Abstract
Context:
Multipotent stromal cells are isolated from various fetal sources and studied for their phenotypic characterization and ability to differentiate into different lineages.
Aims:
In this study, we aimed to isolate mesenchymal stem or stromal cells (MSCs) from villous chorion, expand under clinical scale level, compared the potency with other source of fetal-derived MSCs and studied their differentiation capabilities to form all three germ layers.
Subjects and Methods:
Placenta obtained from C-section was used to isolate villous chorion-MSCs (VC-MSCs) were expanded up to tenth passage and their characteristics were assessed by proliferation rate and phenotypic characterization using fluorescence-activated cell sorting and also expanded MSCs were analyzed for differentiated into all three germ layers by cytochemical staining.
Results:
Stem cell isolated from VC yielded up to 2.16 × 109 cells at second passage and 3.06–4.23 × 104 cells/cm2 at tenth passage. The total yield of cells with all three sources analysis showed that VC has a low yield at second passage compared to amniotic membrane and Wharton's jelly, but the VC-MSCs yield significant amount in lesser days. The phenotypic characterization revealed positive for CD73, CD90, and CD105 and negative for CD79, CD34, CD45, human leukocyte antigen-DR proving their stemness even at tenth passage. They can able to differentiate into ectodermic neural cells, endodermic hepatocytes, and mesodermal differentiation of chondrocytes, adipocytes, and osteogenic cells proving their ability to differentiate into all three germ layers.
Conclusions:
This result suggests that the VC-MSCs are ideal source of stem cells with similar characteristics such as other adult stem cells. Thus, VC-derived MSCs can be potential clinical source in regenerative medicine.
Keywords: Amniotic membrane, Cluster of differentiation, multipotent stromal cells, passage, villous chorion, Wharton's jelly
INTRODUCTION
Mesenchymal stem or stromal cells (MSCs) which are multipotent in nature have been isolated from different sources and their potential in differentiating into various germ layers has been extensively studied. Unlike embryonic cells, MSCs are ethically complaint, but their differentiation potential is limited from later. The basic characteristics of MSCs are spindle shaped and plastic adherence, each adult stem cells isolated from various sources show different proliferation potential and differentiation characteristics.[1]
Many clinical applications using adult progenitor cells in cellular therapy and regenerative medicine have been showing promising results.[2] Although MSCs can be isolated from several sources such as bone marrow (BM), adipose, Wharton's jelly (WJ), umbilical cord blood (UCB), and menstrual blood (MB), each source has its own credibility in obtaining, culturing, expanding, and differentiating.[3] For clinical use, BM is one of the earliest sources of MSCs, which has been extensively studied for various degenerative diseases and shown good results, but comparing the invasive procedure and the amount of MSCs obtained is minimal. Adipose-derived MSCs, on the other hand, can be expanded in in vitro culture conditions, but their invasive procedure and autologous to recover from all patients to treat are problematic.[4] WJ-derived MSCs can be isolated in quite a few numbers and their application with respect to cellular therapy in treating various degenerative and metabolic disorders are intriguing.[5,6] Other sources such as UCB and MB all have their flaws in obtaining the numbers for effective clinical therapy.
Recently, placenta-derived MSCs are been studied elaborately for their potency in immunomodulating and promising therapeutic applications.[7] Placental-derived MSCs can able to differentiate into all three germ layers, namely, adipogenic, chondrogenic, osteogenic, myogenic, and neurogenic cells under in vitro conditions.[8] Since human placenta is discarded after birth, the cells are easily accessible without ethical concerns, here we have chosen placental villous chorion (VC) from the fetal part as a source of MSCs. Chorionic villi are the innermost layer of placenta, it has four subtrophoblast layers developed during the first trimester, and they continue to grow throughout the pregnancy enriching the fetus with nutrition and blood supply from mother.[9]
In previous studies, we isolated and expanded MSCs derived from WJ and amniotic membrane and their potential to differentiate into mesodermal lineages such as adipocyte, chondrogenic, and osteogenic cells is significant in therapeutic applications.[10,11] In the present study, we have used villous chorinic-derived MSCs to study the characteristics by isolating, expanding, and comparing it with later expanded MSCs. Furthermore, to evaluate their differentiation capacity and an effort was made to establish all three germ layers in in vitro conditions.
SUBJECTS AND METHODS
Fetal source
The developing fetus is connected to the mother by placenta-fetomaternal organ. The fetal and maternal portions of placenta are known as VC and decidua basalis, respectively. The decidua basalis is anchored to the cytotrophoblastic shell (external layer from fetus side) with the anchoring villi which hold the both portions of placenta together. Placenta (n = 5) irrespective of the sex of baby was collected from full-term births after cesarean section was obtained from the C-section delivery process with parental permission and institutional guidelines.
Cell isolation
The fetal portion of the placenta was cut into approximately 1 cm2 and washed in Dulbecco's phosphate-buffered saline (DPBS) (Himedia, India), decontaminated thoroughly with 70% alcohol for 2 min, and again washed twice with DPBS to remove all traces of blood debris. Single-cell suspensions VC were made by mincing and flushing the tissue parts through a 100 μm nylon filter (Falcon, Becton, CA) with washing solution.
Culture of villous chorion-multipotent stromal cells
Single-cell suspensions of chorionic villus were cultured in Dulbecco's Modified Eagle Medium (DMEM)-nutrient mixture Ham's F-12 (1:1) with GlutaMax (1X), 2.438 g/L sodium bicarbonate, sodium pyruvate (DMEM/F12+; Gibco, USA), and 10% fetal bovine serum (FBS; Gibco, USA) supplemented with 3 ng/mL bFGF (Sigma, USA), MSC culture medium. Tissue culture flasks were coated with 1% gelatin for 30 min at room temperature in a 5% CO2 humidified atmosphere at 37°C. Cells were plated in tissue culture grade T-75 flask (Nunc, Denmark). After 7 days, nonadherent cells were removed, and the medium was refreshed. When grown to confluency, adherent cells were detached with trypsin/ethylenediaminetetraacetic acid for 5 min at 37°C and expanded in culture flasks (T-175 and Hyperflask, Corning, NY, USA).
Seeding density
Harvested cells at P0 were seeded at density of 3000 cells/cm2 for P1 in T-175 tissue culture flasks (Corning, NY, USA) and for P2 in hyperflasks (Corning, NY, USA). Similarly, 3000 cells/cm2 were plated at P3 to P10 in T-175 tissue culture flasks. The harvested cells were suspended in a cryoprotectant solution composed of 90% complete media and 10% dimethyl sulfoxide (Origen Biomedical, USA) and stored in the vapor phase of a liquid nitrogen tank until further use.
Cell proliferation assay
The proliferation was measured as previously described with some modifications.[12] Estimated growth efficiency and proliferation potential of the chorionic villus MSCs were determined based on the total cumulative population doubling level (CPDL) using the formula CPDL = ln (Nf/Ni) ln2, where Ni is the initial number of cells seeded, Nf is the final number of harvested cells, and ln is the natural log. Cells (3000 cells/cm2) were plated in culture flask and subcultured for 4–5 days. The cells were then counted and 3000 cells/cm2 were replated. To determine the CPDL, the population doubling for each passage was calculated and then added to the population doubling levels of the previous passages.
Flow cytometric analysis
Culture-expanded VC-MSCs derived from the fetal portion of the placenta were phenotypically characterized by flow cytometry (FACSCalibur, BD Biosciences, USA), with 1 × 105 cells were incubated with specific mouse antihuman primary antibodies conjugated to fluorochromes. Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies against CD90, CD73, CD105, CD45, CD34, CD79a, and human leukocyte antigen-DR (HLA-DR) (BD Pharmingen, NJ) were used. Positive cells were identified by comparison with isotypic controls (FITC- and PE-conjugated mouse immunoglobulin G1).
In vitro cell differentiation studies
Tri-lineage differentiation into adipocyte, chondrogenic, and osteogenic cells (mesodermal differentiation)
Methods were based on the protocol described by Aurich et al. with certain modifications.[13] Osteoblast differentiation was induced by culturing VC-MSCs in DMEM/F12+ supplemented with 10% FBS, 10-8 M dexamethasone (Sigma-Aldrich), 30 μg/ml ascorbic acid (Sigma-Aldrich), and 10 mM β-glycerophosphate (Sigma-Aldrich) for 3 weeks. Fresh medium was replenished every 3 days. Calcium accumulation was assessed by von Kossa staining. The differentiated cells were washed with phosphate-buffered saline and fixed with 10% formalin for 30 min. The fixed cells were incubated with 5% silver nitrate for 60 min under UV light and then treated with 2.5% sodium thiosulfate for 5 min and images were captured using Olympus microscope CKX41 (Japan).
Adipogenic differentiation
VC-MSCs were cultured for 21 days in DMEM/F12+ supplemented with 10% FBS, 1 μM dexamethasone, 0.5 mM isobutyl methyl xanthine, 1 μg/ml insulin, and 100 μM indomethacin (Sigma-Aldrich). Inducing factors were added to the replenished medium every 3 days. Cells were fixed in 10% formalin for 20 min. Two hundred microliters of oil red O staining solution was added and incubated for 10 min at room temperature.[13] The cells were rinsed five times with distilled water and images were captured using microscope.
Chondrogenic differentiation
VC-MSCs were cultured in STEMPRO (Invitrogen) chondrogenesis differentiation medium (chondrocyte differentiation basal medium with chondrogenesis supplement). Chondrogenesis differentiation medium was replenished every 3 days. For staining, cells were fixed with 4% formalin for 30 min. Safranin O staining solution was added and incubated for 5 min according to manufacturer's instructions. The cells were rinsed with distilled water and images were captured using microscope.
Differentiation into neurocyte-like cells (ectodermal differentiation)
According to an established protocol, cells were serum deprived for 36 h at the initial induction, then treated with neurogenic medium.[14,15] Medium changes were performed thrice weekly. Neurogenic medium consisted of Dulbecco's modified Eagle's medium -Low Glucose (DMEM- LG) and 5% FBS with 2.0–10; 5 mol/L b-mercaptoethanol (2ME, Sigma), MEM-NEAA (a 200-fold dilution of the stock), and GlutaMAX (a 200-fold dilution of the stock).
Differentiation into hepatocyte-like cells (endodermal differentiation)
For hepatogenic differentiation, tenth-passage cells were used. The suspension was inoculated at a density of 1.0 × 104/cm2. Induction procedures were based on described in previous publications with slight modifications.[15,16] At the initial induction, cells were serum deprived for 2 days, in DMEM-LG supplemented with 20 ng/mL epidermal growth factor and 20 ng/mL bFGF, before induction by a two-step protocol. Differentiation was induced by treating MSCs with step-one differentiation medium, consisting of DMEM-LG supplemented with 20 ng/mL HGF and 20 ng/mL bFGF, nicotinamide 0.61 g/L, for 10 days, followed by treatment with step-two maturation medium, consisting of IMDM supplemented with 20 ng/mL oncostatin M, 1 mol/L dexamethasone, and 50 mg/mL ITS premix, thereafter. Medium changes were performed twice weekly, and hepatogenesis was assessed by in vitro assays for functions that are characteristic of liver cells.
Statistical analysis
All statistical analyses were performed using Prism Software (GraphPad Software, Inc. USA). Results were expressed as mean ± standard deviation (SD) for illustration. The statistical significance of proliferation potential among harvested cells was determined using two-way ANOVA. P < 0.0001 was considered statistically significant.
RESULTS AND DISCUSSION
Multipotent stromal cells were initially identified in the BM. Consequently, they were also evidence in fat tissue, blood, and the placenta.[17] It is believed that these cells have the ability to regenerate mesenchymal tissues and blood cells. A number of studies have shown that these cells are not immunogenic and have immunomodulating capacities. The mechanism of this is not clear; it is only known that MSC can constrain the action of T-lymphocytes as well as the differentiation and proliferation of monocytes. These biological properties make them extremely interesting in cellular therapy.[18]
The most significant source of mesenchymal stem cells studied was BM. However, the harvesting and expansion of BM-MSCs to higher passage level raise many complications. To overcome these disadvantages, alternative fetal sources are explored where MSCs could be isolated and expanded. Among the fetal sources, an ideal source is human placenta, of which amniotic membrane and the placental villi are the potential stromal cell sources.[19,20] Amniotic mesenchymal cell (AM) and chorionic mesenchymal cell (CM) are probably derived from the extraembryonic mesodermis. CM cells are much less investigated than amniotic cells. AMs are preferably isolated from the amnion at term from the reflected portion of the membranes, to minimize the presence of maternal cells. Chorionic cells are isolated from the membranes and from the fetal portion of the placenta, after the mechanical and enzymatic removal of the trophoblast layer using dispase. MSCs can be isolated by explants type cultures, from the chorionic plate and the chorionic villi although in this and latter case, the risk of maternal contamination is higher. Both AM and CM have good adhesion and proliferation capacity on the plastic surface of culture containers for a limited number of passages.[17,21]
There are different protocols reported previously in terms of isolation, characterization, and expansion of MSCs, but all MSCs exhibit the minimum criteria proposed by International Society for Cellular Therapy (ISCT). ISCT has proposed minimum criteria to define MSCs. These cells (a) should exhibit plastic adherence, (b) possess specific set of cell surface markers, i.e., cluster of differentiation CD73, CD90, CD105, and lack expression of CD14, CD34, CD45, and HLA-DR, and (c) have the ability to differentiate in vitro into adipocyte, chondrocyte, and osteoblast.[22] These characteristics are valid for all MSCs although few differences exist in MSCs isolated from various tissue origins.
However, the use of MSCs for therapeutic application is based on their subsequent large-scale in vitro expansion. A fast and efficient protocol for generation of large quantities of MSCs is required to meet the clinical demand and biomedical research needs. Furthermore, well-characterized cell lines, constantly growing while maintaining their typical properties, are highly valuable for research. Here, we studied clinical-scale expansion of VC-MSCs up to passage ten levels. The growths of the cells were observed for the first 24 h, at which time the cells were mostly round and oval. After 24 h incubation, most of the cells were adherent to the flask and became fibroblast-like cells and spindle. Approximately, 5.7–8.2 × 106 cells were isolated primarily from the VC (n = 5) at the end of passage 0 after 5–6 days. Adherent cells with fibroblastic morphology could be observed as early as 24 h after seeding. Cells were trypsinized and were seeded into 3000 cells/cm2 in T-175 flasks. Once confluency achieved 0.5 × 108–1.36 × 108, adherent cells were harvested in 4–5 days. After successive passages with a 1:3 split ratio, 0.58 × 109–2.16 × 109 P2 MSCs were harvested within 5 days and cryopreserved at the end of the expansion period. Viability of the freshly harvested cells was >98% in all cases [Graph 1]. The harvested cells from isolated VC from one placenta using current method yields 0.95–1.18 × 104 cells/cm2 at P0, 2.63–4.97 × 104 cells/cm2 at P1, 3.49–4.77 × 104 cells/cm2 at P2, and 3.06–4.23 × 104 cells/cm2 at P10 accordingly are shown in Table 1. These results suggest that the growth of cells of VC-MSCs exhibits near limitless potential, considering the application of these cells in clinical trials will meet the required higher cell numbers.
Graph 1.
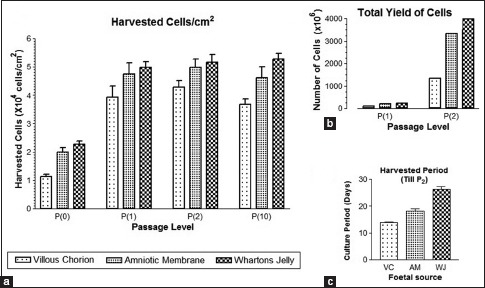
Effect of seeding density and cell proliferation growth kinetics of villous chorion-mesenchymal stem or stromal cells. Comparison of the growth rate and duration of different fetal-derived mesenchymal stem or stromal cells on (a) Harvested cells/cm2. Interaction of P = 0.8954, 0.63% of total variation. (b) Total yield of cells. Interaction P < 0.0001. (c) Harvested period, a significant difference at P < 0.05. Results represent the average mean of five culture replicates (n = 5)
Table 1.
Expansion and comparison summary of villous chorion multipotent stromal cells
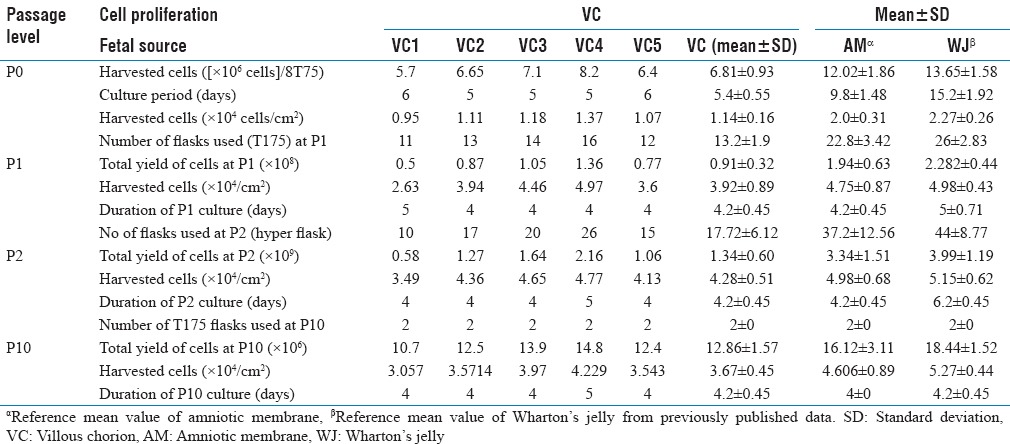
We also investigated cell proliferation rate and effect of seeding density on expansion of VC-MSCs in the culture media was carried out as mentioned in the materials and methods. VC-MSCs were cultured in T75, T175, and Hyper flasks at 2000, 3000, 4000, and 5000 cells/cm2 starting from first passage and continued until fifth passage. We observed seeding densities at 3000 cells/cm2 provided the higher cell proliferation and overall yield (P < 0.05) and successfully isolated and characterized VC-MSCs. In brief, the placental minced tissue was seeded in basal culture medium and subcultured until tenth passage; with a seeding density of 3000 cells/cm2. We could expand these cells to a clinical quantity of 2.16 × 109 cells at second passage within 14 days. The mean SD of VC 1.34 × 109 ± 0.60 was inferior in total yield but harvested in fewer days compared to other fetal sources. The second passage amniotic membrane and WJ total yield mean standard deviation was 3.34 × 109 ± 1.51 and 3.99 × 109 ± 1.19 and duration of culture was average of 18 and 26 days, respectively.[10,11] Moreover, the rate of VC-MSCs proliferation was stable until P10. This is the first report of clinical-scale expansion of VC-MSCs from a single placenta to quantities of 2.16 × 109 cells at second passage level. In fact, by adding just one more round of expansion, we should be able to generate sufficient quantities of MSCs to treat ~150–185 patients from a single placenta, considering that a 70 kg patient needs approximately 2 × 106 MSCs per kilogram body weight for transplantation[23] though an optimal MSC dose needs to be evaluated for the different indications.
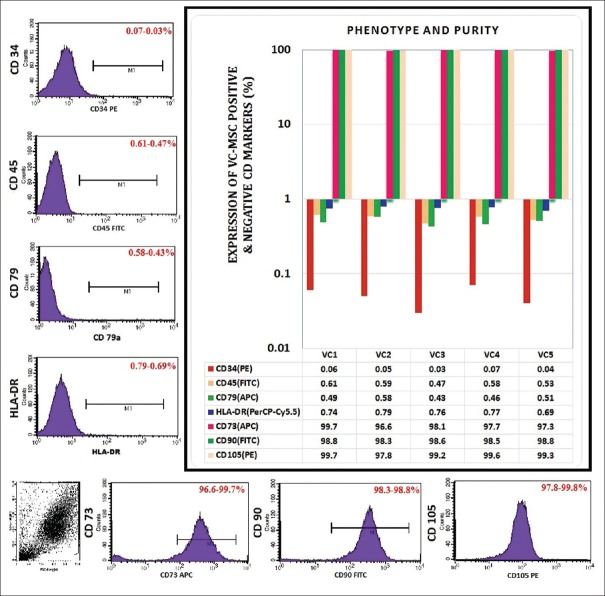
From first passage onward, flow cytometry showed homogeneous reactivity was negative for CD34, CD45, and HLA-DR and was consistently positive for the MSC markers such as CD44, CD90, and CD105, which are known to be expressed on MSCs [Figure 1]. Percent expression of these markers was quite similar and also moderately stable between in all P1, P3, P7, and P10 cell population. In detail, the VC-derived MSCs immunophenotype characterization indicated that positive for MSCs markers such as CD73 (96.6%–99.7%), CD90 (98.3%–98.8%), and CD105 (97.8%–99.8%). Furthermore, MSCs display a distinctive pattern of cell surface antigens such as CD73, CD90, and CD105 markers. Antigens that are not typically found on MSCs include CD79 (0.58%–0.43%), CD34 (0.07%–0.03%), CD45 (0.61%–0.47%), and HLA-DR (0.79%–0.69%). These cells were found to have an immunophenotype characteristic of MSCs and are capable of self-renewal.
Figure 1.
Representative phenotype analysis of villous chorion-mesenchymal stem or stromal cells after expansion when labelled with antibodies against human antigens CD34 (PE), CD45 (FITC), human leucocyte antigen-DR (PerCP-Cy5.5), CD79 (APC) as negative markers and CD73 (APC), CD90 (FITC), and CD105 (PE) as specific markers; color-shaded histogram represents positive reactivity with the indicated antibody
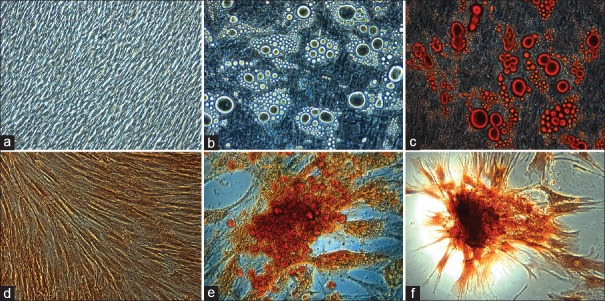
Subsequently, human MSCs have the capacity to differentiate into all the three lineages, i.e., ectoderm, mesoderm, and endoderm, by employing suitable media and growth supplements which promote lineage differentiation. We evaluated that VC can differentiate into cell types of mesodermal lineage such as adipogenic, chondrogenic, and osteogenic and besides due to their multipotency, VC-MSCs can also differentiate into ectodermal lineage such as neurons and endodermal lineage such as hepatocytes. To prove the differentiation capability of VC, MSCs into various mesodermal mesenchymal lineages, adipogenic, chondrogenic, and osteogenic differentiation protocols were performed by changing the induction medium as described in materials and methods. For adipogenic differentiation, small fat droplets in the cytoplasm could be observed after 21 days of induction [Figure 2a–c]. Induced cells morphology gradually became larger and expanded. Lipid droplets that stained with oil red accumulated in the cytoplasm of positive cells [Figure 2c], and the number of stained cells increased in a time-dependent manner. The differentiated unstained cells were shown in Figure 2b; in contrast, no fat droplet was detected in the control [Figure 2a]. To confirm the chondrogenesis potential of the VC-MSCs [Figure 2d–f], positive staining of expanded collagen fibers stained with safranin O [Figure 2f] and chondrocyte pellet [Figure 2e] indicated that MSCs were successfully differentiated to chondrocytes with each individual donor samples and no changes were observed in undifferentiated or control cells [Figure 2d].
Figure 2.
The adipogenic differentiation potential of villous chorion-mesenchymal stem or stromal cells and small lipid droplets in the cytoplasm stained with oil red staining (a-c). The chondrogenic differentiation potential and positive staining of collagen fibers with safranin O stain (d-f)
For osteogenic differentiation, cells started to change morphologically as early as 7 days in an inducing culture medium [Figure 3a–c]. The cells lost their typical fibroblast appearance and showed a rounder, more cuboidal shape [Figure 3b]. The ability of VC-MSCs to differentiate into osteocytes was grown under osteogenic differentiation conditions acquired calcium accumulation was assessed by von Kossa staining; the intense black staining was detected between osteogenic cells [Figure 3c]. Calcium deposition was not observed in cells cultured under control conditions [Figure 3a]. These findings suggested that VC-MSCs could be induced to differentiate into osteoblast-like cells. To determine whether VC-MSCs possess the capability to differentiate into neural lineages [Figure 3d–f], the cells were induced based on the method of Vourc’h et al.[14] After exposure to neural induction medium, VC-MSCs exhibited typical morphological changes: They retracted their cytoplasm, formed a compact cell body, and exhibited multiple extensions [Figure 3e]. At day 14, the neural-like cells had a sharp and elongated shape with secondary networks that expressed the neural marker [Figure 3f]. In contrast, VC-MSCs from untreated control cultures did not have any morphological changes and did not express the neural marker [Figure 3d]. These data suggested that VC-MSCs had the potential of differentiate into neurocyte-like cells.
Figure 3.
The osteogenic differentiation potential of villous chorion-mesenchymal stem or stromal cells and calcium accumulation was assessed by von Kossa staining (a-c). The ectodermal differentiation potential and the morphological appearance of villous chorion-mesenchymal stem or stromal cells changed dramatically after exposure in neural induction medium (d-f)
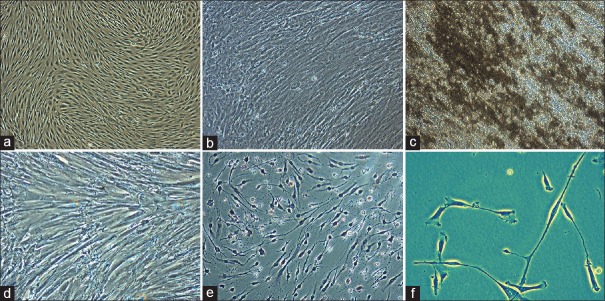
To assess their hepatic differentiation potential, VC-MSCs were induced in the hepatogenic-differentiation medium as described in materials and method. At the initial induction, cells started to shrink and turned from a long spindle shape into a short and thick-shaped morphology [Figure 4a–d]. Cells underwent a marked morphological change with maintenance of induction. At 7th day of induction, 60% of the cells began to show the transition from bipolar fibroblast-like morphology to a round or polygonal shape [Figure 4b]. With the continuous exposure to hepatic differentiating medium, most of the VC-MSCs had turned into oval cells that were increasingly similar to hepatocytes in appearance at day 21 [Figure 4c and d] compared to control cells [Figure 4a] indicating that VC-MSCs can be induced to differentiate into hepatocyte-like cells.
Figure 4.
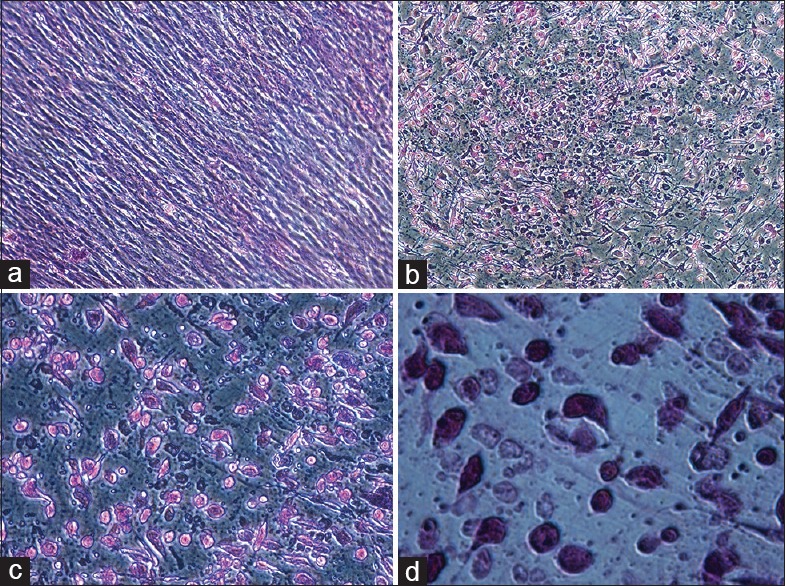
Endodermal differentiation potential of villous chorion-mesenchymal stem or stromal cells (a-d). For hepatogenic differentiation, villous chorion-mesenchymal stem or stromal cells were induced in appropriate induction medium for 21 days. (a) Untreated cells. (b-d) Phase-contrast photograph of induced cells with magnification of ×10, ×20, and ×40 respectively
Among the well-known biological activities of mesenchymal stem cells, it can be isolated from numerous sources, but clinical grade MSCs isolated in large scale for cellular therapy for various disease conditions are in demand; considering the number of cells produced and dose to be given for effective therapy. Here, we have shown that VC is one such source which has the potential to replicate in less number of days for clinical grade expansion and differentiate into all three germ layers. Purity and phenotypic characterization of MSCs isolated from VC analyzed by flow cytometry proved that the surface markers characteristics are typically similar to any other MSCs. These observations have led to conclude that VC MSCs are valuable sources of MSCs.
Collectively, these characteristics of VC-MSCs, comprising their comprehensive proliferative potential and the capacity to differentiate into several cell types, make them an attractive tool in regenerative medicine. This is exclusively evident in the fields of cytology and gene therapy, ensuing in considerable progression in the number of clinical trials based on the use of MSCs. Apparently, these cells might be simply derived from different tissues and expanded in culture in higher numbers that give the prospect to create a tissue-engineered concept containing these cells and reintroduce them into a patient.[24] Due to these significant features, the use of MSCs in clinical trials for cellular therapy is quite promising. It has been documented that these cells engraft effectively in patients and cause favorable effects. Subsequently, learning further about their properties will be a promising to start new, more progressive, and improved treatment strategies for many diseases, even those which appear to be incurable at present. Besides, significant results with MSCs in cellular therapy have been reported, each patient is genetically different and may give a different response to treatment, and carry variable predisposition to different diseases, specifically targeted strategies using autologous MSCs, may be designed. Nevertheless, still it is a long way to go before using these cells as a regularly functional therapy in clinics.[1]
However, this was the first report of VC, in which MSCs from fetal placenta had been isolated at clinical-scale expansion and proved transdifferentiation capacity. We confirmed that VC-MSCs can be differentiated into chondrocytes, osteogenic, adipogenic, neurogenic, and hepatogenic linages using respective induction media supplemented with cocktails of growth factors to form mesoderm, ectoderm, and endoderm of embryonic layers, respectively. In future, better understanding the mechanism by which pluripotent-like characteristics of VC MSCs should be further investigated.
CONCLUSIONS
The fetal portion of the placenta containing villous chorion derived MSCs were similar to amniotic membrane and wharton's jelly derived MSCs in terms of morphology and cell-surface antigen expression. More importantly, in addition to traditional mesodermal differentiation, the isolated VC-MSCs are also capable of a differentiating into neurocytes and hepatocytes. Hence, in this study, we attempted rapid clinical-scale expansion of VC-MSCs which would allow these fetus-derived stem cells to be used for various allogeneic cell-based transplantations and tissue engineering.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–30. [PubMed] [Google Scholar]
- 2.Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013;28:387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Via AG, Frizziero A, Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012;2:154–62. [PMC free article] [PubMed] [Google Scholar]
- 4.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton's jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692–712. doi: 10.3390/ijms140611692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Zheng P, Wang X, Dai G, Cheng H, Zhang Z, et al. A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther. 2014;5:57. doi: 10.1186/scrt446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, et al. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–7. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Wulf GG, Viereck V, Hemmerlein B, Haase D, Vehmeyer K, Pukrop T, et al. Mesengenic progenitor cells derived from human placenta. Tissue Eng. 2004;10:1136–47. doi: 10.1089/ten.2004.10.1136. [DOI] [PubMed] [Google Scholar]
- 9.Castellucci M, Kosanke G, Verdenelli F, Huppertz B, Kaufmann P. Villous sprouting: Fundamental mechanisms of human placental development. Hum Reprod Update. 2000;6:485–94. doi: 10.1093/humupd/6.5.485. [DOI] [PubMed] [Google Scholar]
- 10.Jaianand K, Balaji P. Isolation, characterization and scale-up of foetal amniotic membrane derived multipotent stromal cells for therapeutic applications. Int J Pharm Biol Sci. 2015;06:376–85. [Google Scholar]
- 11.Jaianand K, Balaji P. Clinical prospects of scale-up foetal Wharton's jelly derived multipotent stromal cells to fulfil the therapeutic demands. Int J Pharm Biol Sci. 2015;06:882–94. [Google Scholar]
- 12.Park SB, Seo MS, Kang JG, Chae JS, Kang KS. Isolation and characterization of equine amniotic fluid-derived multipotent stem cells. Cytotherapy. 2011;13:341–9. doi: 10.3109/14653249.2010.520312. [DOI] [PubMed] [Google Scholar]
- 13.Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56:405–15. doi: 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vourc’h P, Romero-Ramos M, Chivatakarn O, Young HE, Lucas PA, El-Kalay M, et al. Isolation and characterization of cells with neurogenic potential from adult skeletal muscle. Biochem Biophys Res Commun. 2004;317:893–901. doi: 10.1016/j.bbrc.2004.03.121. [DOI] [PubMed] [Google Scholar]
- 15.Jiao F, Wang J, Dong ZL, Wu MJ, Zhao TB, Li DD, et al. Human mesenchymal stem cells derived from limb bud can differentiate into all three embryonic germ layers lineages. Cell Reprogram. 2012;14:324–33. doi: 10.1089/cell.2012.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–84. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 17.In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 18.Mihu CM, Mihu D, Costin N, Rus Ciuca D, Susman S, Ciortea R. Isolation and characterization of stem cells from the placenta and the umbilical cord. Rom J Morphol Embryol. 2008;49:441–6. [PubMed] [Google Scholar]
- 19.Pasquinelli G, Tazzari P, Ricci F, Vaselli C, Buzzi M, Conte R, et al. Ultrastructural characteristics of human mesenchymal stromal (stem) cells derived from bone marrow and term placenta. Ultrastruct Pathol. 2007;31:23–31. doi: 10.1080/01913120601169477. [DOI] [PubMed] [Google Scholar]
- 20.Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79:528–35. doi: 10.1097/01.tp.0000149503.92433.39. [DOI] [PubMed] [Google Scholar]
- 21.Delorme B, Chateauvieux S, Charbord P. The concept of mesenchymal stem cells. Regen Med. 2006;1:497–509. doi: 10.2217/17460751.1.4.497. [DOI] [PubMed] [Google Scholar]
- 22.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 23.Bartmann C, Rohde E, Schallmoser K, Pürstner P, Lanzer G, Linkesch W, et al. Two steps to functional mesenchymal stromal cells for clinical application. Transfusion. 2007;47:1426–35. doi: 10.1111/j.1537-2995.2007.01219.x. [DOI] [PubMed] [Google Scholar]
- 24.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: A potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–41. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]