Abstract
Background
High‐potency statins reduce cardiovascular events after acute coronary syndromes but remain underused in clinical practice. We examined predictors of nonuse of high‐potency statins after acute coronary syndromes.
Methods and Results
The Stabilization of pLaques usIng Darapladib‐Thrombolysis in Myocardial Infarction (SOLID‐TIMI 52) trial enrolled patients after an acute coronary syndrome in 36 countries between 2009 and 2011. Statin use was strongly encouraged throughout the trial, and statin potency was at the discretion of the treating physician. A high‐potency statin was defined as ≥40 mg atorvastatin, ≥20 mg rosuvastatin, or 80 mg simvastatin daily. Predictors of nonuse of high‐potency statins were examined using logistic regression. Of the patients included (n=12 446), 11 850 (95.2%) were treated with a statin at baseline after acute coronary syndrome (median 14 days), but only 5212 (41.9%) were on a high‐potency statin. Selected patient factors associated with nonuse of high‐potency statins included age ≥75 years (odds ratio 1.39, 95% CI 1.24–1.56), female sex (odds ratio 1.11, 95% CI 1.02–1.22), renal dysfunction (odds ratio 1.17, 95% CI 1.03–1.32), and heart failure during hospital admission (odds ratio 1.43, 95% CI 1.27–1.62). At 3 months after baseline, only 49% of patients had low‐density lipoprotein cholesterol <70 mg/dL. Among the 5490 patients (59%) who were not on a high‐potency statin at 3 months, lower low‐density lipoprotein cholesterol was a predictor of nonuse of a high‐potency statin after a median of 2.3 years (odds ratio 1.15 for 10 mg/dL decrease, 95% CI 1.11–1.19).
Conclusion
Despite the widespread use of statins after acute coronary syndromes, most patients are not treated with high‐potency statins early and late after the event, including patients at the highest risk of recurrent cardiovascular events.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01000727.
Keywords: acute coronary syndrome, guideline, secondary prevention, statin therapy
Subject Categories: Secondary Prevention, Cardiovascular Disease, Risk Factors, Acute Coronary Syndromes, Lipids and Cholesterol
Introduction
Randomized trials have consistently demonstrated that administration of a high‐potency statin regimen reduces the risk of recurrent cardiovascular events in patients after an acute coronary syndrome (ACS).1, 2, 3, 4 Based on this evidence, existing ACS management guidelines recommend the use of a high‐potency statin regimen in all patients after an ACS, regardless of their baseline lipid profile.5, 6, 7 Despite these recommendations, retrospective studies have highlighted that high‐potency statins remain underutilized in secondary prevention and are not prescribed for 50% to 70% of patients following hospitalization with ACS.8, 9, 10, 11, 12, 13 It remains unclear if there are patient characteristics that may influence clinicians' decisions to administer a high‐potency statin regimen. Better understanding of these features is relevant to identifying potential barriers that can be addressed and thus lead to changes in practice that could translate to improved patient outcomes.
We examined patient characteristics associated with nonuse of a high‐potency statin regimen in a large, multinational, contemporary, randomized trial population after ACS.
Methods
The study design of the SOLID‐TIMI 52 (Stabilization of pLaques usIng Darapladib‐Thrombolysis in Myocardial Infarction 52) trial has been described previously.14 In brief, the SOLID‐TIMI 52 trial was a double‐blind, placebo‐controlled, phase III trial that enrolled 13 026 patients stabilized after an ACS and randomized to either oral darapladib (a selective lipoprotein‐associated phospholipase A2 inhibitor) or matching placebo. Patients were considered eligible for inclusion if they had been hospitalized with an ACS (ST‐segment elevation myocardial infarction [STEMI], non‐STEMI, or unstable angina pectoris) in the 30 days prior to randomization. All participants were required to have at least 1 additional predictor of cardiovascular risk, as follows: age ≥60 years, history of MI prior to the qualifying event, significant renal dysfunction (estimated glomerular filtration rate 30–59 mL/min per 1.73 m2), diabetes mellitus requiring pharmacotherapy, or polyvascular disease (including carotid or peripheral arterial disease).14 Relevant exclusion criteria included planned or completed coronary artery bypass grafting surgery for the qualifying event, known liver disease, severe renal impairment (estimated glomerular filtration rate <30 mL/min per 1.73 m2), and current New York Heart Association class III or IV heart failure.15
High‐Potency Statin Therapy
During the SOLID‐TIMI 52 trial, the use of guideline‐recommended therapies was strongly encouraged and subsequently reinforced through the distribution of performance reports that were sent to the sites. Site‐level and regional reports were sent to sites every 3 months, and patient‐level reports were sent to sites every 6 months. These reports included detailed information on the use of guideline‐recommended therapies and the low‐density lipoprotein (LDL) cholesterol levels achieved at individual sites. The former included the percentage of patients who were treated with any statin, whereas the latter included the percentage of patients who had achieved an LDL cholesterol concentration <70 or <100 mg/dL. Ultimately, the decision to treat with a statin and the selected dose were at the discretion of the treating physician.
The current analysis was restricted to patients for whom baseline data regarding the use of a high‐potency statin were available. A high‐potency statin regimen was defined as ≥40 mg atorvastatin, ≥20 mg rosuvastatin, or 80 mg simvastatin daily. As observed in past clinical trials,4 these regimens are likely to achieve >50% reduction in LDL cholesterol. Patients who were not on a statin or who were administered low‐ or moderate‐potency statin regimens were considered the comparator. The median time from hospital admission with ACS to randomization was 14 days; therefore, the majority of patients were initiated on statin therapy prior to their baseline visit. Because LDL cholesterol concentration at the baseline visit reflected, in part, patients who had been recently initiated on statin therapy, we also examined whether achieved LDL cholesterol at 3 months influenced the decision to alter the statin regimen in patients who were not being administered a high‐potency statin at that time. Prior use of a statin in the 8 weeks prior to the ACS was also captured on the case report form. The protocol and amendments were approved by the ethics committee, and all patients provided written informed consent.
Statistical Analysis
Baseline characteristics were compared using chi‐square tests for categorical variables and Wilcoxon rank sum tests for continuous variables. A logistic regression model with forward selection was used to identify independent predictors associated with nonuse of a high‐potency statin after ACS (using a P value of <0.05 for entry in the model). Variables considered for inclusion were age ≥75 years, female sex, nonwhite race, body mass index (continuous), hypertension, hyperlipidemia, diabetes mellitus, peripheral arterial disease, prior MI, statin use in the 8 weeks prior to the ACS event, elevated cardiac biomarkers (troponin or creatine kinase MB), non‐STE–ACS (versus STEMI), percutaneous coronary intervention for the index event, heart failure during ACS admission, estimated glomerular filtration rate <60 mL/min per 1.73 m2 at baseline using the Modification of Diet in Renal Disease formula, and use of a nonstatin lipid‐modifying drug at baseline. Baseline LDL cholesterol concentration was not considered for inclusion in the model because patients could have been initiated on statin therapy in the few days prior to baseline blood draw. However, achieved LDL cholesterol concentration at 3 months was examined to determine whether it was an independent predictor of use of a high‐potency statin regimen at the end‐of‐treatment visit (median 2.3 years).
Results
Of the 13 026 patients enrolled in SOLID‐TIMI 52, 12 446 patients (96%) had information regarding type and dose of statin at the baseline visit (median 14 days, interquartile range 6–23 days) (Figure 1). Of these patients, 11 850 (95.2%) were reported to be on a statin at baseline after ACS, but only 5212 (41.9%) were reported on a high‐potency statin. Among the minority of patients (n=596, 4.8%) who were not on any type of statin at the baseline visit, the primary reason reported by the investigator was known intolerance of or contraindication to statin therapy (n=243).
Figure 1.
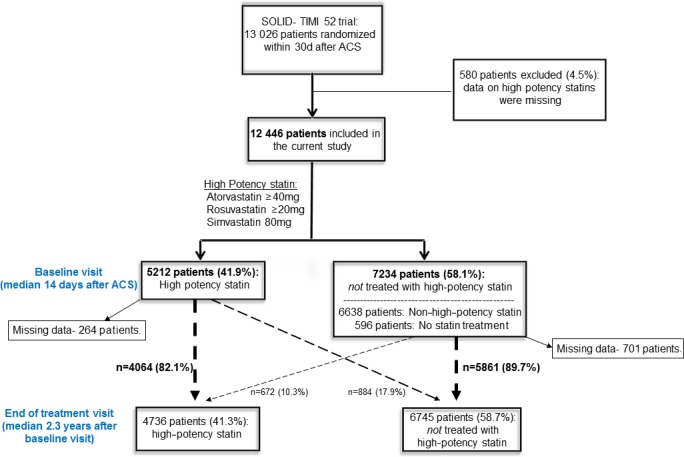
Flow diagram for patients in SOLID‐TIMI 52 included in the current analysis. ACS indicates acute coronary syndrome; SOLID‐TIMI 25, Stabilization of pLaques usIng Darapladib‐Thrombolysis in Myocardial Infarction 52.
Patients not treated with a high‐potency statin regimen at baseline were older (median age 65 versus 63 years), more likely to be female (27.5% versus 23.1%), more likely to be hospitalized with non‐STE–ACS as their qualifying event (57.0% versus 51.0%), less likely to undergo percutaneous coronary intervention for the qualifying event (71.0% versus 84.4%), and less likely to be treated with other evidence‐based therapies including aspirin (95.8% versus 97.3%), P2Y12 receptor inhibitors (84.3% versus 93.7%), and beta blockers (86.0% versus 89.1%; P<0.001 for each) (Table 1).
Table 1.
Baseline Characteristics of Patients Who Were (n=5212, 41.9%) or Were Not (n=7234, n=58.1%) on a High‐Potency Statin at Baseline (Median 14 Days After ACS) in the SOLID‐TIMI 52 Trial
| Characteristic | Total (n=12 446) | High‐Potency Statin at Baseline (n=5212) | Not on High‐Potency Statin at Baseline (n=7234) | P Value |
|---|---|---|---|---|
| Age, years, median (IQR) | 64.0 (59.0–71.0) | 63.0 (58.0–69.0) | 65.0 (60.0–71.0) | <0.001 |
| Age ≥60 years | 9243 (74.3) | 3663 (70.3) | 5580 (77.1) | <0.001 |
| Age ≥75 years | 1749 (14.1) | 583 (11.2) | 1166 (16.1) | <0.001 |
| Male | 9258 (74.4) | 4010 (76.9) | 5248 (72.5) | <0.001 |
| BMI (kg/m2), median (IQR) | 27.6 (24.8–31.0) | 27.9 (25.1–31.4) | 27.3 (24.6–30.7) | <0.001 |
| Race | <0.001 | |||
| White | 10 433 (83.8) | 4600 (88.3) | 5833 (80.6) | |
| Black | 291 (2.3) | 92 (1.8) | 199 (2.8) | |
| Asian | 1522 (12.2) | 403 (7.7) | 1119 (15.5) | |
| Other | 200 (1.6) | 117 (2.2) | 83 (1.1) | |
| Regiona | <0.001 | |||
| North America | 2635 (21.2) | 1218 (46.2) | 1417 (53.8) | |
| South America | 910 (7.3) | 327 (35.9) | 583 (64.1) | |
| Western Europe | 3495 (28.1) | 1353 (38.7) | 2142 (61.3) | |
| Eastern Europe | 3664 (29.4) | 1722 (47.0) | 1942 (53.0) | |
| Asia Pacific | 1742 (14.0) | 592 (34.0) | 1150 (66.0) | |
| Current smoker | 2366 (19.0) | 1061 (20.4) | 1305 (18.1) | 0.001 |
| Hypertension | 9103 (73.1) | 3751 (72.0) | 5352 (74.0) | 0.012 |
| Hyperlipidemia | 7936 (63.8) | 3487 (66.9) | 4449 (61.5) | <0.001 |
| Diabetes mellitus | 4256 (34.2) | 1904 (36.5) | 2352 (32.5) | <0.001 |
| Peripheral arterial disease | 1041 (8.4) | 470 (9.0) | 571 (7.9) | 0.025 |
| Prior MI | 3826 (30.7) | 1623 (31.1) | 2203 (30.5) | 0.41 |
| Prior PCI | 2915 (23.4) | 1293 (24.8) | 1622 (22.4) | 0.002 |
| Statin treatment 8 weeks prior to index event | 5300 (43.0) | 2408 (47.2) | 2892 (40.0) | <0.001 |
| Index event | ||||
| Type of event | <0.001 | |||
| Unstable angina | 1521 (12.2) | 436 (8.4) | 1085 (15.0) | |
| Non‐STEMI | 5263 (42.3) | 2224 (42.7) | 3039 (42.0) | |
| STEMI | 5662 (45.5) | 2552 (49.0) | 3110 (43.0) | |
| Treatment for index event | ||||
| Catheterization | 10 671 (85.7) | 4814 (92.4) | 5857 (81.0) | <0.001 |
| PCI prior to randomization | 9539 (76.6) | 4400 (84.4) | 5139 (71.0) | <0.001 |
| Fibrinolytic | 1146 (9.2) | 441 (8.5) | 705 (9.7) | 0.015 |
| Time from index event to randomization, days, median (IQR) | 14.0 (6.0–23.0) | 13.0 (5.0–22.0) | 15.0 (7.0–23.0) | <0.001 |
| Heart failure at admission | 1587 (12.8) | 474 (9.1) | 1113 (15.4) | <0.001 |
| Laboratories at baseline | ||||
| eGFR <60 mL/min/1.73 m2 | 1434 (11.7) | 511 (10.0) | 923 (13.0) | <0.001 |
| LDL cholesterol, mg/dL, median (IQR) | 74.9 (57.1–96.9) | 70.3 (52.5–91.1) | 78.8 (60.6–101.5) | <0.001 |
| HDL cholesterol, mg/dL, median (IQR) | 42.5 (35.9–50.2) | 40.9 (34.7–48.3) | 42.9 (36.7–51.0) | <0.001 |
| Total cholesterol, mg/dL, median (IQR) | 148.6 (125.5–176.4) | 140.9 (119.7–168.0) | 153.7 (130.9–182.2) | <0.001 |
| Triglycerides, mg/dL, median (IQR) | 133.6 (100.9–182.3) | 129.2 (97.3–176.1) | 136.3 (102.6–186.7) | <0.001 |
| Lp‐PLA2 activity, nmol/min/mL (either baseline or screening), median (IQR) | 111.6 (92.5–133.6) | 108.1 (89.8–130.0) | 114.1 (94.7–136.0) | <0.001 |
| Concomitant medical therapy at baseline | ||||
| Aspirin | 11 994 (96.4) | 5069 (97.3) | 6925 (95.8) | <0.001 |
| P2Y12 inhibitor | 10 978 (88.2) | 4884 (93.7) | 6094 (84.3) | <0.001 |
| Beta blocker | 10 861 (87.3) | 4643 (89.1) | 6218 (86.0) | <0.001 |
| ACEI or ARB | 10 284 (82.6) | 4465 (85.7) | 5819 (80.5) | <0.001 |
| Nonstatin lipid‐modifying drugb | 894 (7.2) | 411 (7.9) | 483 (6.7) | 0.01 |
Data are reported as n (%) unless otherwise specified. The percentage is for column except for region (see below). ACE I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; Lp‐PLA2, lipoprotein‐associated phospholipase A2; MI, myocardial infarction; PCI, percutaneous coronary intervention; SOLID‐TIMI 25, Stabilization of pLaques usIng Darapladib‐Thrombolysis in Myocardial Infarction 52; STEMI, ST‐segment elevation myocardial infarction.
Percentages are per each region except for the total population. Patients in Australia and New Zealand are included in the Asia Pacific category. Patients in Israel and South Africa are included in the Western Europe category.
Nonstatin lipid‐modifying drugs included bile acid sequestrants, cholesterol absorption inhibitors, fibric acid, and nicotinic acid.
Independent Predictors of Nonuse of a High‐Potency Statin Regimen
Through forward selection modeling, multiple predictors were identified that were independently associated with the nonuse of a high‐potency statin regimen at the baseline visit after ACS (Table 2). Among these predictors were age ≥75 years (odds ratio [OR] 1.39, 95% CI 1.24–1.56), female sex (OR 1.11, 95% 1.02–1.22), nonwhite race (OR 1.89, 95% CI 1.69–2.10), estimated glomerular filtration rate <60 mL/min per 1.73 m2 (OR 1.17, 95% CI 1.03–1.32), and the absence of statin therapy during the 8 weeks prior to hospitalization (OR 1.43, 95% CI 1.32–1.54). In addition, factors pertaining to the qualifying event that were associated with the nonuse of a high‐potency statin regimen included hospitalization with a non‐STE–ACS rather than STEMI as the qualifying event type (OR 1.15, 95% CI 1.06–1.24), absence of percutaneous coronary intervention for the qualifying event (OR 1.92, 95% CI 1.74–2.12), and heart failure during hospital admission (OR 1.43, 95% CI 1.27–1.62). Additional predictors are shown in Table 2. Qualitatively consistent results were observed when all analyses were repeated excluding patients taking simvastatin 80 mg daily (n=328).
Table 2.
Independent Predictors of the Nonuse of High‐Potency Statins at the Baseline Study Visit (Using a Forward Logistic Regression Model With a P Value of <0.05 for Entry Criteria in the Model)
| Variable | OR (95% CI) | P Valuea | Chi‐Square |
|---|---|---|---|
| No PCI for index event | 1.92 (1.74–2.12) | <0.001 | 277.0 |
| Nonwhite (vs white) | 1.89 (1.69–2.10) | <0.001 | 145.5 |
| No statin treatment 8 weeks prior to index date | 1.43 (1.32–1.54) | <0.001 | 64.9 |
| Age ≥75 years | 1.39 (1.24–1.56) | <0.001 | 53.1 |
| No biomarkers (troponin or CK‐MB) positive in index event | 1.47 (1.29–1.68) | <0.001 | 51.2 |
| Heart failure during hospital admission | 1.43 (1.27–1.62) | <0.001 | 31.9 |
| No diabetes mellitus | 1.21 (1.12–1.31) | <0.001 | 19.2 |
| NSTE‐ACS (vs STEMI) in index event | 1.15 (1.06–1.24) | <0.001 | 11.5 |
| No peripheral arterial disease | 1.21 (1.06–1.39) | 0.005 | 7.9 |
| eGFR <60 mL/min/1.73 m2 | 1.17 (1.03–1.32) | 0.013 | 7.0 |
| Female sex | 1.11 (1.02–1.22) | 0.017 | 5.7 |
CK‐MB indicates creatine kinase MB; eGFR, estimated glomerular filtration; NSTE‐ACS, non–ST‐elevation acute coronary syndrome; OR, odds ratio; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
The same independent predictors were identified when a threshold of P<0.10 was used for entry into the model with the addition of body mass index (OR 0.99 for 1‐U increase, 95% CI 0.99–1.00; P=0.07). Of note, identical predictors were identified when a backward selection model was used.
Long‐Term Treatment With High‐Potency Statin After ACS
After 3 months from the baseline visit (median 93 days, interquartile range 91–98 days), little change was observed in the use or nonuse of high‐potency statins since baseline. Among the 7234 patients who were not on a high‐potency statin at baseline, only 251 patients (4.4%) had been started on a high‐potency statin regimen after 3 months, and of the 5212 patients who were treated with a high‐potency statin at baseline, 445 patients (10.2%) discontinued this treatment after 3 months.
When reassessed at the end‐of‐treatment visit (median 2.3 years), of the 7234 patients who were not on a high‐potency statin regimen at baseline, 672 (10.3%) were subsequently initiated on a high‐potency statin regimen. In contrast, of the 5212 patients who were on a high‐potency statin regimen at baseline, 884 patients (17.9%) subsequently discontinued the use of a high‐potency statin by the end‐of‐treatment visit (Figure 1). Consequently, of the 12 446 patients included in this analysis, at the end‐of‐treatment visit, 11 481 had data on high‐potency statin use. Of these, 4736 (41.3%) were receiving a high‐potency statin.
Achieved LDL Concentration as a Predictor of Statin Intensification
At 3 months following the baseline visit, 7698 of 9345 patients (82%) had achieved an LDL cholesterol concentration <100 mg/dL, and 4576 patients (49%) had achieved an LDL cholesterol concentration <70 mg/dL (Figure 2, Table 3). Of note, 6273 patients (67%) had LDL <70 mg/dL or were treated with high‐potency statins, whereas the remaining 3072 patients (33%) had LDL ≥70 mg/dL and were not treated with high‐potency statins (Figure 2).
Figure 2.
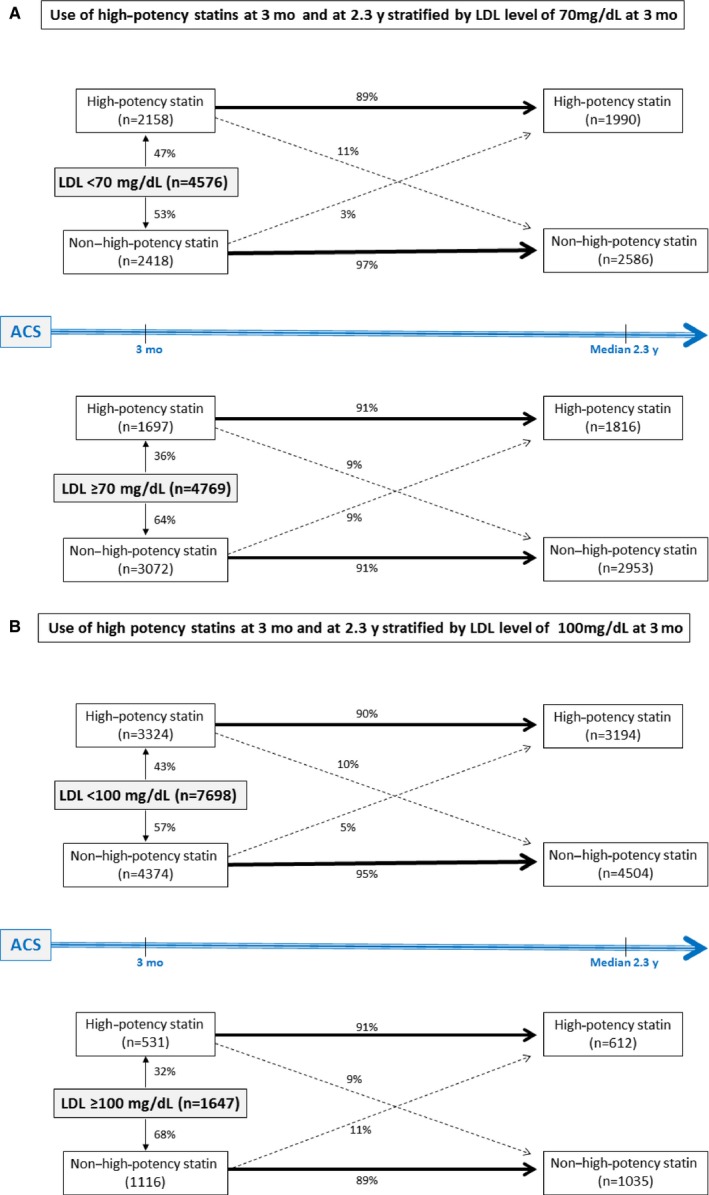
Use of high‐potency statins at the end‐of‐treatment visit stratified by LDL cholesterol concentration at the month 3 study visit. A, LDL cholesterol concentration of 70 mg/dL as the threshold. B, LDL cholesterol concentration of 100 mg/dL as the threshold. Data represent 9345 patients for whom both LDL cholesterol level/high‐potency statin status at 3 months and high‐potency statin status at the end‐of‐treatment visit were available. ACS indicates acute coronary syndrome; LDL, low‐density lipoprotein.
Table 3.
Use of High‐Potency Statins at 3 Months and at the End‐of‐Treatment Visit (Median 2.3 Years) According to LDL Cholesterol Level at 3 Months After Baseline
| LDL cholesterol level at 3 months after baseline, mg/dL | High‐Potency Statin at 3 Months After Baseline (n=3855) | Non–High‐Potency Statin at 3 Months After Baseline (n=5490) | ||
|---|---|---|---|---|
| High‐Potency Statin at End of Treatment (n=3463) | Non–High‐Potency Statin at End of Treatment (n=392) | High‐Potency Statin at End of Treatment (n=343) | Non–High‐Potency Statin at End of Treatment (n=5147) | |
| <70 (n=4576) | 1912 | 246 | 78 | 2340 |
| ≥70 (n=4769) | 1551 | 146 | 265 | 2807 |
| <100 (n=7698) | 2979 | 345 | 215 | 4159 |
| ≥100 (n=1647) | 484 | 47 | 128 | 988 |
Data represent 9345 patients for whom both LDL cholesterol level/high‐potency statin status at 3 months and high‐potency statin status at the end‐of‐treatment visit were available. LDL indicates low‐density lipoprotein.
Of the 5490 patients (59%) who were not on a high‐potency statin regimen at 3 months, a lower achieved LDL cholesterol concentration at that time was an independent predictor of nonuse of a high‐potency statin regimen at the end‐of‐treatment visit (adjusted OR 1.15 for 10‐mg/dL decrease, 95% CI 1.11–1.19, P<0.001) (Tables 4 and 5).
Table 4.
Predictors of the Nonuse of High‐Potency Statins at the End‐of‐Treatment Visit (Median 2.3 Years) Among Patients Who Were Not Treated With High‐Potency Statins at 3 Months
| Variable | OR (95% CI) |
|---|---|
| Age ≥75 years | 1.96 (1.30–2.97) |
| Nonwhite (vs white) | 3.24 (2.10–5.00) |
| No biomarkers (troponin or CK‐MB) positive in index event | 1.58 (1.07–2.34) |
| HDL‐C (10 mg/dL increase) | 0.88 (0.80–0.96) |
| LDL‐C (10 mg/dL decrease) | 1.15 (1.11–1.19) |
Variables included in the model are the same variables used in Table 2 with the addition of HDL‐C at 3 months, LDL‐C at 3 months, and triglycerides at 3 months. CK‐MB indicates creatine kinase MB; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Table 5.
Predictors of Nonuse of High‐Potency Statins at the End‐of‐Treatment Visit (Median 2.3 Years) Among Patients Who Were Treated With High‐Potency Statins at 3 Months
| Variable | OR (95% CI) |
|---|---|
| Age ≥75 years | 0.45 (0.28–0.73) |
| Nonwhite (vs white) | 2.55 (1.89–3.44) |
| No biomarkers (troponin or CK‐MB) positive in index event | 1.58 (1.09–2.30) |
| No statin treatment 8 weeks prior to index date | 1.56 (1.23–1.97) |
Variables included in the model are the same variables used in Table 2 with the addition of HDL‐C at 3 months, LDL‐C at 3 months, and triglycerides at 3 months. CK‐MB indicates creatine kinase MB.
Intensification or discontinuation of a high‐potency statin regimen from 3 months to the end‐of‐treatment visit was infrequent (Figure 2). Only 265 (9%) of the 3072 patients who did not achieve an LDL cholesterol concentration <70 mg/dL and only 128 (11%) of the 1116 patients who did not achieve an LDL cholesterol of <100 mg/dL were initiated on a high‐potency statin regimen by the end‐of‐ treatment visit (Figure 2).
Discussion
Despite the widespread use of statins after ACS, the current findings demonstrate that only a minority of such patients are treated with a high‐potency statin regimen. These observations were made shortly after the ACS, based on the treatment received during the index hospitalization or at discharge before enrolling in the trial, as well as during the trial. The SOLID‐TIMI 52 trial was a large, well‐characterized, multinational trial in which adherence to evidence‐based therapies was strongly encouraged through distribution of regular performance reports to study sites. Notably, many of the patient characteristics that were associated with failure to administer a high‐potency statin were features that, paradoxically, are often associated with higher patient risk including older age, renal dysfunction, and heart failure. In addition, both female sex and nonwhite race were associated with the absence of high‐potency statin use, even after adjusting for age and relevant comorbidities. This study highlights the need to intensify the educational process of physicians, both in hospitals and in the community, who are treating patients during and after ACS. It demonstrates that the crossover between use and nonuse of high‐potency statins over time is very low and emphasizes the importance of treatment with high‐potency statins during the initial hospitalization for ACS.
High‐potency statin regimens remain underutilized in clinical practice in patients after ACS10, 11, 12, 13; however, the reasons for this observation remain incompletely understood. Clinical trials have consistently demonstrated that a higher potency statin regimen reduces the risk of recurrent cardiovascular events compared with a low‐ or moderate‐potency regimen in patients after ACS.1, 2, 3 Moreover, both the relative efficacy and safety of this therapy have been demonstrated across a variety of patient subgroups, including women and the elderly, and regardless of baseline LDL concentration.4 Nevertheless, discussion continues about the appropriate use of statins, including high‐potency regimens, in certain patient populations. In 2013, the American College of Cardiology and American Heart Association guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults endorsed the use of a high‐potency statin regimen in high‐risk patients for secondary prevention but recommended the use of a moderate‐potency statin regimen in patients aged >75 years.5 To support this recommendation, the authors remarked on a relative paucity of data within this age group in existing randomized trials, although good evidence actually shows that the efficacy of a high‐potency statin regimen is consistent regardless of patient age.1, 2, 3 Moreover, in the past few years, a causal relationship between statin use and the development of diabetes mellitus has been suggested.16 However, enrollment in the SOLID‐ TIMI 52 trial occurred between 2009 and 2011, so neither factor would have affected statin use in the current study. Although some guidelines that were available at the time of the trial recommended the routine use of high‐potency statins in patients after ACS,7, 17, 18 other guidelines focused primarily on achieving LDL cholesterol concentrations <100 or <70 mg/dL in high‐risk patients.19, 20 We observed, however, that the use of high‐potency statins was also low in patients who had not achieved desired LDL cholesterol target goals. In the current large‐scale study of patients with ACS, multiple predictors were identified that were independently associated with the failure to use a high‐potency statin regimen. Some of these same factors were also identified in a study by Javed and colleagues in a registry population of patients admitted with ACS, including older age, female sex, renal dysfunction, and the absence of statin therapy prior to the ACS.13 Moreover, prior studies have shown that women and African American patients are less likely to receive evidence‐based therapies.8 Although research to elucidate the barriers to therapy in these patient groups is ongoing, the current findings underscore the need to better understand these observations. Notably, in the current study, patients who were not treated with a high‐potency statin regimen were also less likely to receive other evidence‐based therapies including aspirin, P2Y12 inhibitors, or beta blockers, suggesting that the same characteristics that influence high‐potency statin use may also influence the use of other therapies. However, the decreased use of other therapies was less apparent than it was for high‐potency statin use, suggesting additional factors may also be at play.
Some limitations of the current post hoc analysis warrant consideration. The study population was from a multinational randomized trial and thus was restricted to participants who met study entry criteria. Generalizability of the current findings to other study populations requires validation. Nonetheless, the current findings are notable, given that only a minority of moderate‐ to high‐risk patients received high‐potency statins despite being enrolled at sites that were carefully selected based on anticipated performance. In addition, we cannot exclude the existence of other confounding factors that may have influenced high‐potency statin use. To that end, information on patient socioeconomic status and statin cost across regions was not captured; therefore, we cannot ascertain whether patient income, insurance, or resources may have influenced clinicians' willingness to prescribe a high‐potency statin regimen. Nevertheless, because many statins were generic at the time of the trial, cost should not have played a major role in the decision‐making process. The practice of informing the sites of their patients' LDL cholesterol levels every 6 months would be expected to have increased the percentage of patients on high‐potency statins. Finally, during the course of the study, not all guidelines recommended the use of high‐potency statins and rather focused on LDL cholesterol goals; however, the use of high‐potency statins remained low in patients with LDL cholesterol >100 mg/dL.
In conclusion, despite the widespread use of statins after ACS and the demonstrated clinical benefits of high‐potency statins, most patients are not treated with high‐potency statin regimens early and late after the event, including many patients at the highest risk of recurrent cardiovascular events. Our results emphasize the need to better implement ACS guideline‐recommended therapies for patients with an indication for use.
Disclosures
Cannon discloses research grants (all >10K) from Amgen, Arisaph, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen, Merck, and Takeda and consulting fees from Alnylam, Amgen, Arisaph, Astra Zeneca, Bristol‐Myers, Bristol‐Myers Squibb, GlaxoSmithKline, Kowa, Merck, Takeda, Lipimedix*, Pfizer, Regeneron* and Sanofi* (* denotes >10K). Braunwald discloses grant support to his institution from GlaxoSmithKline, Astra Zeneca, Novartis, Duke University, Merck, and Daiichi Sankyo; uncompensated consultancies and lectures with Merck and Novartis; consultancies with The Medicines Company, Sanofi Aventis and Theravance; and personal fees for lectures from Daiichi Sankyo, Menarini International, Bayer and Medscape. Steen discloses moderate compensation from Sanofi/Regeneron. Dalby is on advisory boards for Aspen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Novartis, Sanofi, and Servier. He has received honoraria from AstraZeneca and Servier and travel sponsorships from Bayer, Boehringer‐Ingelheim, Novartis, and Sanofi. Daga is an employee and shareholder of GlaxoSmithKline. Lukas is an employee and shareholder of GlaxoSmithKline. O'Donoghue discloses grants from Eisai, GlaxoSmithKline, AstraZeneca, Merck, and Janssen. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2017;6:e004332. DOI: 10.1161/JAHA.116.004332.)
References
- 1. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM; Pravastatin or Atorvastatin Evaluation and Infection Therapy‐ Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 2. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 3. de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E; Investigators . Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. [DOI] [PubMed] [Google Scholar]
- 4. Cholesterol Treatment Trialists Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomized trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 6. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association for Clinical Chemistry . 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 7. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D; ESC Committee for Practice Guidelines . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 8. Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA. 2011;305:1882–1889. [DOI] [PubMed] [Google Scholar]
- 9. Farkouh ME, Boden WE, Bittner V, Muratov V, Hartigan P, Ogdie M, Bertolet M, Mathewkutty S, Teo K, Maron DJ, Sethi SS, Domanski M, Frye RL, Fuster V. Risk factor control for coronary artery disease secondary prevention in large randomized trials. J Am Coll Cardiol. 2013;61:1607–1615. [DOI] [PubMed] [Google Scholar]
- 10. Rosenson RS, Kent ST, Brown TM, Farkouh ME, Levitan EB, Yun H, Sharma P, Safford MM, Kilgore M, Muntner P, Bittner V. Underutilization of high‐intensity statin therapy after hospitalization for coronary heart disease. J Am Coll Cardiol. 2015;65:270–277. [DOI] [PubMed] [Google Scholar]
- 11. Albert NM, Birtcher KK, Cannon CP, Goff DC Jr, Mulgund J, Liang L, Fonarow GC. Factors associated with discharge lipid‐lowering drug prescription in patients hospitalized for coronary artery disease (from the Get With the Guidelines database). Am J Cardiol. 2008;101:1242–1246. [DOI] [PubMed] [Google Scholar]
- 12. Arnold SV, Kosiborod M, Tang F, Zhao Z, Maddox TM, McCollam PL, Birt J, Spertus JA. Patterns of statin initiation, intensification, and maximization among patients hospitalized with an acute myocardial infarction. Circulation. 2014;129:1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Javed U, Deedwania PC, Bhatt DL, Cannon CP, Dai D, Hernandez A, Peterson ED, Fonarow GC. Use of intensive lipid‐lowering therapy in patients hospitalized with acute coronary syndrome: an analysis of 65,396 hospitalizations from 344 hospitals participating in Get With the Guidelines (GWTG). Am Heart J. 2011;161:418–424. [DOI] [PubMed] [Google Scholar]
- 14. O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CP; SOLID‐TIMI 52 Investigators , Steen DL. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID‐TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–1015. [DOI] [PubMed] [Google Scholar]
- 15. O'Donoghue ML, Braunwald E, White HD, Serruys P, Steg PG, Hochman J, Maggioni AP, Bode C, Weaver D, Johnson JL, Cicconetti G, Lukas MA, Tarka E, Cannon CP. Study design and rationale for the Stabilization of pLaques usIng Darapladib‐Thrombolysis in Myocardial Infarction (SOLID‐TIMI 52) trial in patients after an acute coronary syndrome. Am Heart J. 2011;162:613–619. [DOI] [PubMed] [Google Scholar]
- 16. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Task Force for Diagnosis and Treatment of Non‐ST‐Segment Elevation Acute Coronary Syndromes of European Society of Cardiology , Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández‐Avilés F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W. Guidelines for the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. [DOI] [PubMed] [Google Scholar]
- 18. European Association for Cardiovascular Prevention & Rehabilitation , Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D; ESC Committee for Practice Guidelines (CPG) 2008‐2010 and 2010‐2012 Committees . ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 19. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction); American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association of Cardiovascular and Pulmonary Rehabilitation; Society for Academic Emergency Medicine . ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. [DOI] [PubMed] [Google Scholar]
- 20. Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC Jr; 2004 Writing Committee Members , Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST‐Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. [DOI] [PubMed] [Google Scholar]


