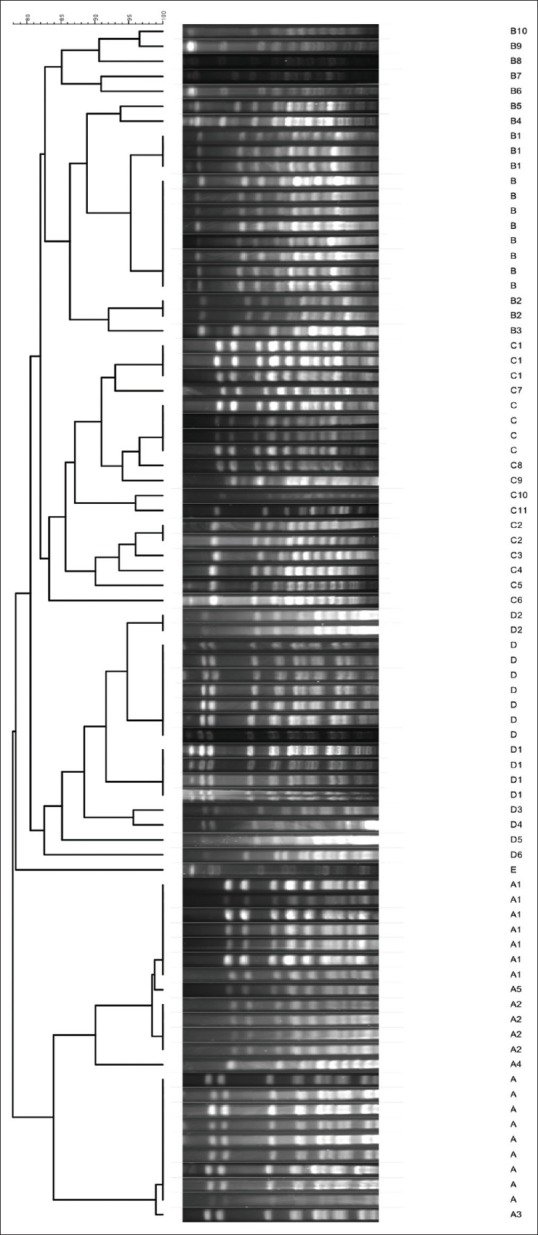
Pulsed-field gel electrophoresis dendrogram of Acinetobacter baumannii isolates
Table 5.
Distribution of pulsed-field gel electrophoresis pattern, anti-microbial susceptibility and Class 1 integron of Acinetobacter baumannii isolates
DISCUSSION
Over the last decade, Acinetobacter has emerged to become an important cause of nosocomial infections in many parts of the world.[8,9] A. baumannii is typically resistant to various antimicrobial agents such as penicillins, cephalosporines, macrolides, aminoglycosides, tetracyclines and fluoroquinolones.[10] Multidrug-resistant Acinetobacter spp. is alert pathogens, mostly in ICUs and is related with outbreaks of infection, which require epidemiologic monitoring as a measure for controlling nosocomial infection.[11] In this study, the majority of Acinetobacter spp. was isolated from the ICU. These results are in consistent with that reported by Al Masoudi et al.[9] in our study showed that the highest number of isolates related to sputum 66%, similar to with other studies.[12,13] In this study we studied susceptibility pattern of the 100 clinical isolates of A. baumannii to 20 different antibiotics as common therapeutic drugs in hospitalized patients. Resistance ratios each antimicrobial agent was all above 50%. The susceptibility of clinical isolates to tigecycline (93%), polymyxin B (82%), minocycline (77%), colistin (72%) is move that 70%. Only these four antimicrobial agents had a tolerable killing ability of clinical A. baumannii from this hospital. Therefore, treatments of these infections are complicated. The resistance of the A. baumannii isolates showed that 29% were MDR and twenty-one (21%) isolates were XDR. The frequency of MDR isolates was reported by Mohajeri et al.[8] in 2015 at Kermanshah, Iran, were 93% among A. baumannii isolates, the same as this study. The prevalence of MDR and XDR causes epidemic in different geographic area. The inclusion of MDR is related to other genetic factors. It seems resistance was through the different ways such as deficiency in the enzyme, in the cell wall, or via plasmid and chromosome that in our study did not include. Most of the MDR isolates belonged to patients in ICU which admits patients. In the ICU indiscriminate use of antimicrobial agents, long hospital stay, prolonged use of catheters and ventilators, implants and other synthetic instruments, lead to pressure select resistant strains which were colonized in susceptible patients.[14]
Class 1 integron being the most common among the clinical isolates of Gram-negative Bacteria, including acinetobacters.[10] The results related to Class 1 integron were in accordance with other geographical regains including European countries (43%),[15] and China (51.9%).[16] Horizontal gene transfer plays a significant role in cases increase the resistant antibiotics with Class 1 integron and publishing antibiotics gene in Class 1 integron. In the Mirnejad et al.'s study, 42% of MDR isolates contained Class 1 integron,[7] the same as this study. In a study in 2009 on 97 A. baumannii in the United States, 80% of isolates were MDR,[17] in contrast with our study. In the present study, there was a significant association between resistance to tobramycin, cefepime, ceftazidime and polymyxin B with integron carriage. No significant association was found between presence of integrons and resistance to antibiotics. These results suggest that resistance to antibiotics is independent of resistance gene cassettes carried by integron Class 1. It seems resistance was through the different ways such as deficiency in the enzyme, in the cell wall, or vial plasmid and chromosome that in our study did not include, which is compatible with studies done by Japoni et al.[18] PFGE is the gold standard technique to investigate the molecular epidemiology of Bacteria.[19] By PFGE method, we obtained 5 clones that the Clone A was involved in the majority of outbreaks in Kermanshah. It occurred at ICU and Isolates within this clone were mainly positive for Class 1 integron (%83). Clone B was the second most common pattern involved in outbreaks. Most of the A. baumannii infections were caused by a few predominant clones. Most of MDR and XDR phenotypes were presented in the Clones A, B, and C. It is possible that the transfer of these clones to other wards by staff or hospital equipments. A high prevalence of the Clone A, B, and C in different parts of the health-care system showed that hospitalized patients should be highly careful to prevent the spread of these clones. As expected, there was no significant relation among strains in term of correlation between Class 1 integron and MDR. This indicates that this gene could be role in common of the A. baumannii in ICU ward. More investigations are required to find putative source of wide distribution of Class 1 integrons. It is difficult to prove whether patients were colonized or infected by A. baumannii especially, where there are not previous epidemiologically records.
CONCLUSION
This study showed that resistance is also more pronounced in the ICU. Infections of ICU are serious problems that can compromise patient's survival and the outcome of reconstructive treatment. Since did not detect any association between resistances to different classes of antibiotics with integrons. Monitoring of drug resistance with use of coexist gene integrase PCR and other IS element are seems very important to plan specific infection control measures. PFGE analysis is helpful for the detection of common strains in different wards and prevention of further spread of these pulsotypes to other hospital environment. Different patterns of PFGE among hospitalized show wide distribution of integrons which reflect the issue in the case of endemic. The high level of similarity between some pulsotypes in A. baumannii strains showed genetic correlation between them. Further investigations are required for analyzing other PCR bands on a wider range of bacterial collection to detect other integrated gene cassettes.
Financial support and sponsorship
Kermanshah University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We gratefully acknowledge the Vice-Chancellor for Research and Technology, Kermanshah University of Medical Sciences, for financial support of this study resulting from MSc Medical Microbiology thesis of Mahsa Eghbalimoghadam, Kermanshah University of Medical Sciences, Iran (Grant No. 94294).
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joly-Guillou ML. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect. 2005;11:868–73. doi: 10.1111/j.1469-0691.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 3.Qi C, Scheetz MH, Malczynski M. Characterization of Acinetobacter baumannii genotypes recovered from patients with repeated colonization or infection. Diagn Microbiol Infect Dis. 2009;65:1–6. doi: 10.1016/j.diagmicrobio.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Peymani A, Farajnia S, Nahaei MR, Sohrabi N, Abbasi L, Ansarin K, et al. Prevalence of class 1 integron among multidrug-resistant Acinetobacter baumannii in Tabriz, Northwest of Iran. Pol J Microbiol. 2012;61:57–60. [PubMed] [Google Scholar]
- 5.Oh JY, Kim KS, Jeong YW, Cho JW, Park JC, Lee JC. Epidemiological typing and prevalence of integrons in multiresistant Acinetobacter strains. APMIS. 2002;110:247–52. doi: 10.1034/j.1600-0463.2002.100307.x. [DOI] [PubMed] [Google Scholar]
- 6.Manchanda V, Sanchaita S, Singh N. Multidrug resistant Acinetobacter. J Glob Infect Dis. 2010;2:291–304. doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirnejad R, Mostofi S, Masjedian F. Antibiotic resistance and carriage class 1 and 2 integrons in clinical isolates of Acinetobacter baumannii from Tehran, Iran. Asian Pac J Trop Biomed. 2013;3:140–5. doi: 10.1016/S2221-1691(13)60038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohajeri P, Farahani A, Feizabadi MM, Norozi B. Clonal evolution multi-drug resistant Acinetobacter baumannii by pulsed-field gel electrophoresis. Indian J Med Microbiol. 2015;33:87–91. doi: 10.4103/0255-0857.148390. [DOI] [PubMed] [Google Scholar]
- 9.Al Masoudi SB, Aly Magda MM, Al Humidi NQ, Halwani MA. Incidence and prevalence of Acinetobacter baumannii in King Fahd General Hospital, Saudi Arabia. Life Sci J. 2013;10:1702–10. [Google Scholar]
- 10.Taherikalani M, Maleki A, Sadeghifard N, Mohammadzadeh D, Soroush S, Asadollahi P, et al. Dissemination of class 1, 2 and 3 integrons among different multidrug resistant isolates of Acinetobacter baumannii in Tehran hospitals, Iran. Pol J Microbiol. 2011;60:169–74. [PubMed] [Google Scholar]
- 11.Fazeli H, Taraghian A, Kamali R, Poursina F, Nasr B. Molecular identification and antimicrobial resistance profile of Acinetobacter baumannii isolated from nosocomial infections of a teaching hospital in Isfahan, Iran. Avicenna J Clin Microbiol Infect. 2014;1:e21489. [Google Scholar]
- 12.Omer MI, Gumaa SA, Hassan AA, Idris KH, Ali OA, Osman MM, et al. Prevalence and resistance profile of Acinetobacter baumannii clinical isolates from a private hospital in Khartoum, Sudan. Am J Microbiol Res. 2015;3:76–9. [Google Scholar]
- 13.Esposito S, Leone S. Antimicrobial treatment for intensive care unit (ICU) infections including the role of the infectious disease specialist. Int J Antimicrob Agents. 2007;29:494–500. doi: 10.1016/j.ijantimicag.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Arabestani MR, Safari M, Roshanaii G, Alikhani MY. Prevalence of class 1, 2 and 3 integrons among extensive drug resistance Acinetobacter baumanii strains isolated from intensive care units in Hamadan, West Province, Iran. Iran J Med Microbiol. 2014;8:8–14. [Google Scholar]
- 15.Martinez-Freijo P, Fluit AC, Schmitz FJ, Grek VS, Verhoef J, Jones ME. Class I integrons in gram-negative isolates from different European hospitals and association with decreased susceptibility to multiple antibiotic compounds. J Antimicrob Chemother. 1998;42:689–96. doi: 10.1093/jac/42.6.689. [DOI] [PubMed] [Google Scholar]
- 16.Gu B, Tong M, Zhao W, Liu G, Ning M, Pan S, et al. Prevalence and characterization of class I integrons among Pseudomonas aeruginosa and Acinetobacter baumannii isolates from patients in Nanjing, China. J Clin Microbiol. 2007;45:241–3. doi: 10.1128/JCM.01318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taitt CR, Leski TA, Stockelman MG, Craft DW, Zurawski DV, Kirkup BC, et al. Antimicrobial resistance determinants in Acinetobacter baumannii isolates taken from military treatment facilities. Antimicrob Agents Chemother. 2014;58:767–81. doi: 10.1128/AAC.01897-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Japoni S, Japoni A, Farshad S, Ali AA, Jamalidoust M. Association between existence of integrons and multi-drug resistance in Acinetobacter isolated from patients in Southern Iran. Pol J Microbiol. 2011;60:163–8. [PubMed] [Google Scholar]
- 19.Gouby A, Carles-Nurit MJ, Bouziges N, Bourg G, Mesnard R, Bouvet PJ. Use of pulsed-field gel electrophoresis for investigation of hospital outbreaks of Acinetobacter baumannii. J Clin Microbiol. 1992;30:1588–91. doi: 10.1128/jcm.30.6.1588-1591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


