Abstract
Background
Aldosterone may have adverse effects in the myocardium and vasculature. Treatment with an aldosterone antagonist reduces cardiovascular risk in patients with acute myocardial infarction complicated by heart failure (HF) and left ventricular systolic dysfunction. However, most patients with acute coronary syndrome do not have advanced HF. Among such patients, it is unknown whether aldosterone predicts cardiovascular risk.
Methods and Results
To address this question, we examined data from the dal‐OUTCOMES trial that compared the cholesteryl ester transfer protein inhibitor dalcetrapib with placebo, beginning 4 to 12 weeks after an index acute coronary syndrome. Patients with New York Heart Association class II (with LVEF <40%), III, or IV HF were excluded. Aldosterone was measured at randomization in 4073 patients. The primary outcome was a composite of coronary heart disease death, nonfatal myocardial infarction, stroke, hospitalization for unstable angina, or resuscitated cardiac arrest. Hospitalization for HF was a secondary endpoint. Over a median follow‐up of 37 months, the primary outcome occurred in 366 patients (9.0%), and hospitalization for HF occurred in 72 patients (1.8%). There was no association between aldosterone and either the time to first occurrence of a primary outcome (hazard ratio for doubling of aldosterone 0.92, 95% confidence interval 0.78‐1.09, P=0.34) or hospitalization for HF (hazard ratio 1.38, 95% CI 0.96‐1.99, P=0.08) in Cox regression models adjusted for covariates.
Conclusions
In patients with recent acute coronary syndrome but without advanced HF, aldosterone does not predict major cardiovascular events.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00658515.
Keywords: acute coronary syndrome, aldosterone, morbidity/mortality
Subject Categories: ACE/Angiotension Receptors/Renin Angiotensin System, Acute Coronary Syndromes
Introduction
Aldosterone is a mineralocorticoid hormone ordinarily secreted by the adrenal gland to maintain physiologic levels of sodium, potassium, and blood volume. In heart failure, secretion of aldosterone may become maladaptive, promoting excessive sodium retention and blood volume. In addition, sustained high levels of aldosterone may exert direct adverse effects on the arterial wall by promoting vascular remodeling with fibrosis and calcification1, 2, 3 and in myocardium by decreasing contractility and promoting coronary vasoconstriction4, 5 and fibrosis that may provide the substrate for arrhythmias.6, 7, 8 Acting in the central nervous system, aldosterone may stimulate neural pathways that promote hypertension and sodium retention.9, 10, 11
Plasma aldosterone concentrations are elevated in patients with acute myocardial infarction.12, 13 Prior small studies have associated elevated aldosterone at the time of acute myocardial infarction with increased short‐ and long‐term risk of adverse cardiovascular outcomes.13, 14, 15, 16 However, these studies have had 2 principal limitations. First, aldosterone levels are in flux during the early period after myocardial infarction,12 affected by changing hemodynamic, neurohumoral, and pharmacologic conditions. Therefore, measurements of aldosterone immediately following myocardial infarction may not reflect the steady‐state levels attained when patients are clinically stable and evidence‐based treatments have been implemented. Second, prior studies included patients with congestive heart failure and low ejection fraction, for whom a risk associated with elevated aldosterone and a benefit of aldosterone antagonism have been clearly established.17 However, a majority of patients with an acute coronary syndrome (ACS) do not develop heart failure. It is unknown whether aldosterone levels predict subsequent cardiovascular risk in such patients. The recent Aldosterone Lethal Effects Blockade in Acute Myocardial Infarction Treated With or Without Reperfusion to Improve Outcome and Survival at Six Months' Follow‐Up (ALBATROSS) trial failed to demonstrate a benefit of early treatment with aldosterone antagonists after ACS in patients without overt heart failure, but did not assess the relationship of aldosterone levels to outcomes or the interaction of treatment assignment and aldosterone on outcomes.18
In this report we sought to bridge a gap in evidence by examining the relationship between plasma aldosterone and the risk of cardiovascular events in a large cohort of patients who were clinically stable after recent ACS and without advanced stages of heart failure.
Methods
Study Population
The dal‐OUTCOMES trial was a randomized, double‐blind trial comparison of dalcetrapib, a cholesteryl ester transfer protein (CETP) inhibitor, with placebo in 15 871 patients with recent ACS (acute myocardial infarction or unstable angina pectoris). Trial design and principal results have been described previously.19, 20 The study was performed between 2008 and 2012 at 935 sites in 27 countries; the institutional review board of each site approved the study, and all subjects provided informed consent. Qualifying patients were at least 45 years of age, were clinically stable at the time of randomization, and had completed all planned coronary revascularization procedures. Importantly, the trial excluded patients with New York Heart Association Class III or IV symptoms of heart failure and those with Class II symptoms and left ventricular ejection fraction ≤40%; ie, patients who would ordinarily have an indication for treatment with an aldosterone antagonist were excluded. Other exclusions included uncontrolled hypertension (systolic blood pressure ≥180 mm Hg and/or diastolic blood pressure ≥110 mm Hg despite treatment) or serum creatinine >2.2 mg/dL (194.5 μmol/L). Randomization and acquisition of baseline laboratory data occurred 4 to 12 weeks (median 6 weeks) after the index ACS and when patients were deemed clinically stable.
The current post hoc analysis comprises 4073 patients who were among the first to be enrolled in dal‐OUTCOMES and had a measurement of plasma aldosterone at randomization. The analysis cohort excluded 59 patients who were treated with an aldosterone antagonist either at randomization or during the follow‐up period. Aldosterone was measured in dal‐OUTCOMES because another CETP inhibitor, torcetrapib, had been shown to raise aldosterone levels and to promote hypertension.21, 22 After the independent Data Safety and Monitoring Board determined that dalcetrapib had no effect on aldosterone concentrations, measurements were not performed on subsequent patients enrolled in the dal‐OUTCOMES trial.
Aldosterone Measurements
Aldosterone was measured in the supine position at randomization. Analysis was performed by radioimmunoassay (DSL‐8600 Active® Aldosterone kit; Diagnostic Systems Laboratory, Webster, TX) with a linear range of 69 to 4450 pmol/L, intra‐assay coefficient of variation 4% to 8%, and interassay coefficient of variation 7% to 10%.
Cardiovascular Outcomes
The primary outcome in this analysis, as in the parent clinical trial, was a composite of coronary heart disease death, nonfatal myocardial infarction, ischemic stroke, hospitalization for unstable angina, and resuscitated cardiac arrest. Prespecified secondary outcomes included all‐cause mortality, unanticipated coronary revascularization, and hospitalization for congestive heart failure. The components of the primary endpoint, all‐cause mortality and unanticipated coronary revascularization, were adjudicated by a blinded independent Event Adjudication Committee. Hospitalization for congestive heart failure was based on investigator reports. Of note, dalcetrapib treatment had no significant effect on any of these outcome measures.
Statistical Analysis
The distribution of aldosterone was displayed as a histogram and examined to determine the need for transformation to reduce the influence of skew. Demographics, medical history, and laboratory and treatment variables were evaluated in each quartile of the aldosterone distribution.
Kaplan‐Meier curves were constructed for the primary and secondary ischemic outcomes across quartiles of aldosterone in order to graphically evaluate differences in the survival curves for increasing levels of aldosterone. Cox proportional hazards regression was used for both unadjusted and adjusted inference about the association between aldosterone (measured continuously) and clinical outcomes. Hence, hazard ratios for all outcomes, along with P‐values and 95% confidence intervals, were based on the Wald statistic. A log2 transformation of aldosterone was used to reduce skew; thus, hazard ratios for each outcome measure are interpretable as the relative hazard for a doubling of baseline aldosterone concentration. Adjusted analyses included covariates for demographics, medical history, laboratory results, and treatment variables, which were chosen on the basis of a statistically significant relationship with aldosterone level or on the basis of clear clinical importance of the variable and its association with cardiovascular endpoints. Based on these criteria, models were adjusted for the following 17 variables: age, sex, race, history of hypertension, diabetes, current smoking, history of cardiovascular event (stroke, myocardial infarction, or coronary revascularization procedure) prior to the index ACS event, myocardial infarction at the index ACS event, presence or absence of New York Heart Association Class I or II symptoms of heart failure at randomization, heart rate, QRS duration, estimated glomerular filtration rate, and treatment with an angiotensin‐converting enzyme inhibitor or receptor blocker, β‐blocker, diuretic, or dalcetrapib. Results are reported as 95% confidence intervals and 2‐sided P‐value with P<0.05 considered statistically significant.
Results
The distribution of aldosterone levels at baseline is shown in Figure 1. Median aldosterone was 267 pmol/L; interquartile range was 201 to 371 pmol/L.
Figure 1.
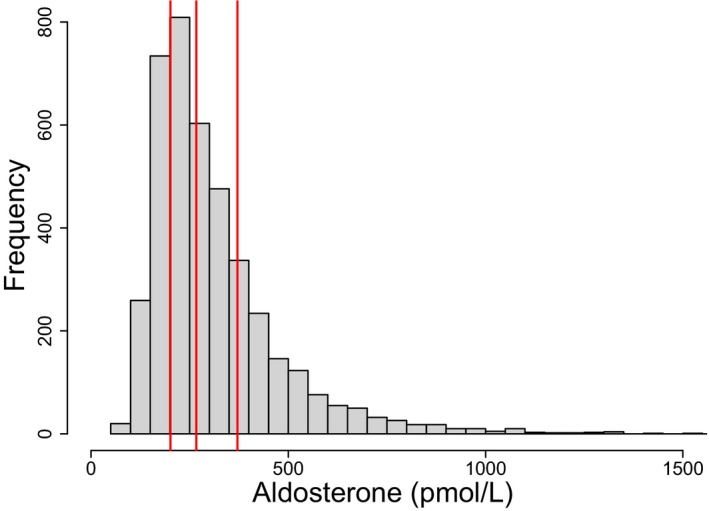
Distribution of plasma aldosterone concentrations. Measurements were made 4 to 12 weeks after an index ACS event. Median and interquartile range are indicated in red.
Table 1 shows baseline characteristics of the patients in aggregate and according to quartile of aldosterone, along with the association between each characteristic and mean aldosterone concentration. There was broad use of evidence‐based treatments for ACS, including dual antiplatelet therapy, statins, β‐blockers, and angiotensin‐converting enzyme inhibitors or receptor blockers. A large majority of patients (97.5%) were free of any symptoms of heart failure with usual activities. NYHA Class II symptoms were present in 102 patients (2.5%). Higher baseline levels of aldosterone were associated with female sex, white race, history of hypertension or diabetes, higher heart rate or QRS duration, lower estimated glomerular filtration rate, and treatment with a diuretic. Lower baseline levels of aldosterone were associated with current smoking, myocardial infarction at index ACS, and treatment with an angiotensin converting enzyme inhibitor or receptor blocker.
Table 1.
Baseline Characteristics According to Quartile of Aldosterone and Association With Aldosterone Levels
| All n=4073 | Aldosterone Quartiles | Association With Aldosterone | |||||
|---|---|---|---|---|---|---|---|
| Quartile 1 n=1029 | Quartile 2 n=1020 | Quartile 3 n=1021 | Quartile 4 n=1003 | Estimate (CI)a n=4073 | P Value | ||
| Demographics | |||||||
| Age, mean | 60.3±9.1 | 60.3±8.9 | 60.6±9.3 | 60.4±9.1 | 60.0±9.1 | 0.21 (−0.42 to 0.84) | 0.52 |
| Female, n (%) | 812 (19.9) | 193 (18.8) | 197 (19.3) | 196 (19.2) | 226 (22.5) | 17.57 (2.69‐32.46) | 0.02 |
| Race, n (%)b | |||||||
| White | 3756 (92.2) | 938 (91.2) | 939 (92.1) | 937 (91.2) | 942 (93.9) | 27.08 (7.51‐46.66) | 0.007 |
| Black | 72 (1.8) | 27 (2.6) | 18 (1.8) | 15 (1.5) | 12 (1.2) | ||
| Other | 245 (6.0) | 64 (6.2) | 63 (6.2) | 69 (6.8) | 49 (4.9) | ||
| Medical history, n (%) | |||||||
| Hypertension | 2827 (69.4) | 681 (66.2) | 720 (70.6) | 701 (68.7) | 725 (72.3) | 16.2 (4.23‐28.1) | 0.008 |
| Diabetes mellitus | 1025 (25.2) | 255 (24.8) | 243 (23.8) | 249 (24.4) | 278 (27.7) | 16.0 (1.98‐30.1) | 0.03 |
| Current smoking | 869 (21.3) | 869 (21.3) | 189 (18.5) | 189 (18.5) | 194 (19.3) | −14.6 (−27.5 to −1.65) | 0.03 |
| Stroke | 119 (2.9) | 26 (2.5) | 35 (3.4) | 27 (2.6) | 31 (3.1) | 2.81 (−29.4 to 35.1) | 0.86 |
| Myocardial infarction prior to index ACS | 649 (15.9) | 156 (15.2) | 166 (16.3) | 156 (15.3) | 171 (17.1) | 11.7 (−4.47 to 27.8) | 0.16 |
| PCI prior to index ACS event | 632 (15.5) | 149 (14.5) | 162 (15.9) | 152 (14.9) | 169 (16.9) | 12.5 (−0.19 to 29.3) | 0.14 |
| CABG prior to index ACS event | 119 (2.9) | 57 (5.5) | 57 (5.6) | 61 (6.0) | 72 (7.2) | 21.6 (−3.39 to 46.6) | 0.09 |
| Myocardial infarction at index ACS | 3522 (86.5) | 897 (87.2) | 882 (86.5) | 899 (88.1) | 844 (84.2) | −23.2 (−43.3 to −3.03) | 0.02 |
| Clinical and laboratory characteristics at randomization | |||||||
| Systolic BP, mm Hg, mean±SD | 128±17.1 | 128±16.4 | 128±16.7 | 128.5±17.7 | 128±17.5 | 0.08 (−0.25 to 0.42) | 0.63 |
| Diastolic BP, mm Hg, mean±SD | 76.8±9.6 | 76.6±9.3 | 76.6±9.1 | 77.3±10.1 | 76.7±9.8 | 0.053 (−0.54 to 0.65) | 0.86 |
| Body mass index, kg/m2, mean±SD | 28.8±5.0 | 28.6±5.2 | 28.9±4.9 | 28.5±4.9 | 29.1±5.0 | 0.59 (−0.56 to 1.74) | 0.31 |
| HF NYHA I, n (%) | 543 (13.3) | 131 (12.7) | 149 (14.6) | 133 (13.0) | 130 (13.0) | 18.9 (−0.89 to 38.7) | 0.06 |
| HF NYHA II, n (%) | 102 (2.5) | 20 (2.0) | 27 (2.7) | 28 (2.7) | 27 (2.7) | 43.5 (−9.08 to 96.0) | 0.10 |
| Heart rate | 61.3 (11.0) | 60.6 (10.6) | 60.4 (10.4) | 61.4 (11.7) | 62.9 (11.2) | 1.35 (0.83‐1.87) | <0.001 |
| QRS interval | 96.0 (16.9) | 94.5 (14.8) | 96.3 (17.1) | 95.7 (16.2) | 97.4 (19.2) | 0.81 (0.47‐1.15) | <0.001 |
| eGFR, mL/min per 1.73 m2, mean±SD | 81.7±18.5 | 85.3±18.4 | 83.0±18.2 | 81.0±18.1 | 77.5±18.5 | −1.87 (−2.18 to −1.57) | <0.001 |
| Serum potassium, mmol/L, mean±SD | 4.4±0.4 | 4.4±0.4 | 4.4±0.4 | 4.4±0.4 | 4.4±0.4 | 6.96 (−7.96 to 21.9) | 0.36 |
| Medications at randomization, n (%) | |||||||
| ACEI/ARB | 3103 (76.2) | 821 (79.8) | 773 (75.8) | 794 (77.8) | 715 (71.3) | −18.7 (−32.2 to −5.15) | 0.007 |
| Diuretics | 802 (19.7) | 131 (12.7) | 175 (17.2) | 203 (19.9) | 293 (29.2) | 94.0 (75.0‐112.9) | <0.001 |
| β‐Blockers | 3579 (87.9) | 920 (89.4) | 898 (88.0) | 895 (87.7) | 866 (89.3) | −16.4 (−34.4 to 1.52) | 0.07 |
| Randomized to dalcetrapib | 2026 (49.7) | 526 (51.1) | 508 (49.8) | 497 (48.7) | 495 (49.4) | 2.27 (−9.19 to 13.7) | 0.70 |
ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; ACS, acute coronary syndrome; BP, blood pressure; CABG, coronary artery bypass grafting; HF, congestive heart failure; CI, confidence interval; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
For categorical variables, estimate is the difference in mean aldosterone level (pmol/L) between groups. For continuous variables, the estimate is the linear regression coefficient relating a 1‐unit increase in the variable to the associated change in mean aldosterone level.
Race was dichotomized and estimate presented as white/nonwhite due to small percentages of nonwhite patients.
Patients included in this analysis were followed for a median of 37 months. During that time there were 366 patients (9.0%) with a primary outcome, 119 patients (2.9%) who died, 384 patients (9.4%) with unanticipated coronary revascularization, and 72 patients (1.8%) hospitalized for congestive heart failure. Table 2 shows the relationships between aldosterone and primary and secondary outcomes in unadjusted and adjusted models. Figure 2 depicts the time to occurrence of ischemic events or death according to quartiles of aldosterone.
Table 2.
Unadjusted and Adjusted Hazard Ratios for Primary and Secondary Outcomes
| Outcome | Hazard Ratio (CI)a | P Value |
|---|---|---|
| Primary composite endpoint | ||
| Unadjusted | 1.03 (0.88‐1.20) | 0.70 |
| Adjusted | 0.92 (0.78‐1.09) | 0.34 |
| All‐cause mortality | ||
| Unadjusted | 0.92 (0.70‐1.22) | 0.57 |
| Adjusted | 0.79 (0.58‐1.06) | 0.12 |
| Unanticipated coronary revascularization | ||
| Unadjusted | 0.93 (0.80‐1.09) | 0.38 |
| Adjusted | 0.88 (0.75‐1.04) | 0.13 |
| Heart failure hospitalization | ||
| Unadjusted | 1.62 (1.18‐2.22) | 0.003 |
| Adjusted | 1.38 (0.96‐1.99) | 0.079 |
Hazard ratio for doubling of baseline aldosterone; CI, 95% confidence interval.
Figure 2.
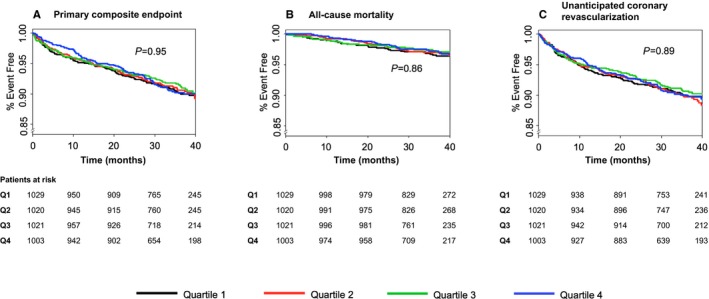
Cumulative freedom from occurrence of the following types of events, according to quartile of aldosterone at baseline. A, Primary endpoint (coronary heart disease death, nonfatal myocardial infarction, ischemic stroke, hospitalization for unstable angina, or resuscitated cardiac arrest). B, All‐cause mortality. C, Unanticipated coronary revascularization. P‐values (log‐rank analysis) indicate the likelihood that at least 1 quartile differs from the others.
Doubling of aldosterone was not associated with risk of a primary endpoint, either in univariate analysis (hazard ratio 1.03, 95% confidence interval [CI] 0.88‐1.20, P=0.70) or after adjustment for the 17 demographic, medical history, laboratory, and drug treatment variables (hazard ratio 0.92, 95% CI 0.78‐1.09, P=0.34). Similarly, there was no significant relationship between aldosterone and either total mortality or unanticipated coronary revascularization.
Because the ALBATROSS trial suggested that early aldosterone antagonism might be beneficial in patients with ST elevation myocardial infarction without heart failure,18 we performed a sensitivity analysis on the 1757 patients who presented with ST elevation myocardial infarction. Similar to the full cohort, a doubling of aldosterone was not associated with the primary composite endpoint in this subset of patients (hazard ratio 0.91, 95% CI 0.70‐1.19, P=0.48).
In univariate analysis, aldosterone was associated with increased risk of hospitalization for heart failure (hazard ratio for doubling of aldosterone concentration 1.62, 95% CI 1.18‐2.22, P=0.003, Table 2). However, after adjustment for the 17 aforementioned demographic, clinical, laboratory, and drug treatment variables, the association was no longer significant (hazard ratio 1.38, 95% CI 0.96‐1.99, P=0.08).
Discussion
Although aldosterone levels predict cardiovascular risk in patients with chronic heart failure,23 most patients with ACS do not develop heart failure. The relationship of aldosterone to prognosis of patients with recent ACS without heart failure at baseline was previously undefined. Here we determined that aldosterone levels do not predict major cardiovascular events in such patients.
The lack of association between aldosterone and recurrent cardiovascular events in this cohort might reflect the high prevalence of treatment with antagonists of other neurohormones including β‐blockers, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers. The present findings may help to explain the neutral results of the ALBATROSS trial that evaluated treatment with aldosterone antagonists beginning immediately after acute myocardial infarction and continuing for 6 months in patients without initial heart failure.18
In 3 important ways, the present analysis differs from and adds to prior studies that examined the relationship of aldosterone levels to outcomes after ACS.12, 13, 14, 24, 25, 26 First, the current analysis cohort is much larger and reflects a globally diverse population with broad application of evidence‐based treatments. Second, in this analysis aldosterone was measured in the subacute period after ACS, whereas in prior studies aldosterone had been measured at the time of presentation with ACS.13, 14, 24, 25 In the immediate period after ACS, left ventricular function, hemodynamics, manifestations of heart failure, and aldosterone levels themselves are in flux.12, 26 Moreover, many patients with ACS begin treatment with an angiotensin‐converting enzyme inhibitor or an angiotensin receptor blocker during hospitalization for ACS, and these drugs suppress aldosterone concentration.12 Thus, information derived from aldosterone levels when patients are at a physiologic and therapeutic steady state may be complementary to measurements of the hormone immediately after ACS. Third, prior analyses of aldosterone after ACS included patients with advanced heart failure and low ejection fraction13, 14 for whom a relation of aldosterone to risk27 and a benefit of treatment with an aldosterone antagonist17 have been established. In contrast, the present analysis was restricted to patients without advanced heart failure, who comprise a majority of those with ACS and in whom a potential benefit of aldosterone antagonists has not been determined.
Strengths and Limitations
Strengths of this analysis include its large sample size, substantial primary event rate, and wide distribution of aldosterone concentrations (~3‐fold difference between the centroids of the first and fourth quartiles).
Among limitations, the timing of aldosterone measurement and duration of follow‐up did not allow us to determine whether aldosterone is related to in‐hospital or early posthospital outcomes following ACS or to long‐term outcomes beyond the 37‐month median follow‐up. We did not measure other neurohormones, including renin and cortisol, which may have interacted with aldosterone to influence the occurrence of cardiovascular events.
Hospitalization for heart failure was determined from investigator reports and was not independently adjudicated. The study protocol did not require measurement of left ventricular ejection fraction, and therefore it was not possible to distinguish heart failure events with preserved ejection fraction from those with reduced ejection fraction. The relatively small number of hospitalizations for heart failure afforded limited power to detect an association with aldosterone after adjustment for covariates. The fact that aldosterone was significantly associated with the risk of hospitalization for heart failure in univariate but not adjusted analysis means that aldosterone does not add to the prediction of heart failure events after other, commonly measured clinical variables have been taken into account. However, this finding does not necessarily imply absence of a biologically important relationship between aldosterone and heart failure events because the influence of aldosterone might be underestimated in multiple regression due to correlation between aldosterone and covariates. Thus, whether long‐term treatment with an aldosterone antagonist would benefit patients with ACS without initial heart failure remains an open question, requiring further randomized clinical trials to provide an answer.
Conclusion
Among patients with ACS but without advanced stages of heart failure, aldosterone levels measured 4 to 12 weeks after the index event are unrelated to the risk of subsequent major cardiovascular events.
Sources of Funding
The dal‐OUTCOMES trial was funded by F. Hoffmann‐La Roche.
Disclosures
Dr Schwartz, through his institution, has received research support from Cerenis, The Medicines Company, Resverlogix, Roche, and Sanofi. Dr Ballantyne has received research support from Abbott Diagnostic, Amarin, Amgen, Eli Lilly, Esperion, Novartis, Pfizer, Otsuka, Regeneron, Roche Diagnostic, Sanofi‐Synthelabo, Takeda, NIH, AHA, and ADA. Dr Barter has received research support from Merck, Pfizer, and the National Health and Medical Research Council of Australia. He has ownership interest in Nil and has received honoraria from Amgen, Astra Zeneca, CSL‐Behring, Lilly, Merck, Novartis, Pfizer, and Sanofi‐Regeneron. Dr Kallend was an employee of F. Hoffmann‐La Roche at the time the dal‐OUTCOMES study was performed and data were collected. Dr Leiter has received research funding from Aegerion, Amgen, The Medicines Company, Merck, Pfizer, Regeneron, and Sanofi. Dr Leitersdorf is consulting for Novartis, Astra Zeneca, Sanofi, and Amgen. Dr Nicholls has received research support from Amgen, Anthera, Eli Lilly, Cerénis, AstraZeneca, Novartis, Resverlogix, and Sanofi‐Regeneron. He is a consultant for Amgen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Merck, Takeda, Roche, Pfizer, and Sanofi‐Regeneron. Dr Tardif has received research support from Amarin, AstraZeneca, DalCor, Eli‐Lilly, Hoffmann‐LaRoche, Merck, Pfizer, Sanofi, and Servier and honoraria from Hoffmann‐LaRoche, Pfizer, Servier, and Valeant. A patent was submitted based on the findings of genotype‐dependent results of dalcetrapib on clinical outcomes, and Dr Tardif is mentioned as an author. Dr Olsson has received research grants from Amgen, AstraZeneca, Karobio, Merck, Pfizer, Roche, and Sanofi; consultation fees from AstraZeneca, MSD, Merck, Pfizer, and Roche. Dr McMurray, through his institution, has received funding from Abbvie, Amgen, Cardiorentis, GSK, Novartis, Pfizer, Roche, and Sanofi‐Aventis. Dr Kittelson has received consulting fees for work on advisory and steering committees for Bayer Healthcare and Pfizer and for work on data and safety‐monitoring committees from Cystic Fibrosis Foundation Therapeutics, Novo Nordisk, Genentech, and BioMarin Pharmaceuticals.
(J Am Heart Assoc. 2017;6:e004119. DOI: 10.1161/JAHA.116.004119.)
Portions of these data were presented in abstract form at the American Heart Association Scientific Sessions, November 7–11, 2015, in Orlando, FL.
References
- 1. McCurley A, McGraw A, Pruthi D, Jaffe IZ. Smooth muscle cell mineralocorticoid receptors: role in vascular function and contribution to cardiovascular disease. Pflugers Arch. 2013;465:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nappi JM, Sieg A. Aldosterone and aldosterone receptor antagonists in patients with chronic heart failure. Vasc Health Risk Manag. 2011;7:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chai W, Garrelds IM, de Vries R, Batenburg WW, van Kats JP, Danser AH. Nongenomic effects of aldosterone in the human heart: interaction with angiotensin II. Hypertension. 2005;46:701–706. [DOI] [PubMed] [Google Scholar]
- 5. Fujita M, Minamino T, Asanuma H, Sanada S, Hirata A, Wakeno M, Myoishi M, Okuda H, Ogai A, Okada K, Tsukamoto O, Koyama H, Hori M, Kitakaze M. Aldosterone nongenomically worsens ischemia via protein kinase C‐dependent pathways in hypoperfused canine hearts. Hypertension. 2005;46:113–117. [DOI] [PubMed] [Google Scholar]
- 6. Dabrowski R, Szwed H. Antiarrhythmic potential of aldosterone antagonists in atrial fibrillation. Cardiol J. 2012;19:223–229. [DOI] [PubMed] [Google Scholar]
- 7. Mountantonakis S, Deo R. Biomarkers in atrial fibrillation, ventricular arrhythmias, and sudden cardiac death. Cardiovasc Ther. 2012;30:e74–e80. [DOI] [PubMed] [Google Scholar]
- 8. Swedberg K, Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Shi H, Vincent J, Pitt B; EMPHASIS‐HF Study Investigators . Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS‐HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–1603. [DOI] [PubMed] [Google Scholar]
- 9. Geerling JC, Kawata M, Loewy AD. Aldosterone‐sensitive neurons in the rat central nervous system. J Comp Neurol. 2006;494:515–527. [DOI] [PubMed] [Google Scholar]
- 10. Gomez‐Sanchez EP. Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology. 1986;118:819–823. [DOI] [PubMed] [Google Scholar]
- 11. Sakai RR, McEwen BS, Fluharty SJ, Ma LY. The amygdala: site of genomic and nongenomic arousal of aldosterone‐induced sodium intake. Kidney Int. 2000;57:1337–1345. [DOI] [PubMed] [Google Scholar]
- 12. Udell JA, Morrow DA, Braunwald E, Swedberg K, Bode C, Rifai N, Brunel PC, Prescott MF, Ren F, Hoffman EB, Scirica BM. Inhibition of the renin‐angiotensin system reduces the rise in serum aldosterone in acute coronary syndrome patients with preserved left ventricular function: observations from the AVANT GARDE‐TIMI 43 trial. Clin Chem. 2013;59:959–967. [DOI] [PubMed] [Google Scholar]
- 13. Mignano A, Pitruzzella V, Arnone G, Arnone MT, Rotolo A, Assennato P, Novo G, Corrado E, Novo S. Prognostic role of aldosterone in patients with acute coronary syndrome: short and medium term follow‐up. J Cardiovasc Med (Hagerstown). 2014;15:27–32. [DOI] [PubMed] [Google Scholar]
- 14. Beygui F, Montalescot G, Vicaut E, Rouanet S, Van Belle E, Baulac C, Degrandsart A, Dallongeville J; OPERA Investigators . Aldosterone and long‐term outcome after myocardial infarction: a substudy of the French nationwide Observatoire sur la Prise en charge hospitaliere, l'Evolution a un an et les caRacteristiques de patients presentant un infArctus du myocarde avec ou sans onde Q (OPERA) study. Am Heart J. 2009;157:680–687. [DOI] [PubMed] [Google Scholar]
- 15. Hillaert MA, Lentjes EG, Beygui F, Kemperman H, Asselbergs FW, Nathoe HM, Agostoni P, Voskuil M, Ivanes F, Jude B, Bertrand ME, Pasterkamp G, van der Graaf Y, Doevendans PA, Montalescot G, Van Belle E. Measuring and targeting aldosterone and renin in atherosclerosis—a review of clinical data. Am Heart J. 2011;162:585–596. [DOI] [PubMed] [Google Scholar]
- 16. Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautiere K, Collet JP, Beygui F, Hennache B, Ennezat PV, Juthier F, Richard F, Dallongeville J, Hillaert MA, Doevendans PA, Jude B, Bertrand M, Montalescot G, Van Belle E. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33:191–202. [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 18. Beygui F, Cayla G, Roule V, Roubille F, Delarche N, Silvain J, Van Belle E, Belle L, Galinier M, Motreff P, Cornillet L, Collet JP, Furber A, Goldstein P, Ecollan P, Legallois D, Lebon A, Rousseau H, Machecourt J, Zannad F, Vicaut E, Montalescot G; ALBATROSS Investigators . Early aldosterone blockade in acute myocardial infarction: the ALBATROSS Randomized Clinical Trial. J Am Coll Cardiol. 2016;67:1917–1927. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS; dal‐OUTCOMES Investigators . Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. [DOI] [PubMed] [Google Scholar]
- 20. Schwartz GG, Olsson AG, Ballantyne CM, Barter PJ, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Shah PK, Tardif JC, Chaitman BR, Duttlinger‐Maddux R, Mathieson J; dal‐OUTCOMES Investigators . Rationale and design of the dal‐OUTCOMES trial: efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 2009;158:896–901.e3. [DOI] [PubMed] [Google Scholar]
- 21. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez‐Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B; ILLUMINATE Investigators . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. [DOI] [PubMed] [Google Scholar]
- 22. Forrest MJ, Bloomfield D, Briscoe RJ, Brown PN, Cumiskey AM, Ehrhart J, Hershey JC, Keller WJ, Ma X, McPherson HE, Messina E, Peterson LB, Sharif‐Rodriguez W, Siegl PK, Sinclair PJ, Sparrow CP, Stevenson AS, Sun SY, Tsai C, Vargas H, Walker M III, West SH, White V, Woltmann RF. Torcetrapib‐induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br J Pharmacol. 2008;154:1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82:1730–1736. [DOI] [PubMed] [Google Scholar]
- 24. Beygui F, Collet JP, Benoliel JJ, Vignolles N, Dumaine R, Barthelemy O, Montalescot G. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST‐elevation myocardial infarction. Circulation. 2006;114:2604–2610. [DOI] [PubMed] [Google Scholar]
- 25. Palmer BR, Pilbrow AP, Frampton CM, Yandle TG, Skelton L, Nicholls MG, Richards AM. Plasma aldosterone levels during hospitalization are predictive of survival post‐myocardial infarction. Eur Heart J. 2008;29:2489–2496. [DOI] [PubMed] [Google Scholar]
- 26. Vantrimpont P, Rouleau JL, Ciampi A, Harel F, de Champlain J, Bichet D, Moye LA, Pfeffer M. Two‐year time course and significance of neurohumoral activation in the Survival and Ventricular Enlargement (SAVE) Study. Eur Heart J. 1998;19:1552–1563. [DOI] [PubMed] [Google Scholar]
- 27. Rouleau JL, Packer M, Moye L, de Champlain J, Bichet D, Klein M, Rouleau JR, Sussex B, Arnold JM, Sestier F. Prognostic value of neurohumoral activation in patients with an acute myocardial infarction: effect of captopril. J Am Coll Cardiol. 1994;24:583–591. [DOI] [PubMed] [Google Scholar]


