Abstract
Chemotherapy is associated with male infertility. Cisplatin (cis-diamminedichloro-platinum (II) (CDDP) as a chemotherapy medication used to treat a number of cancers has been reported to most likely induce testicular toxicity. Administration of antioxidants, such as pentoxifylline (PTX) may reduce some Adverse Drug Reactions (ADRs) of CDDP. Therefore, this study investigated the potentially protective effects of PTX on CDDP-induced testicular toxicity in adult male rats. For this purpose, 42 male rats were randomly divided into 7 groups. The rats were orally pretreated with PTX at the 3 doses of 75, 150, and 300 mg/kg once a day for 14 successive days. On the 14th day of the study, they were intraperitoneally (IP) administered with a single dose of CDDP (7 mg/kg). Finally, the sperm/testis parameters, serum levels of reproductive hormones, including testosterone, Luteinizing Hormone (LH), and Follicle Stimulating Hormone (FSH) as the pivotal endocrine factors controlling testicular functions, and histopathological changes of testis tissue were examined. Pretreatment with the two doses of 75 and 150 mg/kg PTX indicated significant increases in the sperm count and motility induced by CDDP administration. The right and significantly left testis weights were decreased following the treatment with 300 mg/kg of PTX plus CDDP. However, 75 mg/kg of PTX plus CDDP showed the best near-to-normal histopathological features. The results demonstrated that PTX alone enhanced some parameters, such as the sperm count, while reducing other parameters, including sperm fast motility and germ layer thickness. Furthermore, despite testosterone or LH levels, the mean serum FSH level was significantly augmented by the doses of 75 and 150 mg/kg. It was concluded that PTX administration cannot reduce CDDP-induced testicular toxicity even at high doses (e.g., 300 mg/kg), while it seemed to partially intensify CDDP toxicity effects at a dose of 75 mg/kg. Thus, further research is required in this regard.
Keywords: Chemotherapy, Testicular toxicity, Antioxidant, Histopathological feature, Sperm motility
INTRODUCTION
Infertility and its related problems are a major issue in today’s marital affairs (1). According to statistics, 35% of infertility cases are related to men, while 25% relate to both partners (2). The inability to produce a sufficient number of healthy and active sperms is the most common cause of male infertility (3,4). Here, it should be pointed out that cancer chemotherapy drugs, antibiotics, toxins, pesticides, radiation, stress, air pollution, inadequate levels of vitamins, and administration of glucocorticoids are the factors contributing to poor sperm production and consequent male infertility (5–8). Chemotherapy and radiation therapy are associated with changes in the male genital tract. In chemotherapy, drugs with alkalizing properties have the highest dose-dependent ADRs on testicles (6).
CDDP is a chemotherapy medication that forms DNA adducts resembling bifunctional alkylating agents. CDDP can be used to treat various tumors, including ovarian, testicular, bladder, head and neck, lung, and cervical and endometrial tumors, as well as sarcoma and lymphoma (9,10). However, due to its harmful effects on the kidneys, peripheral nerves, inner ear, and testicles, the use of this drug has been limited (11–14). The main reason for the restriction in its therapeutic efficacy is its toxic effects on the reproductive system causing severe testicular damage, which can be identified with the death of zoogenic cells in the process of spermatogenesis and sperm DNA damage (15,16).
PTX is a derivative of methyl xanthine that can be applied for the treatments of peripheral vascular disease, cerebrovascular disease, or conditions causing topical disturbance on microcirculation due to its effects on blood flow and glutathione (GSH) (17). This compound can enhance chemotactic responses of neutrophils, while it may hinder the phagocyte and superoxide productions of neutrophils and monocytes (18,19). PTX is a hemorheologic active compound, which is used for the treatment of intermittent claudication in the foot and other vascular disorders and can inhibit phosphodiesterase (PDE), which leads to augmented levels of intracellular cAMP (17,20–22). Due to playing a role in improving sperm function, PTX has attracted much attention. cAMP plays a role in the control of motile spermatozoa. Nevertheless, its numerous protective mechanisms are less clear (20,21).
PTX beneficial effects on sperm function and male infertility have been noted in several studies (23,24). Yet, there are some controversies in its beneficial effects on sperm function and semen parameters (25,26). Furthermore, research has shown that PTX has anti-inflammatory and antioxidant effects in addition to its useful effects on blood circulation (27). Thus, given the role of oxidants in the incidence of testicular toxicity induced by CDDP and PTX useful properties, it was decided to examine PTX protective effects on CDDP-induced testicular toxicity.
MATERIALS AND METHODS
Animals
A total of 42 male Wistar rats (180~200 g) housed under the standard condition, including 12 hr of darkness, 12 hr of light, and a temperature of 25 ± 2°C with free access to adequate amount of food and water in order to comply with the required situation was employed. All the animal experiments were performed according to the ethical guidelines suggested by the animal ethics committee of Yasuj University of Medical Sciences (Yasuj, Iran).
Treatment
The animals were randomly divided into 7 groups and treated for a period of 14 consecutive days. The rats were weighed once prior to the study and once before being sacrificed. Group A served as the control, which was daily gavaged with Distilled Water (DW). Group B received DW orally once a day for 14 days, while being once injected with 7 mg/kg of CDDP intraperitoneally (IP) on the 14th day of the study. Group C received 75 mg/kg of PTX orally once a day for 14 days, while being once injected with 7 mg/kg of CDDP intraperitoneally (IP) on the 14th day of the study. Group D received 150 mg/kg of PTX orally once a day for 14 days, while being once injected with 7 mg/kg of CDDP intraperitoneally (IP) on the 14th day of the study. Group E received 300 mg/kg of PTX orally once a day for the period of 14 days, while being once injected with 7 mg/kg of CDDP intraperitoneally (IP) on the 14th day of the study. Group F received 150 mg/kg of PTX orally once a day for the 14-day period. Group G received 300 mg/kg of PTX orally once a day for 14 days.
Sperm quality evaluation
To examine the sperm quality, first, the tail of the epididymis was cut and kept in T6 medium. Then, it was divided into pieces and hence, the sperm was inserted into T6. Morphology, number (count), and movement (motility) of the sperms were examined in all the study groups (28,29).
Hormone measurement
The rats in all the groups were weighed on the 1st and 14th days of the study. After the 14-day period, the weighed rats were anesthetized with ketamine (40 mg/kg)/xylazine (10 mg/kg) and their abdomens were opened to take their blood samples from their hearts and examine their serum testosterone, LH, and FSH levels.
Histopathological examination
All the animals were sacrificed and their testicular tissues were fixed by 10% neutral buffered formalin, embedded in paraffin, rehydrated, and cut into 5 μm-thick sections using a rotary microtome. The sections were stained with hematoxylin and eosin (H&E), mounted in Canada balsam, and then evaluated with a light microscope to determine any histological changes. Furthermore, epididymis was used to assess sperm parameters.
Statistical analysis
The Statistical Package for Social Sciences (SPSS 22, IBM, NY, USA) software was utilized for the statistical analysis. The variables in terms of homogeneity of variance and a normal distribution were examined. If they contained a normal distribution and equal variance, analysis of variance (ANOVA) would be used and otherwise, Kruskal-Wallis one-way test would be applied. To specify the different groups, Tukey test was employed. The significance tests of p<0.05 were considered.
RESULTS
Body weight changes
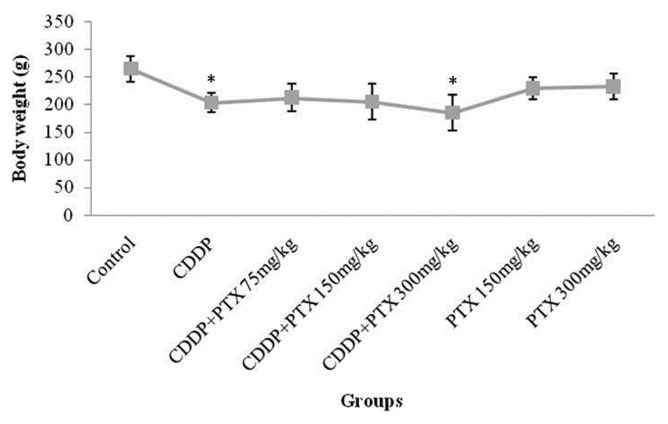
The mean body weights of the rats were significantly reduced after treatment with CDDP or CDDP combined with PTX at a dose of 300 mg/kg. However, 75 mg/kg of PTX could inhibit the animal’s weight decrease. Moreover, administration of PTX alone showed no significant changes in the body weight (Fig. 1). The animal weight reduction could indicate toxicity during the treatment.
Fig. 1.
The mean weight of the animals at the beginning and end of the * indicated significant (p<0.05) difference compared with control.
Changes in sperm parameters
• Sperm count
The data demonstrated that the number of sperms in the group treated with CDDP (218.66 ± 8.99) significantly reduced compared to that of the control group. The data also suggested that a concurrent administration of CDDP and PTX at a dose of 300 mg/kg with a mean of 90 ± 30.35 caused a significant reduction in the sperm count (p≤0.01). On the other hand, administration of PTX alone at a dose of 150 mg/kg with a mean of 495.66 ± 102.08 led to a significant enhancement of the sperm counts of the treatment group compared to those of the control group. Furthermore, significant increase and decrease were observed in the group receiving PTX combined with CDDP at the doses of 75 and 300 mg/kg with the means of 444.50 ± 19.96 and 90 ± 30.35 compared to the group receiving CDDP alone, respectively. A significant difference was also observed with the coadministration of 150 mg/kg PTX and CDDP compared to the group receiving CDDP alone. Also, with the increasing dose of PTX up to 300 mg/kg, a significant decline in the sperm count was witnessed as compared to the control group (Table 1).
Table 1.
Mean average (mean ± SD) of rats’ sperm parameters in treated groups
| Sperm parameters | Control | CDDP | CDDP + PTX (75 mg/kg) | CDDP + PTX (150 mg/kg) | CDDP + PTX (300 mg/kg) | PTX (150 mg/kg) | PTX (300 mg/kg) |
|---|---|---|---|---|---|---|---|
| Sperm count (×106/mm3) | 340.75 ± 22 | 218.66 ± 8.99* | 444.50 ± 19.96*## | 256.75 ± 13.98*# | 90 ± 30.35**## | 495.66 ± 102.08*# | 231 ± 15.63* |
| Sperm morphology | |||||||
| Normal (%) | 86 ± 9.78 | 93.33 ± 1.96 | 81.25 ± 15.76 | 81.25 ± 15.41 | 96.66 ± 0.33 | 97 ± 0.01 | 94.60 ± 2.40 |
| Non-normal (%) | 14 ± 8.82 | 6.66 ± 1.89 | 18.75 ±14.64 | 18.77 ± 15.43 | 3.33 ± 0.32 | 3 ± 0.02 | 5.40 ± 2.41 |
| Sperm motility | |||||||
| Fast (%) | 33.75 ± 7.78 | 0*** | 0*** | 0*** | 1 ± 1*** | 8.33 ± 3.33**### | 8.20 ± 1.98**### |
| Slow (%) | 52.50 ± 7.77 | 26 ± 10.03* | 14.25 ± 1.10**# | 1.75 ± 0.62***### | 4 ± 2.08***### | 38.33 ± 4.51*# | 55.60 ± 6.58 |
| Non motility (%) | 13.75 ± 1.49 | 74 ± 12.71** | 68.75 ± 17.92** | 98.25 ± 4.77**# | 95 ± 3.65**# | 53.33 ± 5.02**# | 36.20 ± 7.47*# |
p<0.05,
p<0.01,
p<0.001 compared to control;
p<0.05,
p<0.01,
p<0.001 compared to CDDP-treated group.
• Sperm shape
The results demonstrated that the shapes of the sperm cells in the treated compared to the control group were not significantly different (Table 1).
• Sperm fast motility
Table 1 displays the mean and SD of the studied factors of the sperms isolated from the testicular tissues of the selected groups. Sperm fast motility in the control group was statistically significant (p<0.001) compared to those of the other studied groups; however, the difference was not statistically significant in the groups receiving different doses of PTX combined with CDDP compared to the CDDP-administrated group.
• Sperm sluggish motility
Table 1 shows that the average percentage of the sluggish motilities of the sperms in the control group is 52.50 ± 7.77. In addition, statistically significant differences can be seen in the group receiving CDDP combined with the different PTX doses of 75, 150, and 300 mg/kg with the averages of 14.25 ± 1.10, 1.75 ± 0.62, and 4 ± 2.08, respectively. The data obtained from 300 mg/kg PTX were not significantly different compared with that of the control group. Moreover, a significant reduction was found in the groups receiving the different PTX doses of 75, 150, and 300 mg/kg combined with CDDP as compared with the CDDP group.
Non-motile sperms
The percentage of non-motile sperms in the control group was 13.75 ± 1.49 in comparison with the CDDP-receiving group and the group of CDDP combined with the different PTX doses of 75, 150, and 300 mg/kg with the averages of 68.75 ± 17.92, 98.25 ± 4.77 and 95 ± 3.65, respectively, thus representing a statistically significant difference. Yet, the data of the group administrated with 150 and 300 mg/kg PTX did not reveal significant differences from that of the control group. Furthermore, no significant differences were observed in the groups receiving different doses of PTX plus CDDP as compared with the CDDP-receiving group (Table 1).
Changes in testis parameters
• Left testis weight
Table 1 depicts a significant decrease in the left testicular weights of the groups receiving CDDP alone (1.25 ± 0.06 g) and a combination of CDDP and 300 mg/kg PTX (1.20 ± 0.08 g) compared with that of the control group (1.53 ± 0.02 g; p = 0.041). No statistically significant differences were observed in the left testis weights of the groups administered with different doses of PTX plus CDDP compared to the group receiving CDDP alone.
• Right testis weight
The right testicular weight in the CDDP-administered group was 1.24 ± 0.05 g in comparison with that of the control group (1.51 ± 0.03 g), thus revealing a statistically significant decrease (p = 0.037). However, the right testis weights of the groups receiving CDDP and 300 mg/kg PTX were reduced compared to that of the control group though this reduction was not statistically significant (p = 0.098). Besides, no significant differences were observed in the right testicular weights of the groups receiving different doses of PTX plus CDDP compared with that of the CDDP group (Table 2).
Table 2.
Mean average (mean ± SD) of rats’ testes parameters in treated groups
| Testis parameters | Control | CDDP | CDDP + PTX (75mg/Kg) | CDDP + PTX (150mg/kg) | CDDP + PTX (300mg/kg) | PTX (150mg/kg) | PTX (300mg/kg) |
|---|---|---|---|---|---|---|---|
| Left testis weight (g) | 1.53 ± 0.02 | 1.25 ± 0.06* | 1.34 ± 0.03 | 1.35 ± 0.04 | 1.20 ± 0.08*# | 1.48 ± 0.11 | 1.49 ± 0.02 |
| Right testis weight (g) | 1.51 ± 0.03 | 1.24 ± 0.05* | 1.37 ± 0.04 | 1.33 ± 0.06 | 1.23 ± 0.09 | 1.47 ± 0.12 | 1.49 ± 0.01 |
| Seminiferous tubule diameter (μm) | 268.63 ± 13.49 | 330.08 ± 28.80 | 267.95 ± 12.06 | 296.95 ± 21.06 | 275.09 ± 14.16 | 258.63 ± 11.49 | 251.94 ± 10.07 |
| Germinal cell layer (μm) | 262.69 ± 14.22 | 73.24 ± 6.67*** | 57.88 ± 3.64***# | 68.20 ± 6.35*** | 82.83 ± 6.33*** | 48.03 ± 5.49***# | 54.6 ± 4.02***# |
| Spermatogonia cell number (mm2) | 84.5 ± 4.23 | 115.25 ± 3.24** | 108.58 ± 6.78** | 116.33 ± 3.85** | 156.33 ± 13.59**# | 140.01 ± 8.49**# | 139.83 ± 11.41**# |
| Leydig cell (mm2) | 5.33 ± 0.61 | 5.33 ± 0.37 | 5.25 ± 0.39 | 5.17 ± 0.45 | 5.01 ± 0.36 | 5.01 ± 0.49 | 4.5 ± 0.33 |
| Sertoli cell (mm2) | 4.02 ± 0.36 | 4.5 ± 0.31 | 3.75 ± 0.21# | 5.24 ± 0.31 | 4.33 ± 0.28# | 4.02 ± 0.38 | 4.02 ± 0.27 |
p<0.05,
p<0.01,
p<0.001 compared to control;
p<0.05 compared to CDDP-treated group.
• Seminiferous tubule diameter
According to the data, the mean seminiferous tubule diameters of the treated groups compared with that of the control group indicated no statistically significant differences (Table 2). There was a decline in the mean seminiferous tubule diameters of the groups treated with different doses of PTX combined with CDDP with the means of 267.95 ± 12.06, 296.95 ± 21.06, and 275.09 ± 14.16, compared to the group treated with CDDP alone (330.08 ± 28.80), respectively, but the reduction was not statistically significant.
• Zoogenic (germ) layer diameter
Table 2 exhibits that the diameters of the germ layers in all the groups were significantly decreased (p<0.001) as compared to that of the control group. Additionally, a statistically significant reduction was found in the groups pretreated with a dose of 75 mg/kg PTX plus CDDP with a mean of 57.88 ± 3.64 compared to that of the CDDP group (73.24 ± 6.67).
• Number of spermatogonial cells
The numbers of spermatogonia in the group receiving 300 mg/kg of PTX alone (139.83 ± 11.41) and the group treated with CDDP and 300 mg/kg PTX (156.33 ± 13.59) significantly increased compared to that of the control group (84.5 ± 4.23; p<0.01). Furthermore, a statistically significant enhancement was found in the group receiving CDDP and 300 mg/kg PTX compared to the group receiving CDDP alone. Yet, no simultaneous significant differences were observed in the other two groups administered with CDDP and PTX as compared to the group receiving CDDP alone (Table 2).
• Number of Leydig cells
The results were indicative of no significant differences between the studied and control groups based on the number of Leydig cells. In addition, a reduction was observed in the numbers of Leydig cells in the groups treated with different PTX doses of 75, 150, and 300 mg/kg in combination with CDDP with the means of 5.25 ± 0.39, 5.17 ± 0.45, and 5.01 ± 0.36 compared to that of the group treated with CDDP alone (5.33 ± 0.37), respectively, thus revealing a non-statistically significant variation.
• Number of sertoli cells
The numbers of sertoli cells in the studied groups compared with the control group represented no statistically significant differences (Table 1). The groups pretreated with 75 and 300 mg/kg PTX and CDDP at the two doses of 3.75 ± 0.21 and 4.33 ± 0.28 demonstrated a significant decline as compared to the CDDP group (4.5 ± 0.33), while no statistically significant augmentation was discovered in the group receiving 150 mg/kg PTX and CDDP (5.24 ± 0.31).
Hormonal changes
• Serum testosterone level
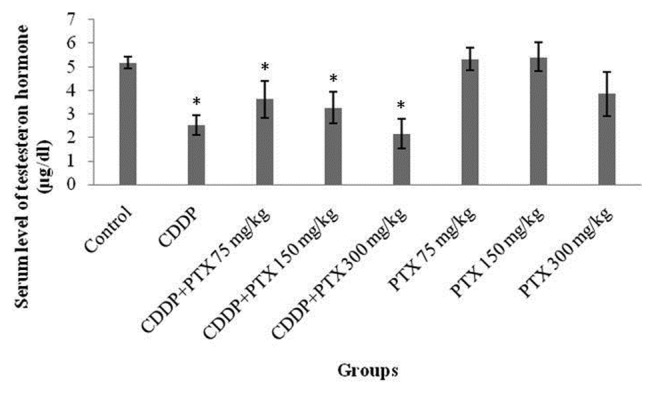
The mean serum testosterone levels in the CDDP and concurrent CDDP- and PTX-receiving groups at the different doses of 75, 150, 300 mg/kg were 2.52 ± 0.42, 3.62 ± 0.71, 3.25 ± 0.67, and 2.15 ± 0.65 μg/dL, respectively, thus representing a high significant reduction in comparison with that of the control group (5.17 ± 0.26 μg/dL; p<0.01). Despite that, the mean serum testosterone levels were slightly increased in the groups receiving 75 or 150 mg/kg of PTX plus CDDP compared with the CDDP group though this enhancement was not statistically significant. In any case, PTX at 300 mg/kg indicated the greatest reduction. Furthermore, testosterone levels in the PTX-administered group showed no significant difference from that of the control group (Fig. 2).
Fig. 2.
Serum testosterone levels in study groups. * Indicated significant (p<0.05) difference compared with control.
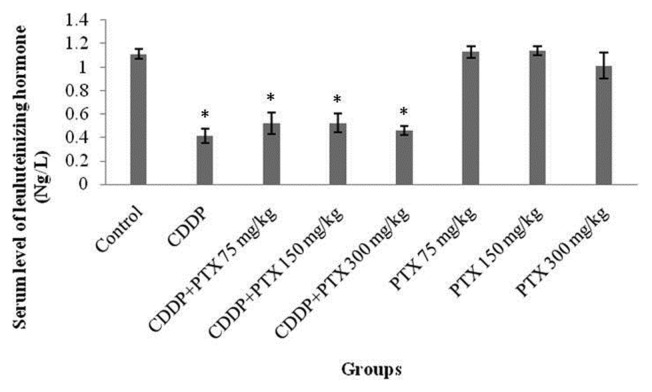
• Measurement of LH level in blood
No statistically significant alleviation was observed in the serum LH levels of the groups receiving different doses of PTX in combination with CDDP as compared to the CDDP group (Fig. 3).
Fig. 3.
The mean luteinizing hormone (LH) in blood. * Indicated significant (p<0.05) difference compared with control.
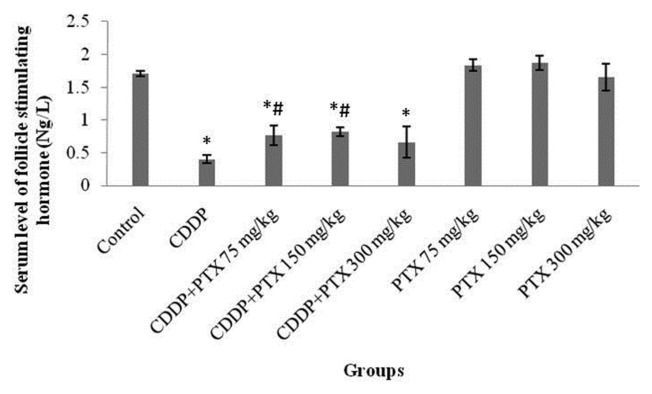
• Measurement of FSH level in blood
Pretreatment with different doses of PTX (75, 150, and 300 mg/kg) combined with CDDP exhibited the means of 0.77 ± 0.15, 0.82 ± 0.07, and 0.66 ± 0.24 Ng/L, respectively, in comparison with 0.4 ± 0.06 Ng/L for the CDDP group. A statistically significant elevation of FSH serum levels was observed in the groups receiving 75 and 150 mg/kg of PTX in combination with CDDP compared with that of the CDDP group. However, no significant changes were indicated at a dose of 300 mg/kg (Fig. 4).
Fig. 4.
The mean follicle stimulating hormone (FSH) in blood. * Indicated significant (p<0.05) difference compared with control; and # indicated significant (p<0.05) differences compared with CDDP group.
• Histopathological analysis
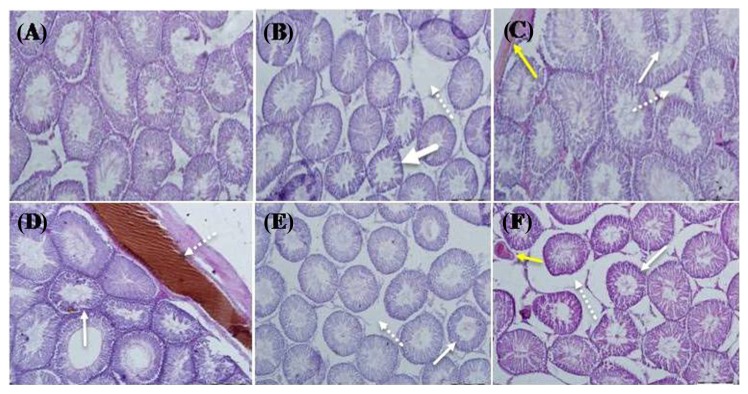
The best near-to-normal histological features were revealed in the group of CDDP plus 75 mg/kg PTX by H & E as compared with those of the control group (Fig. 5C). In this Figure, the continuous arrows depict the diameters of seminiferous tubules and the non-continuous arrows display the normal thickness of the germinal layer. In addition, yellow arrows represent venous congestion (Fig. 5C). The control group portrayed normal seminiferous tubular and germinal epithelium diameters and spaces of seminiferous tubules (Fig. 5A). Decreased seminiferous tubular diameters (continuous arrows) and increased space between the seminiferous tubules (non-continuous arrows) were indicated in the testes of the rats treated with CDDP (Fig. 5B). Reduced seminiferous tubular diameter and thickness of germinal epithelium layer (continuous arrows) besides vascular congestion (non-continuous arrows) were observed in the testis sections of the rats treated with a dose of 150 mg/kg PTX and CDDP (Fig. 2D). Decreased seminiferous tubular diameters (continuous arrows) and increased spaces between the seminiferous tubules caused by the tissue edema (non-continuous arrows) were seen in the testis sections of the rats treated with a dose of 300 mg/kg PTX and CDDP as represented in Fig. 5E. Furthermore, Fig. 5F demonstrates a reduced seminiferous tubular diameter (continuous arrows) and tissue edema in the spaces between seminiferous tubules (non-continuous arrows) in addition to venous congestion (yellow arrows) in the testis sections of the rats treated with a dose of 300 mg/kg PTX.
Fig. 5.
Microscopic views of hematoxylin and eosin (H & E) - stained rat testes with ×40 magnification. (A) Control group. (B) Rats treated with cisplatin (CDDP). (C) Rats treated by CDDP and PTX (75mg/kg). (D) Rats treated by CDDP and PTX (150 mg/kg). (E) Rats treated by CDDP and PTX (300 mg/kg). (F) Rats treated with PTX (300 mg/kg).
DISCUSSION
This research was an experimental study conducted on PTX effects at the different doses of 75, 150, and 300 mg/kg on CDDP-induced testicular toxicity in rats. According to the sperm/testis parameters, pretreatment with the two doses of 75 and 150 mg/kg PTX revealed significant increases in the sperm count and motility against CDDP administration. PTX alone enhanced some parameters, such as the sperm count, but reduced other parameters, including sperm fast motility and germ layer thickness. However, the right and significantly left testis weights were decreased following the treatment with 300 mg/kg of PTX plus CDDP. Histopathological results also indicated the best near-to-normal features of CDDP+75 mg/kg PTX. Furthermore, the levels of reproductive hormones, such as FSH significantly rose at the doses of 75 and 150 mg/kg despite testosterone or LH levels as compared with CDDP level. The serum levels of testosterone, LH, and FS hormones make parts of the regulatory toxicity studies performed on the reproductive system. Therefore, PTX could not reduce CDDP-induced testicular toxicity even at high doses (e.g., 300 mg/kg) although it seemed to partially intensify CDDP toxicity effects at a dose of 75 mg/kg.
It has been reported that CDDP causes irregular seminiferous tubes, reduces seminiferous tubular epithelium and fibrosis around the vessels and melatonin, and helps to dramatically improve these changes by increasing the levels of GSH and glutathione peroxidase (GPX) activities, enhancing serum testosterone levels, and reducing malondialdehyde (MDA) levels (30). MDA results from the lipid peroxidation (LPO) of polyunsaturated fatty acids and serves as a marker for Oxidative Stress (OS). In the previous studies, the antioxidant effects of vitamin C, diphenyldiamine (DPPD), and L-cysteine on the kidney and testicular toxicities of CDDP in rats were evaluated (31,32). In those studies, CDDP was administered at the doses of 2 and 8 mg/kg for 2 weeks. The results of the CDDP-receiving groups showed that the levels of LPO, peroxidase, and superoxide anions significantly augmented. Furthermore, dramatic reductions in the activities of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione transferase were observed. GSH levels were substantially lowered in the testes treated with CDDP compared with that of the control group. The CDDP-induced toxicity resulted from free radicals was biochemically reported to be generated in the tissues. However, the mechanism of CDDP-induced testicular damage is not well understood. Numerous studies have shown that exposure to CDDP can disturb redox balance, which is indicative of the oxidative type of the stress leading to the biochemical and physiological disorders (33,34).
The sperms of mammals contain a unique lipid composition with high levels of polyunsaturated fatty acids, sphin-gomyelin, and plasmalogen. The unusual membrane structures of the sperms are responsible for their flexibilities and performance capabilities. Lipids in spermatozoa are the main precursors of peroxidation, which may stimulate severe abnormalities in the sperm functions (35,36). In some experimental studies, Reactive Oxygen Species (ROS) is known to provide a mechanism for the pathogenesis of CDDP-induced testicular toxicity (37,38). The concept of ROS encompasses oxygen ions, free radicals, and peroxidase, which can cause infertility via two main mechanisms. Firstly, the damage to the sperm membrane can reduce sperm motility and ability to fuse with the ovum. Secondly, the direct damage to the sperm DNA can cause some problems in the paternal genomic contribution to the embryo (39).
Due to the toxic effects of chemotherapy on fertility, researchers have used such techniques as cryopreservation of spermatozoa and Intra-Cytoplasmic Sperm Injection (ICSI) to retain the reproductive potentiality and increase the chances of a successful pregnancy. Nonetheless, there are restrictions on the use of these methods since these options are not practical for patients before maturation and additionally, semen freezing and thawing can reduce their sperm qualities (40). Therefore, alternative methods are required for protecting the epithelia of the seminiferous tubules and spermatogenesis against the testicular toxicity of anti-cancer agents. Although there are some concerns about the concurrent use of antioxidants and anti-cancer drugs due to the risk of reduced effectiveness of cytotoxic drugs, recent evidence suggests that a combination therapy with protective chemical agents can be helpful to overcome such toxic effects on reproduction (32,41). In recent decades, several studies have shown that the administration of antioxidants from plant and food compounds may help decrease some of the side effects of CDDP without affecting its chemical properties or effects (42–44).
Some examples reported from various similar studies include grape seed extract, turmeric extract, caffeic acid in honey, melatonin, vitamin C, vitamin E, carotenoids, and many other materials with antioxidant mechanisms to either fully or partially reduce CDDP toxic effects on different body systems (30,45–50). Administrations of DPPD, L-cysteine, and vitamin C before CDDP injection have been shown to improve histological features and reduce the number of cells involved in apoptosis, which has been highest when using DPPD (31). Although the relevant studies have demonstrated the beneficial effects of antioxidants, such as PTX on the protection of the reproductive system from toxicity, there are some controversies for the use of PTX affecting the sperm function, semen parameters, or reproductive hormones (25–27). Furthermore, little is known about the protective mechanisms of PTX.
In conclusion, CDDP treatment can induce histopathological changes in testicular tissue through OS as evidenced by the enhanced free radicals, DNA fragmentation, and significant decreases in the enzymatic and non-enzymatic antioxidants. However, via pretreatment with antioxidants, PTX can partially protect CDDP-ADRs via different mechanisms, which mainly involve antioxidants and PTX anti-inflammatory properties. In this research, CDDP was shown to induce testicular toxicity at high doses (300 mg/kg) and even a low dose of 75 mg/kg, thus representing different cellular responses for diminishing its cytotoxic effects. Therefore, further research is required to explain these different behaviors.
REFERENCES
- 1.Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13:163–174. doi: 10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- 2.Aryanpur M, Tarahomi M, Sharifi H, Heydari G, Hessami Z, Akhoundi M, Masjedi MR. Comparison of spermatozoa quality in male smokers and nonsmokers of Iranian infertile couples. Int J Fertil Steril. 2011;5:152–157. [PMC free article] [PubMed] [Google Scholar]
- 3.Baker MA, Aitken RJ. The importance of redox regulated pathways in sperm cell biology. Mol Cell Endocrinol. 2004;216:47–54. doi: 10.1016/j.mce.2003.10.068. [DOI] [PubMed] [Google Scholar]
- 4.Amin A, Hamza AA. Effects of roselle and ginger on cisplatin-induced reproductive toxicity in rats. Asian J Androl. 2006;8:607–612. doi: 10.1111/j.1745-7262.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 5.Martenies SE, Perry MJ. Environmental and occupational pesticide exposure and human sperm parameters: a systematic review. Toxicology. 2013;307:66–73. doi: 10.1016/j.tox.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell SJ, Shalet SM. Spermatogenesis after cancer treatment, damage and recovery. J Natl Cancer Inst Monogr. 2005;(34):12–17. doi: 10.1093/jncimonographs/lgi003. [DOI] [PubMed] [Google Scholar]
- 7.Mosher WD, Pratt WF. Fecundity and infertility in the United States, incidence and trends. Fertil Steril. 1991;56:192–193. doi: 10.1016/S0015-0282(16)54469-0. [DOI] [PubMed] [Google Scholar]
- 8.Schill WB. Recent progress in pharmacological therapy of male subfertility-a review. Andrologia. 1979;11:77–107. doi: 10.1111/j.1439-0272.1979.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 9.Chirino YI, Hernández-Pando R, Pedraza-Chaverrí J. Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol. 2004;4:20. doi: 10.1186/1471-2210-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colpi GM, Contalbi GF, Nerva F, Sagone P, Piediferro G. Testicular function following chemo-radiotherapy. Eur J Obstet Gynecol Reprod Biol. 2004;113:S2–S6. doi: 10.1016/j.ejogrb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology. 2005;212:116–123. doi: 10.1016/j.tox.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Bhat SG, Nie Z, Ramkumar V. Cisplatin up-regulates adenosine A(1) receptors in rat testes. Eur J Pharmacol. 1999;382:35–43. doi: 10.1016/S0014-2999(99)00584-1. [DOI] [PubMed] [Google Scholar]
- 13.Ozols RF, Deisseroth AB, Javadpour N, Barlock A, Messerschmidt GL, Young RC. Treatment of poor prognosis nonseminomatous testicular cancer with a “high-dose” platinum combination chemotherapy regimen. Cancer. 1983;51:1803–1807. doi: 10.1002/1097-0142(19830515)51:10<1803::AID-CNCR2820511008>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Stadnicki SW, Fleischman RW, Schaeppi U, Merriam P. Cis-dichlorodiammineplatinum (II) (NSC-119875), hearing loss and other toxic effects in rhesus monkeys. Cancer Chemother Rep. 1975;59:467–480. [PubMed] [Google Scholar]
- 15.Fung C, Vaughn DJ. Complications associated with chemotherapy in testicular cancer management. Nat Rev Urol. 2011;8:213–222. doi: 10.1038/nrurol.2011.26. [DOI] [PubMed] [Google Scholar]
- 16.Hooser SB, van Dijk-Knijnenburg WC, Waalkens-Berendsen ID, Smits-van Prooije AE, Snoeij NJ, Baan RA, Fichtinger-Schepman AM. Cisplatin-DNA adducts formation in rat spermatozoa and its effects on fetal development. Cancer Lett. 2000;151:71–80. doi: 10.1016/S0304-3835(99)00415-2. [DOI] [PubMed] [Google Scholar]
- 17.Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Noyan T, Onem O, Ramazan Sekeroğlu M, Köseoğlu B, Dülger H, Bayram I, Yalçinkaya AS, Bakan V. Effects of erythropoietin and pentoxifylline on the oxidant and antioxidant systems in the experimental short bowel syndrome. Cell Biochem Funct. 2003;21:49–54. doi: 10.1002/cbf.991. [DOI] [PubMed] [Google Scholar]
- 19.Mandell GL. ARDS, neutrophils and pentoxifylline. Am Rev Respir Dis. 1988;138:1103–1105. doi: 10.1164/ajrccm/138.5.1103. [DOI] [PubMed] [Google Scholar]
- 20.Tesarik J, Mendoza C, Carreras A. Effects of phosphodiesterase inhibitors, caffeine and pentoxifylline, on spontaneous and stimulus-induced acrosome reactions inhuman sperm. Fertil Steril. 1992;58:1185–1190. doi: 10.1016/S0015-0282(16)55567-8. [DOI] [PubMed] [Google Scholar]
- 21.Garbers DL, Lust WD, First NL, Lardy HA. Effect of phosphodiesterase inhibitors and cyclic nucleotides on sperm respiration and motility. Biochemistry. 1971;10:1825–1831. doi: 10.1021/bi00786a015. [DOI] [Google Scholar]
- 22.Porter JM, Cutler BC, Lee BY, Reich T, Reichle FA, Scogin JT, Strandness DE. Pentoxifylline efficacy in the treatment of intermittent claudication, multicenter controlled double-blind trial with objective assessment of chronic occlusive arterial disease patients. Am Heart J. 1982;104:66–72. doi: 10.1016/0002-8703(82)90642-1. [DOI] [PubMed] [Google Scholar]
- 23.Oliva A, Dotta A, Multigner L. Pentoxifylline and antioxidants improve sperm quality in male patients with varicocele. Fertil Steril. 2009;91:1536–1539. doi: 10.1016/j.fertnstert.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell DT, Jacobson JD, King A, Chan PJ. Effect of pentoxifylline on tumor suppressor and proto-oncogene apoptosis in sperm. J Assist Reprod Genet. 2002;19:279–283. doi: 10.1023/A:1015725230011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terriou P, Hans E, Giorgetti C, Spach JL, Salzmann J, Urrutia V, Roulier R. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows normal fertilization, pregnancy, and birth after intracytoplasmic sperm injection. J Assist Reprod Genet. 2000;17:194–199. doi: 10.1023/A:1009435732258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faka B, Api M, Fiçicioğlu C, Gürbüz A, Oral O. Pentoxifylline in male-factor infertility, its therapeutic efficacy after oral administration. Acta Eur Fertil. 1994;25:351–353. [PubMed] [Google Scholar]
- 27.Noyan T, Yalcinkaya AS, Sekeroglu MR, Dulger H, Balaharoglu R. Antioxidant effects of pentoxifylline and melatonin in the alloxane-induced diabetic mice. Turk J Biochem. 2004;29:268–272. [Google Scholar]
- 28.Alizadeh N, Abbasi M, Abolhassani F, Amidi F, Mahmoudi R, Hoshino Y, Sato E, Ragerdikashani I. Effects of aminoguanidine on infertile varicocelized rats: A functional and morphological study. Daru. 2010;18:51–56. [PMC free article] [PubMed] [Google Scholar]
- 29.Nikseresht M, Fallahzadeh AR, Toori MA, Mahmoudi R. Effects of pomegranate seed oil on the fertilization potency of rat’s sperm. J Clin Diagn Res. 2015;9:FF01–4. doi: 10.7860/JCDR/2015/12576.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somay A. Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertil Steril. 2009;92:1124–1132. doi: 10.1016/j.fertnstert.2008.07.1758. [DOI] [PubMed] [Google Scholar]
- 31.Nassar A, Omara HM, Sary AEKH, Ahmed E, Ragab SMM. Vitamin C, N, N-diphenyl-p-phenyl-enediamine and L-cysteine ameliorate the pathological and oxidative Stress-induced by cisplatin in the lidneys of male rats. J Free Radic Antioxid. 2012;138:112–121. [Google Scholar]
- 32.Ahmed EA, Omar HM, Abdelghaffar SK, Ragb SM, Nasser AY. The antioxidant activity of vitamin C, DPPD and L-cysteine against Cisplatin-induced testicular oxidative damage in rats. Food Chem Toxicol. 2011;49:1115–1121. doi: 10.1016/j.fct.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Ravi R, Somani SM, Rybak LP. Mechanisms of cisplatin ototoxicity: antioxidant system. Pharmacol Toxicol. 1995;76:386–394. doi: 10.1111/j.1600-0773.1995.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 34.Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril. 1992;57:409–416. doi: 10.1016/S0015-0282(16)54855-9. [DOI] [PubMed] [Google Scholar]
- 35.Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004;2:12. doi: 10.1186/1477-7827-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sikka SC. Oxidative stress and role of antioxidant in normal and abnormal sperm function. Front Biosci. 1996;1:e78–e86. doi: 10.2741/A146. [DOI] [PubMed] [Google Scholar]
- 37.Turk G, Atessahin A, Sonmez M, Ceribasi AO, Yuce A. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil Steril. 2008;89:1474–1481. doi: 10.1016/j.fertnstert.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 38.Atessahin A, Karahan I, Turk G, Gur S, Yılmaz S, Ceribası AO. Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod Toxicol. 2006;21:42–47. doi: 10.1016/j.reprotox.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Tremellen K. Oxidative stress and male infertility-a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 40.Minaei MB, Barbarestani M, Nekoonam S, Abdolvahabi MA, Takzare N, Asadi MH, Hedayatpour A, Amidi F. Effect of Trolox addition to cryopreservation media on human sperm motility. Iran J Reprod Med. 2012;10:99–104. [PMC free article] [PubMed] [Google Scholar]
- 41.Narayana K, Al-Bader M, Mousa A, Khan KM. Molecular effects of chemotherapeutic drugs and their modulation by antioxidants in the testis. Eur J Pharmacol. 2012;674:207–216. doi: 10.1016/j.ejphar.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 42.Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development on side effects. Nutr Cancer. 2000;37:1–18. doi: 10.1207/S15327914NC3701_1. [DOI] [PubMed] [Google Scholar]
- 43.Pérez-Rojas JM, Cruz C, García-López P, Sánchez-González DJ, Martínez-Martínez CM, Ceballos G, Espinosa M, Meléndez-Zajgla J, Pedraza-Chaverri J. Renoprotection by alpha-mangostin is related to the attenuation in renal oxidative/nitrosative stress induced by cisplatin nephrotoxicity. Free Radical Res. 2009;43:1122–1132. doi: 10.1080/10715760903214447. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez PY, Morales BR, García-Cuellar CM, Lopez MR, Calderon OM, Pedraza CJ, Chirino YI. The alpha mangostin prevention on cisplatin-induced apoptotic death in LLC-PK1 cells is associated to an inhibition of ROS production and p53 induction. Chem Biol Interact. 2010;188:144–150. doi: 10.1016/j.cbi.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009;47:1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Saad AA, Yousef MI, El-Shennawy LK. Cisplatin induced damag in kidney genomic DNA and nephrotoxicity in male rats: the protective effect of grape seed proanthocyanidin extract. Food Chem Toxicol. 2009;47:1499–1506. doi: 10.1016/j.fct.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 47.Jariyawat S, Kigpituck P, Suksen K, Chuncharunce A, Chaovanalikit A, Piyachaturawat P. Protection against cisplatin-induced nephrotoxicity in mice by Curcuma comosa Roxb, ethanol extract. J Nat Med. 2009;63:430–436. doi: 10.1007/s11418-009-0345-5. [DOI] [PubMed] [Google Scholar]
- 48.Iraz M, Ozerol E, Gulec M, Tasdemir S, Idiz N, Fadillioglu E, Naziroglu M, Akyol O. Protective effect of caffeic acid phenethyl ester (CAPE) administration on cisplatin-induced oxidative damage toliver in rat. Cell Biochem Funct. 2006;24:357–361. doi: 10.1002/cbf.1232. [DOI] [PubMed] [Google Scholar]
- 49.Choudhury RC, Jagdale MB. Vitamin E protection from potentiation of the cytogenetic toxicity of cisplatin in Swiss mice. J Chemother. 2002;14:397–405. doi: 10.1179/joc.2002.14.4.397. [DOI] [PubMed] [Google Scholar]
- 50.Narayana K, Verghese S, Jacob SS. I-Ascorbic acid partially protects two cycles of cisplatin chemotherapy-induced testis damage and oligo-astheno-teratospermia in a mouse model. Exp Toxicol Pathol. 2009;61:553–563. doi: 10.1016/j.etp.2008.12.001. [DOI] [PubMed] [Google Scholar]