Abstract
Context:
Although existence of a probable association between glycopeptide and biocide resistance among enterococci has often been hypothesized, all studies conducted so far on this subject have been inconclusive.
Aims:
The aim of this study was to explore the possibility of the existence of an association between glycopeptide resistance and reduced susceptibility to biocides among Enterococcus spp.
Settings and Design:
This was a pilot study conducted in a super-speciality hospital situated in New Delhi, India, between June and November, 2015.
Patients and Methods:
Fourteen isolates of Enterococcus spp. obtained from various clinical samples of inpatients were subjected to susceptibility testing by modified Kirby-Bauer disk diffusion method to the following antibiotics: ampicillin (30 μg), gentamicin (120 μg), linezolid (30 μg), teicoplanin (30 μg), and vancomycin (30 μg). Based on the preliminary glycopeptide susceptibility results, all the isolates were classified into glycopeptide-sensitive and glycopeptide-resistant groups, respectively. Isolates belonging to both of these groups were subjected to tube dilution method for determining minimum inhibitory concentration of three biocides, namely, sodium hypochlorite, povidone-iodine, and absolute ethanol, respectively. Minimum bactericidal concentration of these disinfectants was also determined as per standard guidelines.
Statistical Analysis Used:
Not applicable.
Results:
More number of glycopeptide-sensitive strains exhibited reduced susceptibility to sodium hypochlorite than glycopeptide-resistant strains of enterococci. However, more number of glycopeptide-resistant isolates exhibited lower susceptibility to povidone-iodine than glycopeptide-sensitive isolates of enterococci. Both glycopeptide-sensitive and glycopeptide-resistant enterococci were equally susceptible to absolute ethanol.
Conclusions:
It seems that biocide resistance is an important issue and may have links with antibiotic resistance. This study points towards a possible association between glycopeptide resistance and reduced susceptibility to povidone iodine among enterococci. More studies should be conducted in order to further explore this supposedly enigmatic issue.
Keywords: Biocide, enterococci, glycopeptide
INTRODUCTION
Reduced susceptibility to biocides is apparently increasing, but it is more likely to be low level in nature and to concentrations well below those used in hospitals.[1] Concerns about the resistance of Pseudomonas aeruginosa to cationic biocides were expressed 50 years ago, subsequent studies showing that many different types of bacteria could gradually become less susceptible to most biocides over long periods of time.[1] In a study conducted by Chapman in 1998, increased insusceptibility to many biocides, including quaternary ammonium compounds (QACs), chlorhexidine, phenolics, heavy metals and even aldehydes, such as glutaraldehyde, was demonstrated.[2]
Glycopeptide-resistant enterococci (GRE) showing resistance to glycopeptide antibiotics such as vancomycin and/or teicoplanin have been reported from many parts of the world.[3] As these strains also exhibit ampicillin and high-level aminoglycoside resistance, they cause some of the most dreaded infections that are often difficult to treat.[4] Furthermore, these colonized patients contaminate themselves as well as environment, thereby having potential for transfer of GRE from environment to patients.[5,6] As GRE survives for long periods of time on dry surfaces, it is a successful environmental contaminant causing some outbreaks.[7,8]
Several studies have been conducted in the past dwelling into the subject of reduced susceptibility of GRE to biocides. In addition, the existence of a probable association between glycopeptide and biocide resistance has often been hypothesized. However, most of these studies were largely inconclusive. We hereby present a study with the aim of exploring the possibility of existence of an association between glycopeptide resistance and reduced susceptibility to biocides among Enterococcus spp.
PATIENTS AND METHODS
A pilot study was conducted in a super-speciality hospital situated in New Delhi, India, between June and November, 2015. Fourteen isolates of Enterococcus spp. (which were identified up to genus level as per standard procedures)[9] were obtained from various clinical samples of inpatients such as pus, bile, drain fluids, cerebrospinal fluid, urine, blood, and central lines. These isolates were subjected to antibiotic susceptibility testing to the following antibiotics by modified Kirby-Bauer disk diffusion method as per the Clinical and Laboratory Standards Institute Guidelines 2015: ampicillin (30 μg), high-level gentamicin (gentamicin 120 μg), linezolid (30 μg), teicoplanin (30 μg), and vancomycin (30 μg). Based on the preliminary glycopeptide susceptibility results obtained by the aforementioned method, all the isolates were classified into two groups, namely glycopeptide sensitive and glycopeptide resistant (resistant to vancomycin and/or teicoplanin), respectively. Isolates belonging to both these groups were subjected to tube dilution method suggested by Mazzola et al.[10] for determining minimum inhibitory concentration (MIC) of three commonly used biocides in our hospital, namely, sodium hypochlorite (4% available chlorine), 5% w/v povidone-iodine (0.5% w/v available iodine), and absolute ethanol (99.9%), respectively. Minimum bactericidal concentration (MBC) of these disinfectants was determined by subculturing from each of these tubes on to blood agar plates, which were then incubated aerobically at 37°C for 24 h. The highest dilution of disinfectant at which no growth was obtained on blood agar plates was taken as MBC. Similar tests were also performed on ATCC Enterococcus faecalis 29212, which was used as the control strain.
RESULTS
Of the 14 isolates of Enterococcus spp., six were susceptible and eight were resistant to glycopeptide antibiotics. Of the eight glycopeptide-resistant test isolates, seven exhibited resistance to both vancomycin and teicoplanin whereas one isolate was resistant to vancomycin but susceptible to teicoplanin. The susceptibility of glycopeptide-sensitive and glycopeptide-resistant test isolates to ampicillin, high-level gentamicin, and linezolid has been shown in Table 1. The MIC and MBC results of glycopeptide-sensitive and glycopeptide-resistant test isolates and those of ATCC E. faecalis 29212 for the three commonly used biocides in our hospital have been depicted in Table 2 and summarized as follows:
Table 1.
Percentage susceptibility of glycopeptide-sensitive and glycopeptide-resistant test isolates for ampicillin, high-level gentamicin, and linezolid
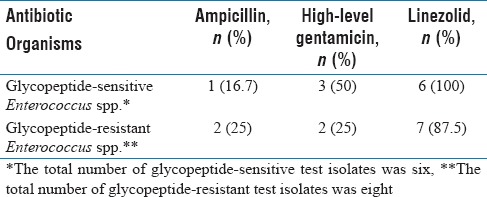
Table 2.
Minimum inhibitory concentration and minimum bactericidal concentration results of glycopeptide-sensitive and glycopeptide-resistant test isolates in comparison to those of control strain for the three commonly used biocides in our hospital
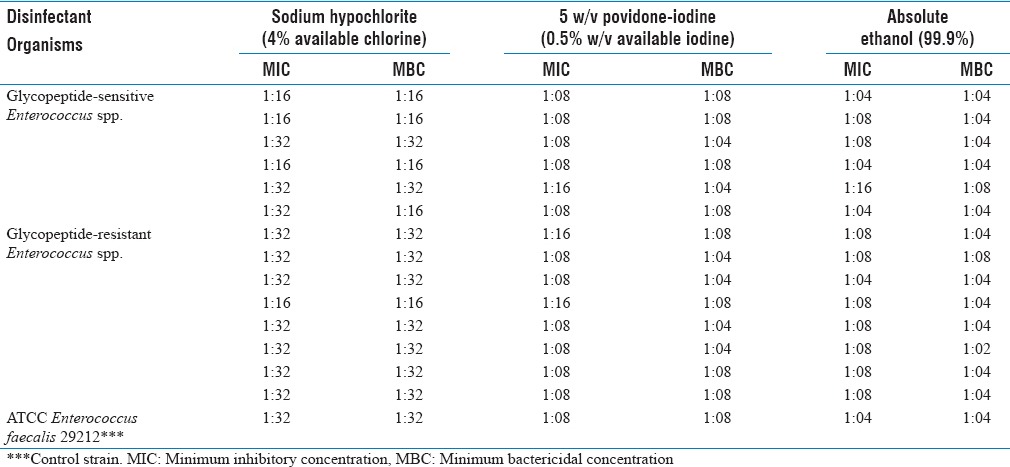
Sodium hypochlorite (4% available chlorine)
Of the six test isolates belonging to glycopeptide-sensitive group, three had higher MIC and MBC values and one isolate had higher MBC value but lower MIC value in comparison to those of the control strain. On the other hand, seven out of the eight glycopeptide-resistant test isolates had the same MIC and MBC values and one isolate had higher MIC and MBC values as compared to those of the control strain.
5% w/v povidone-iodine (0.5% w/v available iodine)
Two out of the six glycopeptide-sensitive test isolates had higher MBC values when compared to that of the control strain. However, five of these test isolates had the same MIC values and one isolate had lower MIC value than that of the control strain. Among the glycopeptide-resistant test isolates, four had higher MIC and MBC values, two had the same MIC and MBC values, and two had lower MIC and same MBC values in comparison to those of the control strain.
Absolute ethanol (99.9%)
None of the test isolates belonging to glycopeptide-sensitive and glycopeptide-resistant groups displayed higher MIC and/or MBC values when compared to those of the control strain.
DISCUSSION
Enterococci are present as commensals in the gastrointestinal tract.[11] Their emergence as important nosocomial pathogens in the last two decades can be attributed to their resistance to many commonly used antimicrobial agents and ease with which they appear to attain and transfer resistant genes.[4] Glycopeptide resistance in enterococci was first reported by Uttley et al. in 1989 from Great Britain.[12] Faecal carriage of GRE is recognized to be frequently associated with serious clinical infection and it is likely that colonization of gastrointestinal tract occurs as a prelude to clinical infection.[4]
Biocide resistance has been a poorly studied subject, possibly due to the belief that such resistance is rare and clinically insignificant. Various recent findings, however, have underlined the importance of biocide resistance as a clinically relevant phenomenon. Outbreaks of biocide-resistant organisms in hospitals have also been described.[13] In Staphylococcus aureus, genetic mechanism for resistance to QACs has been elucidated.[13] In addition, cross resistance between triclosan and antituberculous drugs has been demonstrated in certain strains of mycobacteria.[13] To the best of our knowledge, till date, no association has been established between glycopeptide resistance and reduced biocide susceptibility among Enterococcus spp.
Although preliminary, the biocide MIC and MBC results obtained in the present study provide an insight on this intricate issue. More number of glycopeptide-sensitive strains exhibited reduced susceptibility to sodium hypochlorite (4% available chlorine) than glycopeptide-resistant strains of enterococci. However, more number of glycopeptide-resistant isolates exhibited lower susceptibility to 5% w/v povidone-iodine than glycopeptide-sensitive isolates of Enterococcus spp. Both glycopeptide-sensitive and glycopeptide-resistant enterococci were equally susceptible to absolute ethanol (99.9%).
In a study conducted by Anderson et al., it was observed that both glycopeptide-sensitive and glycopeptide-resistant enterococci were equally susceptible to a wide range of environmental disinfectants and had parallel inactivation rates when challenged with extended dilutions of these products.[14] Gina and Martin also concluded that there was no difference between glycopeptide-sensitive and glycopeptide-resistant strains of Enterococcus spp. in terms of MIC and time–kill studies for several different biocides.[15] In a study conducted by Fraise, the author observed that there was an intrinsic resistance to chemical agents among enterococci although no clear relationship between antimicrobial resistance and resistance to chemical agents could be established. In the same study, it was also observed that some strains of Methicillin-resistant S. aureus which had intermediate resistance to glycopeptides were demonstrated to have decreased susceptibility to some biocides.[13] Although in consonance with all the aforementioned studies, the results obtained in our study are largely inconclusive. However, it seems that there is a possibility of an association between glycopeptide resistance and reduced susceptibility to 5% w/v povidone-iodine. In addition, the reduced susceptibility of more number of glycopeptide-sensitive strains (as compared to glycopeptide-resistant strains) of enterococci to sodium hypochlorite (4% available chlorine) is a matter which needs to be looked into. A plausible explanation for this aberrant finding could be that disc diffusion and automated methods of glycopeptide resistance detection in enterococci are a problem, especially for strains exhibiting lower levels of resistance.[16] Use of lower content (5 μg) discs may solve this problem to some extent. In the present study, since susceptibility to vancomycin was determined by modified Kirby-Bauer disc diffusion method using 30 μg disc instead of 5 μg disc, it is possible that some of the strains expressing lower levels of vancomycin resistance might have been missed and therefore, wrongly classified as glycopeptide (vancomycin) sensitive.
CONCLUSIONS
Although not definite, this study points toward a possible association between glycopeptide resistance and reduced susceptibility to 5% w/v povidone-iodine among enterococci. A major drawback of this study was that the sample size was very small owing to which no tests of significance could be conducted to verify our observations. Furthermore, the use of modified Kirby-Bauer disc diffusion method in our study for determining glycopeptide resistance and grouping enterococci into glycopeptide-sensitive and glycopeptide-resistant strains might have resulted in misclassification of these isolates, thereby leading to some perplexing findings. It seems that biocide resistance is an important issue and may have links with antibiotic resistance. If biocide resistance becomes increasingly common and it is associated with resistance to antimicrobial agents, this could have an impact on increasing the number of untreatable human infections. More studies should be conducted to further explore this supposedly enigmatic issue.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Mrs. Vandana Asthana, Technical Assistant, Department of Microbiology, Govind Ballabh Pant Institute of Postgraduate Medical Education and Research, New Delhi, India, for her contribution to this study.
REFERENCES
- 1.Russell AD. Antibiotic and biocide resistance in bacteria: Introduction. J Appl Microbiol. 2002;92:1S–3S. [PubMed] [Google Scholar]
- 2.Chapman JS. Characterizing bacterial resistance to preservatives and disinfectants. International Biodeterioration and Biodegradation. 1998;41:241–245. [Google Scholar]
- 3.Arthur M, Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993;37:1563–71. doi: 10.1128/aac.37.8.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marothi YA, Agnihotri H, Dubey D. Enterococcal resistance – An overview. Indian J Med Microbiol. 2005;23:214–9. [PubMed] [Google Scholar]
- 5.Morris JG, Jr, Shay DK, Hebden JN, McCarter RJ, Jr, Perdue BE, Jarvis W, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med. 1995;123:250–9. doi: 10.7326/0003-4819-123-4-199508150-00002. [DOI] [PubMed] [Google Scholar]
- 6.DeLisle S, Perl TM. Vancomycin-resistant enterococci: A road map on how to prevent the emergence and transmission of antimicrobial resistance. Chest. 2003;123(5 Suppl):504S–18S. doi: 10.1378/chest.123.5_suppl.504s. [DOI] [PubMed] [Google Scholar]
- 7.Livornese LL, Jr, Dias S, Samel C, Romanowski B, Taylor S, May P, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992;117:112–6. doi: 10.7326/0003-4819-117-2-112. [DOI] [PubMed] [Google Scholar]
- 8.Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol. 1995;16:577–81. doi: 10.1086/647011. [DOI] [PubMed] [Google Scholar]
- 9.Betty AF, Daniel FS, Alice SW, editors. Bailey & Scott's Diagnostic Microbiology. 12th ed. St. Louis, Missouri: Mosby Elsevier; 2007. Streptococcus, Enterococcus and similar organisms; pp. 265–80. [Google Scholar]
- 10.Mazzola PG, Jozala AF, de Lencastre NL, Moriel P, Penna TC. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz J Pharm Sci. 2009;45:241–8. [Google Scholar]
- 11.Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uttley AH, George RC, Naidoo J, Woodford N, Johnson AP, Collins CH, et al. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiol Infect. 1989;103:173–81. doi: 10.1017/s0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraise AP. Susceptibility of antibiotic-resistant cocci to biocides. J Appl Microbiol. 2002;92:158S–62S. [PubMed] [Google Scholar]
- 14.Anderson RL, Carr JH, Bond WW, Favero MS. Susceptibility of vancomycin-resistant enterococci to environmental disinfectants. Infect Control Hosp Epidemiol. 1997;18:195–9. doi: 10.1086/647587. [DOI] [PubMed] [Google Scholar]
- 15.Gina P, Martin SF. Antibiotic and Biocide Resistance in MRSA and VRE. Infection Control and Hospital Epidemiology. 2000;21:284. [Doi: 10.1017/S0195941700054874] [Google Scholar]
- 16.Barry DC. Glycopeptide resistant enterococci – The threat examined. Culture (Oxoid) 1998;19:1–5. [Google Scholar]


