Abstract
Background
Optimal timing of implantation of an implantable cardioverter/defibrillator (ICD) after newly diagnosed heart failure is unclear given that late reverse remodelling may occur. We aimed to analyze left ventricular ejection fraction (LVEF) after diagnosis of an LVEF ≤35% during optimization of heart failure drug therapy.
Methods and Results
One hundred fifty‐six patients with newly diagnosed LVEF ≤35% receiving a wearable cardioverter/defibrillator (WCD) were analyzed. WCD was prescribed for 3 months until first re‐evaluation. Indications for prolongation of WCD wearing period instead of ICD implantation were: (1) LVEF at 3‐month visit 30% to 35%; (2) increase in LVEF of ≥5% compared to the last visit; and (3) nonoptimized heart failure medication. Mean LVEF was 24±7% at diagnosis and 39±11% at last follow‐up (mean, 12±10 months). Whereas 88 patients presented a primary preventive ICD indication (LVEF ≤35%) at 3‐month follow‐up, only 58 showed a persistent primary preventive ICD indication at last follow‐up. This delayed improvement in LVEF was related to nonischemic origin of cardiomyopathy, New York Heart Association functional class at baseline, heart rate, better LVEF after 3 months, and higher dosages of mineralocorticoid receptor antagonist. Twelve appropriate WCD shocks for ventricular tachycardia/ventricular fibrillation occurred in 11 patients. Two patients suffered from ventricular tachycardia/ventricular fibrillation beyond 3 months after diagnosis.
Conclusions
A relevant proportion of patients with newly diagnosed heart failure shows recovery of LVEF >35% beyond 3 months after initiation of heart failure therapy. To avoid untimely ICD implantation, prolongation of WCD period should be considered in these patients to prevent sudden cardiac death while allowing left ventricular reverse remodeling during intensified drug therapy.
Keywords: heart failure, sudden cardiac death, ventricular remodelling, wearable cardioverter/defibrillator
Subject Categories: Arrhythmias, Sudden Cardiac Death, Cardiomyopathy, Heart Failure
Introduction
Current guidelines recommend primary preventive implantable cardioverter/defibrillator (ICD) therapy in patients with symptomatic heart failure and left ventricular (LV) ejection fraction (LVEF) ≤35%, claiming ≥3 months of optimal medical therapy.1, 2 Early implantation after diagnosis does not improve prognosis in patients with ischemic cardiomyopathy (ICM) and nonischemic cardiomyopathy (NICM).3, 4, 5 However, patients with reduced LVEF following myocardial infarction (MI) carry a relevant risk for life‐threatening arrhythmias.6, 7 Moreover, even in patients already implanted with an ICD according to guidelines, LVEF may improve with consecutively reduced arrhythmic risk.8
The wearable cardioverter/defibrillator (WCD; LifeVest, ZOLL, Pittsburgh, USA) was introduced for temporary prevention of sudden cardiac death (SCD) in selected populations and provides secured time for sophisticated risk stratification.9 In the early phase after diagnosis of heart failure with reduced LVEF, titration of heart failure medication is cumbersome, but, however, crucial for optimal elicitation of LVEF recovery and therefore may take time.
The aim of the Prolongation of Reverse remOdelling period to avoid untimely ICD impLantation in newly diagnOsed heart failure usiNG the wearable cardioverter/defibrillator (PROLONG) study was to analyze evolution of LVEF after first diagnosis of a reduced LVEF ≤35% during elaborate optimization of heart failure therapy using the WCD to provide a secured prolongation of the observation period. Special consideration was given to the effects of reverse remodelling and its implications in the decision‐making process for ICD indication.
Methods
All patients being prescribed a WCD at Hannover Medical School (Hannover, Germany) between June 2012 and January 2016 were analyzed retrospectively. The study complies with the Declaration of Helsinki, and patients gave written informed consent approved by the local ethics committee. Patients with newly diagnosed LVEF ≤35% were included in the study. At discharge, patients and treating physicians/cardiologists were instructed to optimize heart failure therapy to target dosages according to current guidelines.10 WCD was prescribed for 3 months until first re‐evaluation irrespective of the underlying etiology of the newly diagnosed heart failure. Clinical workup and decision tree are shown in Figure 1.
Figure 1.
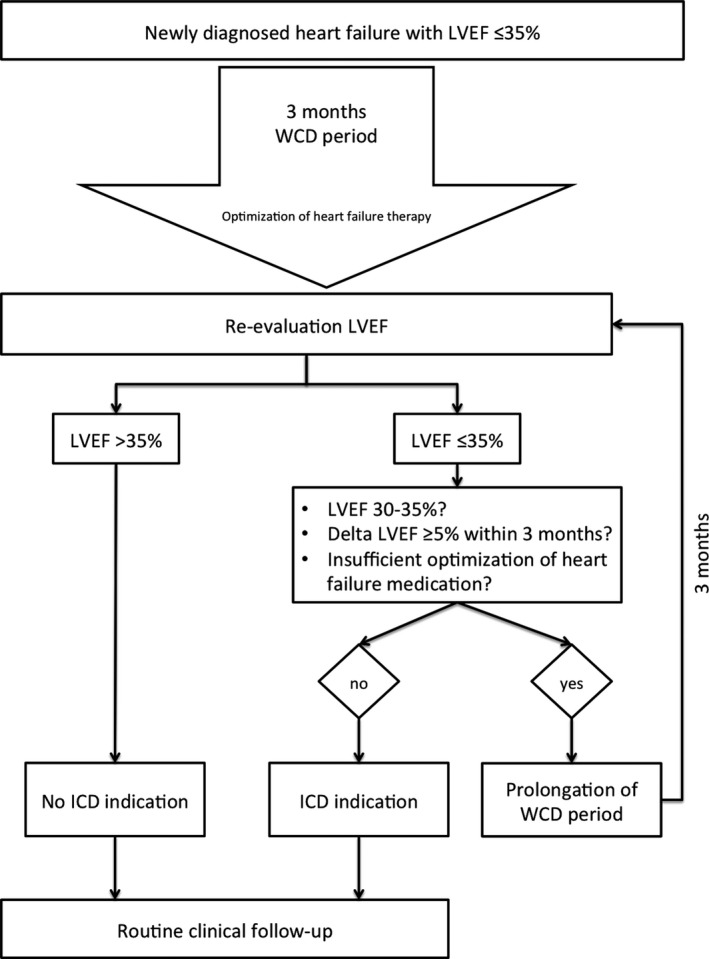
Clinical workup of patients with newly diagnosed heart failure with left ventricular function ≤35%. ICD indicates implantable cardioverter/defibrillator; LVEF, left ventricular ejection fraction; WCD, wearable cardioverter/defibrillator.
Patient characteristics were obtained at baseline. Re‐evaluation visits were scheduled for each patient 3 months after diagnosis. Only patients with complete data sets were analyzed. At each visit, echocardiograms were performed using standard echocardiographic views and LVEF was calculated using Simpson's method. Additionally, detailed medication record was obtained including dosages. Further follow‐up data were collected according to available medical records. A minimum data set, including medical history, drug therapy, and echocardiography, was required at baseline, 3‐month follow‐up, and additional follow‐ups.
Indication for ICD and timing of implantation were left to the discretion of the treating cardiologist. However, indications for prolongation of reverse remodeling period were communicated to all treating physicians. These indications included: (1) LVEF at visit 30% to 35%; (2) increase in LVEF of ≥5% compared to the last visit; and (3) nonoptimized heart failure medication. If waiting period was prolonged, patients were proposed to prolong WCD period as well, commonly for another 3 months, until re‐evaluation.
WCD data were collected via the remote monitoring platform of the manufacturer (LifeVest Network; ZOLL, Pittsburgh, PA).
Patients with recovery of LVEF >35% after 3 months were considered “early improvers,” whereas those with LVEF ≤35% after 3 months were considered “early nonimprovers.” Patients with LVEF ≤35% after 3 months but recovery during prolonged follow‐up were considered “delayed improvers,” whereas those with persistent LVEF ≤35% during long‐term follow‐up were considered “delayed nonimprovers.” Patients having received cardiac resynchronization therapy (CRT) in the course of the study were excluded from analyses of improvers versus nonimprovers because CRT is known to have an important effect on LVEF recovery. Dead patients were considered nonimprovers. Subgroups are shown in Figure 2.
Figure 2.
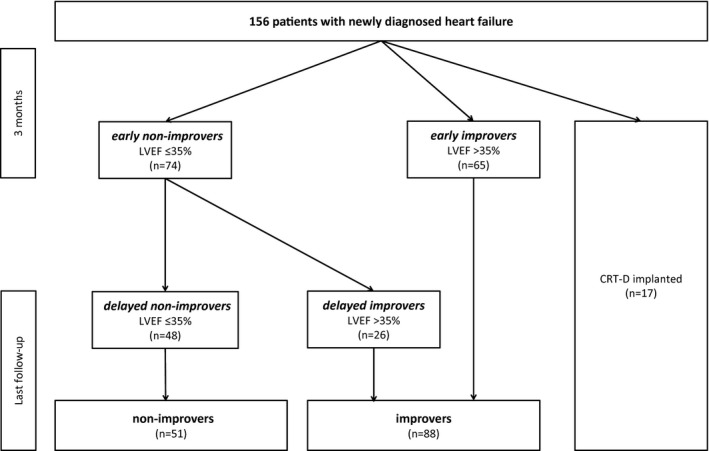
Investigational plan and outcome with respect to different subgroups. CRT‐D indicates cardiac resynchronization therapy defibrillator; LVEF, left ventricular ejection fraction.
Statistical Analysis
Data are presented as mean±SD for continuous variables or as number of cases and percentage for categorical variables. For comparison of continuous variables over time, a paired t test was performed. Categorical variables were compared using a chi‐squared test or McNemar's test, where appropriate. For analysis of longitudinal evolution, an ANOVA for repeated measures was performed. A P<0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics software (version 23; IBM Corp, Armonk, NY).
Results
Between June 2012 and January 2016, 254 patients were prescribed a WCD. One hundred sixty‐seven patients had newly diagnosed LVEF ≤35%. One hundred fifty‐six patients had complete data sets and were included in the study. Reasons for exclusion were nonexistent echocardiography, loss‐to‐follow‐up, or follow‐up in external centers without any data available.
Mean age was 54±15 years, 101 patients (65%) were male, mean N‐terminal prohormone of brain natriuretic peptide (NTproBNP) at diagnosis was 5874±8245 ng/L. Etiology of heart failure was NICM in 86 (55%), ICM in 45 (29%), peripartum cardiomyopathy (PPCM) in 19 (12%), and myocarditis in 6 (4%) patients. Patient characteristics at baseline and 3 month follow‐up are shown in Table 1. Three patients died within the first 3 months of follow‐up; 2 more died during further follow‐up. Investigational plan and outcome are shown in Figure 2. Evolution of LVEF is shown in Figures 3 and 4.
Table 1.
Baseline Characteristics and 3 Months Follow‐up Data of the Study Cohort
| Baseline (n=156) | 3 Months Follow‐up (n=153) | P Value | |
|---|---|---|---|
| NYHA | 2.8±0.7 | 2.0±0.5 | <0.001 |
| LVEF, % | 24±7 | 35±10 | <0.001 |
| LVEF ≤35%, n (%) | 167 (100) | 89 (58) | |
| Δ LVEF from baseline, % | — | 11±11 | |
| Rhythm, n (%) | 0.486 | ||
| Sinus | 136 (87) | 138 (90) | |
| AF | 19 (13) | 13 (8) | |
| Other | 1 (1) | 2 (1) | |
| Heart rate, bpm | 80±18 | 69±14 | <0.001 |
| QRS, ms | 112±26 | 115±27 | 0.081 |
| Beta‐blocker | |||
| N (%) | 149 (96) | 145 (95) | 1.000 |
| Dosage (% from target dose) | 47±26 | 63±30 | <0.001 |
| RAS antagonist | |||
| N (%) | 151 (97) | 152 (99) | 0.250 |
| Dosage (% from target dose) | 45±25 | 65±29 | <0.001 |
| MRA | |||
| N (%) | 140 (90) | 136 (89) | 0.791 |
| Dosage (% from target dose) | 49±21 | 55±27 | 0.001 |
| Diuretics, n (%) | 130 (83) | 123 (80) | 0.388 |
| Ivabradine | |||
| N (%) | 37 (24) | 31 (20) | 0.146 |
| Dosage (% from target dose) | 78±12 | 76±14 | 0.274 |
| Digitalis, n (%) | 19 (12) | 14 (9) | 0.227 |
AF indicates atrial fibrillation; bpm, beats per minute; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association functional class; RAS, renin‐angiotensin system.
Figure 3.
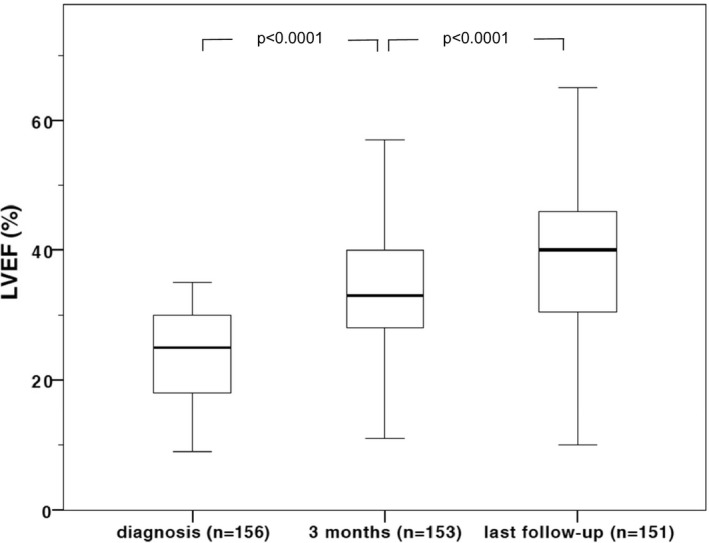
Evolution of left ventricular ejection fraction (LVEF) during follow‐up. Improvement in LVEF was highly significant (P<0.0001).
Figure 4.
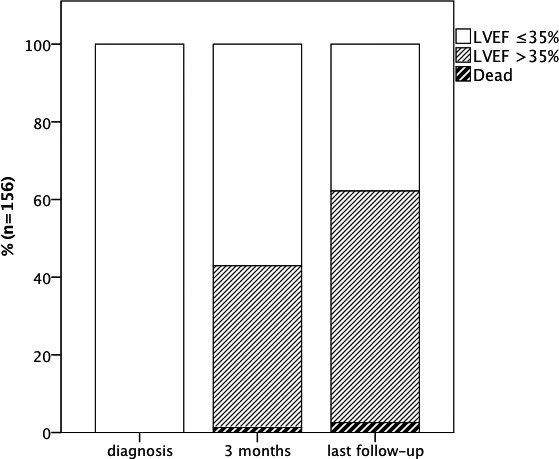
Evolution of the proportion of patients with left ventricular ejection fraction (LVEF) ≤35% versus >35% over time.
Long‐Term Follow‐up
Mean follow‐up was 12±10 (median, 9; range, 1–36) months. Mean New York Heart Association (NYHA) functional class was 1.8±0.5. Mean last LVEF was 39±11% (P<0.001 vs baseline) with a Δ LVEF of 15±12% compared to baseline and 4±7% compared to 3 months follow‐up, respectively. Five (3%) patients died during follow‐up: 3 died in terminal heart failure, 1 died in septicemia, and 1 died from intracranial hemorrhage. Three (2%) patients received a left ventricular assist device (LVAD).
Improver Versus Nonimprover
Comparison of baseline parameters for patients presenting with LVEF ≤35% (nonimprovers) versus >35% (improvers) at last‐follow‐up is presented in Table 2. Patients who received a cardiac resynchronization therapy with a defibrillator (CRT‐D; n=17) were excluded from this analysis. Significant differences in baseline parameters between improvers (n=88) and nonimprovers (n=51) at last follow‐up were only found in age and LVEF (Table 2).
Table 2.
Comparison of Baseline Parameters of Patients Presenting With LVEF ≤35% (Nonimprovers) Versus >35% (Improvers) at Last Follow‐up, Excluding Patients Having Received CRT
| Nonimprovers (n=51) | Improvers (n=88) | P Value | |
|---|---|---|---|
| Male, n (%) | 38 (75) | 53 (60) | 0.088 |
| Age, y | 58±13 | 52±16 | 0.016 |
| LVEF at baseline, % | 23±7 | 25±7 | 0.036 |
| NYHA at baseline, % | 2.8±0.7 | 2.8±0.7 | 0.507 |
| Rhythm at baseline, n (%) | 0.510 | ||
| Sinus | 44 (86) | 78 (89) | |
| AF | 6 (12) | 10 (11) | |
| Other | 1 (2) | 0 | |
| Heart rate at baseline, bpm | 83±20 | 80±17 | 0.379 |
| QRS at baseline, ms | 112±23 | 105±24 | 0.074 |
| NTproBNP at baseline, ng/L | 5666±7263 | 6374±8976 | 0.913 |
| Medication dose (% from target dose) | |||
| Beta‐blocker | 51±27 | 47±27 | 0.834 |
| RAS antagonist | 45±24 | 46±27 | 0.439 |
| MRA | 46±24 | 49±21 | 0.547 |
| Ivabradine | 79±9 | 79±14 | 0.995 |
AF indicates atrial fibrillation; bpm, beats per minute; CRT, cardiac resynchronization therapy; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NTproBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association functional class, RAS, renin‐angiotensin system.
At 3‐month follow‐up, early improvers showed significantly lower NYHA functional class (1.9±0.5 vs 2.3±0.5; P<0.001) and heart rate (66±10 vs 73±19 beats per minute [bpm]; P=0.014) compared to early nonimprovers. Furthermore, LVEF (40±9% vs 27±6%; P<0.001) and Δ LVEF (15±11% vs 4±7%; P<0.001) at 3‐month follow‐up were significantly higher in early improvers. With respect to drug therapy, a higher dosage of mineralocorticoid receptor antagonist (MRA) at 3‐month follow‐up was observed in early improvers (59±26% vs 47±22% of target dose; P=0.006). Evolution of LVEF in overall improvers and overall nonimprovers at last follow‐up is shown in Figure 5.
Figure 5.
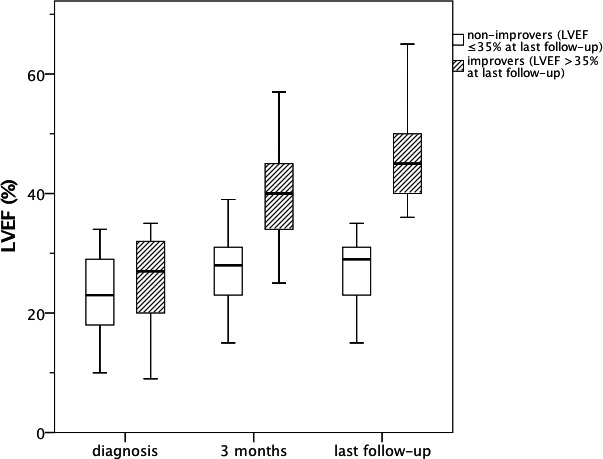
Comparison of evolution in left ventricular ejection fraction (LVEF) in overall improvers (n=88) vs overall nonimprovers (n=51).
Delayed Improvers During Prolongation of WCD Period
To further analyze factors influencing the need for prolongation of LVEF improvement, patients with a persistent LVEF ≤35% (n=74) at the time of 3‐month follow‐up were divided in 2 additional groups: “delayed nonimprovers” with LVEF ≤35% at last follow‐up (n=48) and “delayed improvers” with improvement after more than 3 months (n=26). Both groups did not show significant differences in age, sex, LVEF at diagnosis, NYHA functional class at diagnosis, heart rate at diagnosis, QRS duration at diagnosis, or NTproBNP levels at diagnosis. However, delayed nonimprovers showed more frequently ICM, whereas delayed improvers were more likely to have any NICM (delayed nonimprovers: NICM 26 [54%], ICM 21 [44%], PPCM 1 [2%], and myocarditis 0; delayed improvers: NICM 19 [73%], ICM 4 [15%], PPCM 2 [8%], and myocarditis 1 [4%]; P<0.001). LVEF at 3‐month follow‐up was 26±5% in delayed nonimprovers and 31±4% in delayed improvers, respectively (P<0.001). NYHA functional class at 3‐month follow‐up was 2.3±0.5 in delayed nonimprovers and 2.0±0.3 in delayed improvers (P=0.03). Delayed improvers showed a significantly lower heart rate at 3‐month follow‐up (75±18 vs 65±7 bpm; P=0.03) and were treated with significantly higher dosages of mineralocorticoid receptor antagonist (MRA) at 3‐month follow‐up (47±22% vs 67±24% of target dose; P<0.001).
ICD Indication
Whereas 88 patients (58% of patients alive) presented a primary preventive ICD indication with an LVEF ≤35% at 3‐month follow‐up, only 58 (38% of patients alive) showed a persistent ICD indication at last follow‐up.
At last follow‐up, 59 patients were implanted with an ICD, including 17 with a CRT‐D. ICDs were implanted for primary prevention in 48 (81%) patients and 11 (19%) for secondary prevention, respectively. Median time from diagnosis to ICD implantation was 3 months (range, 1–20; interquartile range, 2). Nine patients refused ICD implantation despite established indication.
Four patients were implanted with a primary preventive ICD (excluding CRT‐D) in external centers before 3‐month follow‐up. However, these patients improved in LVEF >35% during long‐term follow‐up.
WCD Data
In all 156 patients, a WCD was recommended. Forty‐eight patients terminated WCD wearing period before 3‐month follow‐up. Reasons for preliminary termination of WCD period were: incompliance (n=24); early LVEF improvement (n=9); WCD shock/ventricular tachycardia (VT) detected by WCD and subsequent implantation of an ICD (n=8), nonarrhythmic death (n=2 terminal heart failure; n=1 intracranial hemorrhage), skin reaction (n=2), and other intervention (n=1 aortic valve replacement; n=1 LVAD implantation). Sixty‐three patients wore the WCD for more than 3 months (>92 days).
In all patients, cumulative WCD wear time adds up to 42.7 patient‐years. Mean wear time of WCD was 101±89 days; mean wear time per day was 21.7±4.0 hours. Twelve appropriate WCD shocks for VT/VF (ventricular fibrillation) occurred in 11 patients (4 men; 4 NICM, 4 PPCM, and 3 ICM). Episodes occurred between 13 and 161 days after WCD prescription (median after 59 days). Ten VT/VF episodes occurred within 3 months after diagnosis; 2 VT/VF episodes were observed in 2 patients during prolongation period (Figure 6). No asystoles or inappropriate WCD shocks were recorded. Patients with WCD shocks did not show significant differences in baseline parameters compared to those without WCD shock.
Figure 6.
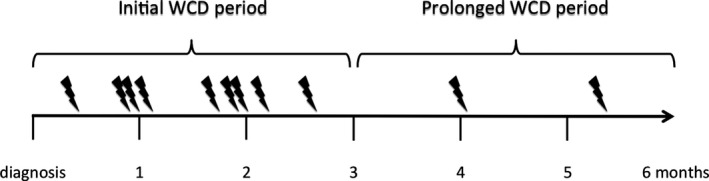
Occurrence of ventricular tachyarrhythmia episodes during early and prolonged WCD period in 11 patients. Note that patients showed life‐threatening arrhythmias in both periods.
Discussion
The major findings of the PROLONG study were: (1) Improvement of LVEF to >35% beyond a 3‐month waiting period occurred in 29 of 89 (33%) patients by prolonged optimization of heart failure therapy. (2) However, patients with LVEF ≤35% showed a relevant risk for life‐threatening ventricular tachyarrhythmias early after diagnosis as well as during prolongation of heart failure therapy optimization.
Reverse Remodeling
Intensive drug therapy, including beta‐blockers and inhibitors of the renin‐angiotensin‐aldosterone system, is one of the cornerstones of heart failure therapy, leading to a significant reduction in morbidity and mortality.2, 11 However, a relevant number of patients is undertreated with some of these drugs.12 Current guidelines recommend primary preventive ICD implantation for patients receiving optimal medical therapy for more than 3 months.1 A recent study on post‐MI patients investigated evolution of LVEF and showed that there is further improvement in LVEF beyond the initial 40 days post‐MI and confirmed the relevant risk for life‐threatening ventricular arrhythmias within the first weeks post‐MI.7 LVEF at presentation, length of hospitalization, previous MI, lateral wall motion abnormality at presentation, and peak troponin were shown to influence LVEF evolution post‐MI.13 Patients with recent diagnosis of NICM likewise showed a good recovery within 6 months after diagnosis.14 In our study including NICM as well as ICM, we could confirm these findings within 3 months, but beyond that, 33% of our patients even improved during prolongation period beyond 3 months after diagnosis, emphasizing the need for elaborate optimization of heart failure therapy. Implanting an ICD already after 3 months in these patients would have been untimely. We observed 4 patients with early ICD implantation in external centers who improved in LVEF >35% during long‐term follow‐up. These patients would have benefited from waiting for completion of reverse remodeling. Accordingly, ICD recipients may improve in LVEF after ICD implantation and subsequently show a very low arrhythmic risk.8, 15, 16 ICD implantation in these patients occurred too early and left the patients only at risk for device‐related complications. According to our data, when re‐evaluating LVEF after 3 months, we propose 3 indications for prolongation of reverse remodeling period: (1) LVEF 30% to 35%; (2) Δ LVEF ≥5%; and (3) insufficient optimization of medical dosages (especially MRA). This indicates that continuous optimization and titration of heart failure medication supports reverse remodeling beyond 3 months of therapy.
A relevant proportion of patients receiving an ICD does not meet evidence‐based criteria for implantation.17 However, patients may already be at risk for SCD during initiation and optimization of heart failure medication. On the other hand, too early ICD implantation exposes the patient to the risk of early and late complications.18, 19 A substudy of the MADIT‐II trial showed a greater benefit of primary preventive ICD implantation after remote MI.20 This might substantiate our hypothesis that ICD should be implanted in those patients having completed reverse remodeling.
Role of Medication
Titration of heart failure medication is challenging.21 Dosages of beta‐blockers, renin‐angiotensin system (RAS) antagonists and MRA were significantly higher at 3‐month follow‐up compared to hospital discharge and were comparable to a recent randomized study on optimization of heart failure medication management.22 We found that delayed improvers had significantly higher dosages of MRA than nonimprovers. That supports the importance of MRA in heart failure therapy and the necessity to reach highest possible dosages.
Some special cardiomyopathies like PPCM present with severe LVEF deterioration at diagnosis and usually recover shortly after initiation of heart failure therapy, but nevertheless patients may be at high risk for ventricular arrhythmias in the early phase of the disease.23 Furthermore, our data show that, especially, patients with any type of NICM seem to particularly benefit from a prolonged and deliberate period of optimization of heart failure medication and thereby omitting untimely ICD implantation.
Overall, we observed a relevant improvement of LVEF during optimization of heart failure medication in our cohort. Whether this improvement is a matter of dosages or rather a matter of time cannot be answered with our available data, mainly attributed to the retrospective character of this study. This point is left to upcoming prospective studies.
Value of the WCD
Current guidelines consider WCD wearing for patients with transient or unknown risk for life‐threatening arrhythmias.2, 24, 25 Arrhythmogenicity is supposed to be elevated during ventricular remodeling.26 During the time of reverse remodeling, patients in our study presented a relevant risk for SCD given that 11 (7%) suffered from VT/VF during the study and received a life‐saving WCD therapy. Of note, 2 ventricular tachyarrhythmias occurred during the prolongation period beyond 3 months after diagnosis, indicating the necessity to prolong WCD wearing to prevent SCD in this period as well.
For patients with ischemic cardiomyopathy, a relevant risk for SCD after newly diagnosed heart failure was shown.6 In contrast to this finding, in our cohort the majority of patients with life‐threatening arrhythmias were diagnosed with NICM. The recently published DANISH trial could confirm the risk of life‐threatening ventricular arrhythmias in the chronic phase of NICM. However, primary preventive ICD implantation did not reduce overall mortality.27 We were focusing on the early phase after newly diagnosed heart failure during establishment of heart failure medication, in contrast to the DANISH trial that included patients on optimal heart failure medication. Given that primary preventive ICD implantation in NICM has become debatable, WCD offers prevention from sudden arrhythmic death during careful optimization of heart failure therapy and may avoid too early or even not mortality reducing ICD implantation in NICM.
Limitations
The current study was a retrospective analysis and it is therefore subject to the known limitations attributed to this study design. Even if we established a standardized workflow for identification and treatment of patients with newly diagnosed heart failure in our department, including prescription of WCD, we cannot exclude a selection bias because our analysis only included patients having received a prescription of WCD with available complete data sets. Nevertheless, even if assuming a relevant selection bias, we still found a relevant proportion of these possibly selected patients with delayed recovery of LVEF, thereby proving the need for prolonged risk stratification. Against the background of limited financial resources in the health care system, prospective studies are needed to address the role of the WCD after newly diagnosed heart failure and to identify possible predictors of LVEF evolution and SCD.
Conclusions
After new diagnosis of heart failure with a severely reduced LVEF, an important number of patients recover after initiation of heart failure therapy. Furthermore, a relevant proportion of patients recover beyond 3 months after initiation of heart failure therapy. For patients with a LVEF of 30% to 35%, Δ LVEF ≥5%, or insufficient optimization of medication dosages, time for reverse remodeling should be prolonged beyond 3 months to avoid ICD implantation, especially in those with nonischemic cardiomyopathy. Nevertheless, according to our data, these patients are at risk during early and prolonged remodeling period and might therefore benefit from WCD during the whole period to prevent SCD.
Disclosures
Duncker received lecture honorary and travel support from ZOLL. Veltmann received lecture honorary, travel support, and a research grant from ZOLL. König, Hohmann, and Bauersachs do not report any conflicts of interest.
(J Am Heart Assoc. 2017;6:e004512. DOI: 10.1161/JAHA.116.004512.)
References
- 1. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ; Authors/Task Force Members, Document Reviewers . 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members, Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 3. Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ; DINAMIT Investigators . Prophylactic use of an implantable cardioverter‐defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. [DOI] [PubMed] [Google Scholar]
- 4. Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, Kornacewicz‐Jach Z, Sredniawa B, Lupkovics G, Hofgärtner F, Lubinski A, Rosenqvist M, Habets A, Wegscheider K, Senges J; IRIS Investigators . Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–1436. [DOI] [PubMed] [Google Scholar]
- 5. Sheppard R, Mather PJ, Alexis JD, Starling RC, Boehmer JP, Thohan V, Pauly DF, Markham DW, Zucker M, Kip KE, McNamara DM; IMAC Investigators . Implantable cardiac defibrillators and sudden death in recent onset nonischemic cardiomyopathy: results from IMAC2. J Card Fail. 2012;18:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solomon SD, Zelenkofske S, McMurray JJV, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, White H, Van de Werf F, Pieper K, Califf RM, Pfeffer MA; Valsartan in Acute Myocardial Infarction Trial (VALIANT) Investigators . Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. [DOI] [PubMed] [Google Scholar]
- 7. Sjöblom J, Muhrbeck J, Witt N, Alam M, Frykman‐Kull V. Evolution of left ventricular ejection fraction after acute myocardial infarction: implications for implantable cardioverter‐defibrillator eligibility. Circulation. 2014;130:743–748. [DOI] [PubMed] [Google Scholar]
- 8. Berthelot‐Richer M, Bonenfant F, Clavel M‐A, Farand P, Philippon F, Ayala‐Paredes F, Essadiqi B, Badra‐Verdu MG, Roux J‐F. Arrhythmic risk following recovery of left ventricular ejection fraction in patients with primary prevention ICD. Pacing Clin Electrophysiol. 2016;39:680–689. [DOI] [PubMed] [Google Scholar]
- 9. Duncker D, Veltman C. The wearable cardioverter/defibrillator—Toy or Tool? J Atr Fibrillation. 2016;8:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines , Popescu BA, McDonagh T, Sechtem U. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt S, Hürlimann D, Starck CT, Hindricks G, Lüscher TF, Ruschitzka F, Steffel J. Treatment with higher dosages of heart failure medication is associated with improved outcome following cardiac resynchronization therapy. Eur Heart J. 2014;35:1051–1060. [DOI] [PubMed] [Google Scholar]
- 12. Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence‐based heart failure therapies on mortality. Am Heart J. 2011;161:1024–1030.e3. [DOI] [PubMed] [Google Scholar]
- 13. Brooks GC, Lee BK, Rao R, Lin F, Morin DP, Zweibel SL, Buxton AE, Pletcher MJ, Vittinghoff E, Olgin JE; PREDICTS Investigators . Predicting persistent left ventricular dysfunction following myocardial infarction: the PREDICTS study. J Am Coll Cardiol. 2016;67:1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, Gorcsan J, Kip KE, Dec GW; IMAC Investigators . Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)‐2 study. J Am Coll Cardiol. 2011;58:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kini V, Soufi MK, Deo R, Epstein AE, Bala R, Riley M, Groeneveld PW, Shalaby A, Dixit S. Appropriateness of primary prevention implantable cardioverter‐defibrillators at the time of generator replacement: are indications still met? J Am Coll Cardiol. 2014;63:2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grimm W, Timmesfeld N, Efimova E. Left ventricular function improvement after prophylactic implantable cardioverter‐defibrillator implantation in patients with non‐ischaemic dilated cardiomyopathy. Europace. 2013;15:1594–1600. [DOI] [PubMed] [Google Scholar]
- 17. Al‐Khatib SM, Hellkamp A, Curtis J, Mark D, Peterson E, Sanders GD, Heidenreich PA, Hernandez AF, Curtis LH, Hammill S. Non‐evidence‐based ICD implantations in the United States. JAMA. 2011;305:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleemann T, Becker T, Doenges K, Vater M, Senges J, Schneider S, Saggau W, Weisse U, Seidl K. Annual rate of transvenous defibrillation lead defects in implantable cardioverter‐defibrillators over a period of >10 years. Circulation. 2007;115:2474–2480. [DOI] [PubMed] [Google Scholar]
- 19. van der Heijden AC, Borleffs CJW, Buiten MS, Thijssen J, van Rees JB, Cannegieter SC, Schalij MJ, van Erven L. The clinical course of patients with implantable cardioverter‐defibrillators: extended experience on clinical outcome, device replacements, and device‐related complications. Heart Rhythm. 2015;12:1169–1176. [DOI] [PubMed] [Google Scholar]
- 20. Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, Burke M, Moss AJ. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082–1084. [DOI] [PubMed] [Google Scholar]
- 21. Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L; Heart Failure Association of the ESC . Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail. 2013;15:1173–1184. [DOI] [PubMed] [Google Scholar]
- 22. Güder G, Störk S, Gelbrich G, Brenner S, Deubner N, Morbach C, Wallenborn J, Berliner D, Ertl G, Angermann CE. Nurse‐coordinated collaborative disease management improves the quality of guideline‐recommended heart failure therapy, patient‐reported outcomes, and left ventricular remodelling. Eur J Heart Fail. 2015;17:442–452. [DOI] [PubMed] [Google Scholar]
- 23. Duncker D, Haghikia A, König T, Hohmann S, Gutleben K‐J, Westenfeld R, Oswald H, Klein H, Bauersachs J, Hilfiker‐Kleiner D, Veltmann C. Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function‐value of the wearable cardioverter/defibrillator. Eur J Heart Fail. 2014;16:1331–1336. [DOI] [PubMed] [Google Scholar]
- 24. Schwab JO, Bänsch D, Israel C, Nowak B. Statement on the use of wearable cardioverter defibrillators. Kardiologe. 2015;9:165–170. [Google Scholar]
- 25. Piccini JP, Allen LA, Kudenchuk PJ, Page RL, Patel MR, Turakhia MP; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing . Wearable cardioverter‐defibrillator therapy for the prevention of sudden cardiac death: a science advisory from the American Heart Association. Circulation. 2016;133:1715–1727. [DOI] [PubMed] [Google Scholar]
- 26. Lip GYH, Heinzel FR, Gaita F, Juanatey JRG, Le Heuzey JY, Potpara T, Svendsen JH, Vos MA, Anker SD, Coats AJ, Haverkamp W, Manolis AS, Chung MK, Sanders P, Pieske B; Document Reviewers ; Gorenek B, Lane D, Boriani G, Linde C, Hindricks G, Tsutsui H, Homma S, Brownstein S, Nielsen JC, Lainscak M, Crespo‐Leiro M, Piepoli M, Seferovic P, Savelieva I. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace. 2016;18:12–36. [DOI] [PubMed] [Google Scholar]
- 27. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp‐Pedersen C, Pehrson S; DANISH Investigators . Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]


