Abstract
Background
Evidence regarding the association of lower extremity peripheral arterial disease with quality of life (QOL) is mainly from selected clinical populations or relatively small clinical cohorts. Thus, we investigated this association in community‐derived populations.
Methods and Results
Using data of 5115 participants aged 66 to 90 years from visit 5 (2011‐2013) of the Atherosclerosis Risk in Communities Study, we quantified the associations of ankle‐brachial index (ABI) with several QOL parameters, including 12‐item Short‐Form Health Survey (SF‐12), after accounting for potential confounders using linear and logistic regression models. Peripheral arterial disease defined by an ABI <0.90 (n=402), was independently associated with a low SF‐12 Physical Component Summary score (−3.26 [95% CI −5.60 to −0.92]), compared to the ABI reference 1.10 to 1.19 (n=1900) but not with the Mental Component Summary score (−0.07 [−2.21 to 2.06]). A low ABI was significantly associated with poorer status of all SF‐12 physical domains (physical functioning, role‐physical, bodily pain, and general health) but only vitality out of 4 mental domains. Similarly, low ABI values were more consistently associated with other physically related QOL parameters (leisure‐time exercise/activity/walking) than mentally related parameters (significant depressive symptoms and hopeless feeling). Lower physical QOL was observed even in individuals with borderline low ABI (0.90 to 0.99; n=426).
Conclusions
Low ABI (even borderline) was independently associated with poor QOL, especially for physical components, in community‐dwelling older adults. QOL is a critical element for older adults, and thus, further studies are warranted to assess whether peripheral arterial disease‐specific management can improve QOL in older populations.
Keywords: aging, atherosclerosis, epidemiology, peripheral vascular disease, quality of life
Subject Categories: Epidemiology, Aging, Quality and Outcomes, Peripheral Vascular Disease, Mental Health
Introduction
Peripheral arterial disease (PAD), commonly identified by an ankle‐brachial index (ABI) less than 0.9, is a growing problem.1 In the United States, PAD affects over 8 million individuals,2 with particularly high prevalence among people aged over 70 years.3, 4 Persons with PAD have 3‐ to 5‐fold higher mortality risk compared with those without PAD. Increased mortality is mainly driven by a higher cardiovascular disease (CVD) risk5, 6 and reflects the underlying systemic atherosclerosis process in PAD patients.7
PAD can also impact quality of life (QOL). As the disease progresses, patients with PAD may develop intermittent claudication and critical limb ischemia (a condition including ischemic rest pain, ulcers, or gangrene, with up to 25% risk of amputation within 1 year of diagnosis),8 leading to the reduction of health‐related QOL. Indeed, several studies have evaluated the impact of PAD on QOL measures,9, 10, 11, 12, 13, 14, 15, 16, 17, 18 but most of these studies have been conducted in small clinic‐based populations with symptomatic PAD9, 10, 11, 12, 13, 14 or in selected populations with specific cardiovascular disease risk profiles.16, 17 The impact of clinical and subclinical PAD on QOL in the general population is thus yet to be characterized.
The aim of this study was to comprehensively investigate the association of PAD—across a spectrum of ABI values—with health‐related QOL among older adults in the community using data from the Atherosclerosis Risk in Communities (ARIC) Study. We hypothesized that lower ABI value, including borderline low ABI (ABI <1.00),19 would be comprehensively associated with reduced health‐related QOL in community‐dwelling older adults. Some people (particularly as they age) may value QOL relatively more than merely survival, and PAD‐specific management (eg, supervised exercise, pharmacotherapy, and revascularization) has been shown to improve QOL in some patients.20, 21 Thus, our findings may have clinical and public health implications.
Methods
Study Population
The ARIC Study is an ongoing prospective cohort originally designed to investigate the natural history of atherosclerosis and related cardiovascular events. The cohort consisted of 15 792 participants aged 45 to 64 at baseline visit in 1987‐1989 from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. The ARIC Study conducted the fifth examination (visit 5) between June 1, 2011 and August 30, 2013, which 6538 participants aged 66 to 90 years attended. Among these visit attendees, we excluded 18 nonwhite/nonblack participants, 868 participants without ABI measurement, and 537 participants with missing information for QOL data and covariates, leaving the final population of 5115 participants for this study. A main reason for missing ABI measurement was home visit provided to participants who were unable to come to the field centers. As anticipated from this aspect, 5115 participants in this study had healthier profiles as compared to those who were excluded from this analysis (Table S1). All participants gave informed consent, and the study was approved by the institutional review board at each study site.
ABI Measurement
ABI was defined as the ratio of systolic blood pressure in the ankle to the systolic pressure in the arm. An oscillometric device, OMRON VP‐1000 plus (Kyoto, Japan), allowed blood pressure to be automatically and simultaneously measured in both ankles and arms by trained and certified technicians after at least 5 minutes of rest.22 The higher value of the right or left arm blood pressure was used as the denominator, and the ABI was calculated for both the right and left legs. The measurement was repeated after 5 minutes, and the mean ABI was recorded for each leg. The lower value of right and left ABI was used for this analysis in general.9, 23 One exception was when both ABIs were greater than 1.0 and at least 1 ABI was higher than 1.3. In this situation, to capture pathophysiological information of high ABI (indicating arterial noncompressibility),2, 24, 25, 26 we used the higher ABI value for our analysis.
Health‐Related Quality of Life
Our primary measure of health‐related QOL relied on the 12‐item Short‐Form Health Survey (SF‐12), which captures both physical and mental elements of health‐related QOL. To assess the robustness of our findings, we also evaluated physical activity and hopeless feeling by self‐report and significant depressive symptoms by use of the Epidemiologic Studies Depression Scale (CES‐D) Short Form.
The 12‐Item Short‐Form Health Survey
The SF‐12v2 questionnaire, adopted in the ARIC study, is designed to assess health‐related QOL, which has been validated across populations27 and is comparable to the original full form of SF‐36.27, 28 It reports 2 summary measurements for physical and mental health‐related QOL, the Physical Component Summary and Mental Component Summary scores. Each summary component was based on 4 domains: Physical Component Summary—physical functioning (the ability to do moderate activities or climb stairs), role‐physical (impaired working ability or daily activities due to physical issue), bodily pain (extent to which pain interferes with daily work), and general health (self‐graded health status); Mental Component Summary—mental health (whether feeling peaceful or depressed), role‐emotional (impaired working ability or daily activities due to emotional issue), social functioning (frequency of social activities interfered by physical or emotional issues), and vitality (whether feeling energetic). All information was based on the respondents’ experience during the previous 4 weeks. The method that defines the calculation of these QOL scores was published elsewhere.29 Briefly, the answer for each question was scaled based on the frequency and intensity of a certain QOL condition using a point between 1 and 5. A higher score indicates better QOL status. A raw QOL score for each domain was calculated by summing the points for relevant questions. Then, the final score for each domain ranging from 0 to 100 was calculated based on the following formula: ([actual raw score−lowest possible raw score]/possible raw score range)×100. The QOL scores used in this study were further standardized to the general US population in 1998, with a mean of 50 and a standard deviation of 10. Thus, scores above and below 50 are above and below the average in the general US population.30
Other Health‐Related QOL Variables
Leisure‐time exercise frequency, activity level compared to peers, and walking frequency were collected by questionnaire during an interview. Participants selected their leisure‐time exercise and walking frequency from 5 categories of “Never,” “Seldom,” “Sometimes,” “Often,” and “Very Often.” “Never” and “Seldom” were defined as “low” for leisure‐time exercise and walking frequency. Similarly, among the 5 categories of “Much less,” “Less,” “The same,” “More,” and “Much more” for the question on leisure‐time activity compared to their peers, “Much less” and “Less” were considered “low” leisure‐time activity.
The CES‐D Short Form is a measurement instrument of depression symptoms, with 11 items selected from 20 items that define the full CES‐D Form. This short‐form CES‐D demonstrates good validity31 and high correlation with the full CES‐D Form (Pearson r=0.95).32 With a scale between 0 and 2 (0=hardly ever or never; 1=some of the time; 2=much or most of the time) for each item, the total CES‐D score ranges between 0 and 22. The score was treated as missing if more than 1 item was missing. Significant depressive symptoms were defined as any CES‐D score ≥9.33 Information about the frequency of feeling hopeless in the past week (none, 1‐2 days, 3‐7 days) was gathered via a simple question asked during the interview. “Hopeless feeling” was defined by any self‐report of feeling hopeless at least 1 day in the previous week.
Covariates
Age, sex, and race were self‐reported. Information regarding each participant's education level (high school or lower vs college or above) was collected during the ARIC visit 1 interview. Subjective economic status was measured based on the MacArthur scale,34 a self‐estimated rank of the family financial status compared to the whole US population and ranked into 10 categories (rank score 1 [lowest] to 10 [highest]), and lower economic status was defined as having a rank score <6 (median score among study participants). The subjective economic status has demonstrated a good predictability for health status.35 Smoking and drinking status were classified as current or not. Body mass index was calculated as weight (kg) divided by the square of height (m). Total cholesterol was assessed via enzymatic methods.36 Glucose level was measured by glucose‐6‐phosphate dehydrogenase methods.37 Diabetes mellitus was defined as fasting glucose level ≥126 mg/dL (≥7 mmol/L), nonfasting glucose level ≥200 mg/dL (≥11.1 mmol/L), medication use for diabetes mellitus, or self‐reported physician diagnosis. Hypertension was defined as a systolic blood pressure (mean of the second and third measurements in sitting position) ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or use of antihypertensive medication. History of coronary heart disease (CHD) or stroke was identified by the clinical history acquired at visit 1 and study‐adjudicated CHD and stroke events between visit 1 and visit 5, respectively. History of heart failure was defined as cases adjudicated by the ARIC physician panel from 2005 onward, hospitalizations with ICD code 428.x prior to 2005, or heart failure diagnosis confirmed with the participant's physician. Lung disease was based on the self‐reported physician diagnosis of emphysema/chronic obstructive pulmonary disease, chronic bronchitis, or asthma. Estimated glomerular filtration rate was derived using the CKD‐EPI equation incorporating serum creatinine level, sex, race, and age.38 Reduced kidney function was defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2.38
Statistical Analysis
According to previous literature,19, 23, 26, 39 ABI was a priori categorized into 6 groups: <0.90, 0.90 to 0.99 (≥0.9 to <1), 1.00 to 1.09 (≥1 to <1.1), 1.10 to 1.19 (≥1.1 to <1.2), 1.20 to 1.29 (≥1.2 to <1.3), and ≥1.30. The category of 1.10 to 1.19 was used as reference because it was the most prevalent category in our study and was used as a reference in a previous meta‐analysis.23 Participants’ baseline characteristics were compared across these 6 ABI groups based on chi‐squared tests and analysis of variance (ANOVA) tests, as appropriate. We used linear regression models to evaluate continuous QOL parameters from the SF‐12 (2 summary components and 8 health‐related domains) and logistic regression models for the other QOL variables (ie, “low” leisure‐time exercise, activity, and walking, significant depressive symptoms, and hopeless feeling). To evaluate the influence of potential confounders, 3 types of models were constructed for each QOL parameter. Model 1 adjusted for demographic variables, age, sex, ethnicity, education level, and economic status. Model 2 further adjusted for CVD risk factors (current smoking/drinking status, body mass index, total cholesterol level, diabetes mellitus, and hypertension) and history of CVD (history of CHD, heart failure, and stroke). Model 3 additionally included the presence of noncardiovascular comorbidities (lung disease and reduced kidney function). Because we are particularly interested in the independent association of ABI with health‐related QOL, we primarily present results derived from Model 3. We repeated the analysis in subgroups according to sex, race, and the status of CVD history, diabetes mellitus, and reduced kidney function. In this subgroup analysis we maintained our analytic frame of 6 ABI categories for continuous QOL parameters. However, to obtain reliable estimates, we dichotomized ABI (<1.00 [low and borderline low]19 and ≥1.00 [as reference]) for dichotomous QOL parameters. Residual normality and equal variance assumptions were confirmed for the major outcome variables, the Physical/Mental Component Summary scores. Due to the ordinal nature of the measurements for each SF‐12 subdomain, the normality assumption did not necessarily hold for every subdomain. Nonetheless, given the sample size and the Central Limit Theorem, the comparison of average subdomain scores across ABI categories would be still valid.40 P<0.05 was considered statistically significant.
Results
Participant Characteristics
The mean age of the 5115 participants was 75.4 (SD 5.0) years, and 21.8% (n=1113) were black. There were 402 participants (7.9%) with a low ABI <0.90, a category considered as PAD, and 426 (8.3%) with a borderline low ABI of 0.90 to 0.99.41 As compared to participants with ABI in the reference range (1.10‐1.19), those with lower ABI values (ABI <1.10) tended to have a worse cardiovascular risk factor profile, namely increased age, lower socioeconomic status, higher prevalence of current smoking, diabetes mellitus, hypertension, history of CVD and chronic lung disease, and reduced kidney function (Table 1). Those with high ABI (≥1.30) also demonstrated a worse risk factor profile and more adverse clinical comorbidity profile with increased age, higher adiposity, higher prevalence of diabetes mellitus, and prior history of CVD compared to those with ABI in the reference range (1.10‐1.19).
Table 1.
Characteristics of the Study Population According to Categories of ABI, the ARIC Study (2011‐2013)
| Characteristics | ABI Categories (n) | |||||||
|---|---|---|---|---|---|---|---|---|
| All (5115) | <0.9 (402) | 0.90 to 0.99 (426) | 1.00 to 1.09 (1234) | 1.10 to 1.19 (1900) | 1.20 to 1.29 (694) | ≥1.3 (459) | P Valuea | |
| Age (y), mean (SD) | 75.4 (5.0) | 77.3 (5.5) | 76.0 (5.6) | 75.3 (5.1) | 75.0 (4.8) | 74.8 (4.9) | 75.5 (4.8) | <0.001 |
| Sex (Females) | 2948 (57.6%) | 214 (53.2%) | 298 (70.0%) | 878 (71.2%) | 1138 (59.9%) | 289 (41.6%) | 131 (28.5%) | <0.001 |
| Blacks | 1113 (21.8%) | 164 (40.8%) | 133 (31.2%) | 352 (28.5%) | 352 (18.5%) | 66 (9.5%) | 46 (10.0%) | <0.001 |
| Educationb | 2819 (55.1%) | 258 (64.2%) | 264 (62.0%) | 732 (59.3%) | 1029 (54.2%) | 339 (48.8%) | 197 (42.9%) | <0.001 |
| Economic statusb | 2367 (46.3%) | 232 (57.7%) | 229 (53.8%) | 642 (52.0%) | 828 (43.6%) | 267 (38.5%) | 169 (36.8%) | <0.001 |
| Current smoker | 297 (5.8%) | 56 (13.9%) | 53 (12.4%) | 77 (6.2%) | 80 (4.2%) | 16 (2.3%) | 15 (3.3%) | <0.001 |
| Current drinker | 2559 (50.8%) | 147 (36.6%) | 179 (42.0%) | 561 (45.5%) | 1040 (54.7%) | 410 (59.1%) | 262 (57.1%) | <0.001 |
| Body mass index (kg/m2), mean (SD) | 28.6 (5.5) | 28.9 (5.9) | 29.5 (6.6) | 29.0 (5.7) | 28.2 (5.1) | 28.0 (4.7) | 29.1 (5.6) | <0.001 |
| Total cholesterol (mmol/L), mean (SD) | 4.7 (1.1) | 4.6 (1.1) | 4.8 (1.1) | 4.8 (1.1) | 4.7 (1.1) | 4.6 (1.0) | 4.4 (1.0) | <0.001 |
| Diabetes mellitus | 1606 (31.4%) | 173 (43.0%) | 142 (33.3%) | 388 (31.4%) | 546 (28.7%) | 205 (29.5%) | 152 (33.1%) | <0.001 |
| Hypertension | 3774 (73.8%) | 347 (86.3%) | 338 (79.3%) | 953 (77.2%) | 1343 (70.7%) | 481 (69.3%) | 312 (68.0%) | <0.001 |
| CHD history | 734 (14.3%) | 115 (28.6%) | 72 (16.9%) | 153 (12.4%) | 229 (12.1%) | 88 (12.7%) | 77 (16.8%) | <0.001 |
| Heart failure history | 239 (4.7%) | 58 (14.4%) | 28 (6.6%) | 56 (4.5%) | 59 (3.1%) | 16 (2.3%) | 22 (4.8%) | <0.001 |
| Stroke history | 174 (3.4%) | 29 (7.2%) | 26 (6.1%) | 36 (2.9%) | 50 (2.6%) | 18 (2.6%) | 15 (3.3%) | <0.001 |
| Lung disease | 566 (11.1%) | 72 (17.9%) | 64 (15.0%) | 155 (12.6%) | 185 (9.7%) | 54 (7.8%) | 36 (7.8%) | <0.001 |
| Kidney functionb | 1424 (27.8%) | 176 (43.8%) | 160 (37.6%) | 351 (28.4%) | 463 (24.4%) | 156 (22.5%) | 118 (25.7%) | <0.001 |
ABI indicates ankle‐brachial index; CHD, coronary heart disease.
P‐value calculated by analysis of variance (ANOVA) test for continuous variables and by chi‐squared test for categorical variables.
Showing the prevalence of lower level, ie, lower education level, lower economic status, and reduced kidney function.
The 12‐Item Short‐Form Health Survey
In the study population the mean scores for the Physical and Mental Component Summary were 46.8 (SD 9.9) and 55.5 (SD 7.5), respectively. Compared to the reference of ABI 1.10 to 1.19, individuals with ABI <1.00 demonstrated a significantly lower Physical Component Summary score (−3.26 [95% CI −5.60 to −0.92] for ABI <0.90 and −2.23 [95% CI −4.07 to −0.39] for ABI 0.90‐0.99) in Model 3 (Figure). This decrement by lower ABI was not significant for the Mental Component Summary score.
Figure 1.
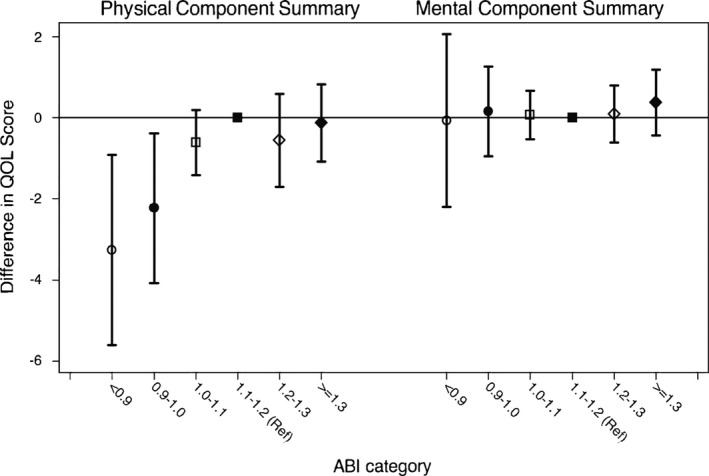
The adjusted difference and 95% CI in quality of life (QOL) scores according to ankle‐brachial index (ABI) categories (Physical/Mental Component Summary from SF‐12). Physical Component Summary and Mental Component Summary scores were adjusted for age, sex, ethnicity, education level, economic status, current smoking/drinking status, body mass index, total cholesterol level, diabetes mellitus, hypertension, history of coronary heart disease, heart failure, and stroke, lung disease, and reduced kidney function.
When we looked at each domain separately, ABI <0.90 was significantly associated with lower scores in all physical component domains, compared to the reference, in Model 3. This was most apparent for the physical functioning score (−3.42 [95% CI −5.50 to −1.35]), followed by the role physical, general health, and bodily pain scores (Table 2 and Table S2). Individuals with ABI 0.90 to 0.99 had lower scores for all physical domains compared to the reference group as well, but the difference reached significance only for the “role‐physical” domain (−1.87 [95% CI −3.58 to −0.16]) and bodily pain domain (−1.59 [95% CI −3.13 to −0.05]). As anticipated, a stronger association was observed between lower ABI values and reduced physical domains in Models 1 and 2 (Tables S3 and S4).
Table 2.
Fully Adjusteda Difference in Quality of Life Scores According to ABI Categories
| QOL Domains | ABI Categories (n) | |||||
|---|---|---|---|---|---|---|
| <0.90 (402) | 0.90 to 0.99 (426) | 1.00 to 1.09 (1234) | 1.10 to 1.19 (1900) | 1.20 to 1.29 (694) | ≥1.30 (459) | |
| Coefficientb (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | Reference | Coefficient (95% CI) | Coefficient (95% CI) | |
| Physical components | −3.26 (−5.60 to −0.92)c | −2.23 (−4.07 to −0.39)c | −0.62 (−1.42 to 0.19) | 0 | −0.56 (−1.71 to 0.59) | −0.13 (−1.09 to 0.82) |
| Physical functioning | −3.42 (−5.50 to −1.35)c | −1.71 (−3.94 to 0.52) | −0.68 (−1.58 to 0.22) | 0 | −0.21 (−1.61 to 1.18) | −0.43 (−1.58 to 0.71) |
| Role physical | −2.76 (−4.98 to −0.55)c | −1.87 (−3.58 to −0.16)c | −0.90 (−2.25 to 0.45) | 0 | −0.45 (−1.48 to 0.58) | −0.01 (−1.64 to 1.62) |
| Bodily pain | −1.99 (−3.77 to −0.22)c | −1.59 (−3.13 to −0.05)c | −0.15 (−0.81 to 0.52) | 0 | −1.01 (−2.48 to 0.45) | −0.03 (−1.42 to 1.35) |
| General health | −2.31 (−3.52 to −1.09)c | −1.55 (−3.30 to 0.20) | −0.16 (−1.36 to 1.04) | 0 | 0.00 (−1.16 to 1.16) | 0.50 (−0.63 to 1.63) |
| Mental components | −0.07 (−2.21 to 2.06) | 0.15 (−0.96 to 1.26) | 0.06 (−0.54 to 0.66) | 0 | 0.09 (−0.62 to 0.79) | 0.37 (−0.44 to 1.19) |
| Mental health | −0.62 (−2.47 to 1.23) | 0.01 (−0.51 to 0.53) | 0.29 (−0.59 to 1.17) | 0 | 0.04 (−1.15 to 1.23) | 0.47 (−0.64 to 1.57) |
| Role emotional | −1.41 (−2.98 to 0.16) | −0.42 (−1.49 to 0.64) | −0.74 (−1.49 to 0.00) | 0 | −0.13 (−0.91 to 0.65) | −0.17 (−0.97 to 0.64) |
| Social functioning | −0.58 (−2.07 to 0.92) | −0.54 (−1.99 to 0.92) | 0.02 (−0.42 to 0.46) | 0 | −0.02 (−1.23 to 1.19) | 0.09 (−1.27 to 1.45) |
| Vitality | −1.54 (−2.25 to −0.83)c | −1.38 (−1.98 to −0.78)c | −0.18 (−0.49 to 0.13) | 0 | −0.31 (−0.88 to 0.26) | 0.42 (−0.03 to 0.88) |
ABI indicates ankle‐brachial index; QOL, quality of life.
Adjusted for age, sex, ethnicity, education level, economic status, current smoking/drinking status, body mass index, total cholesterol, diabetes mellitus, hypertension, history of coronary heart disease (CHD), heart failure, and stroke, lung disease, and reduced kidney function.
Linear regression coefficient. It represents the difference in QOL score between a given ABI category and the reference category (1.10‐1.19).
Indicates significant results.
Compared to physical components, the association with mental domains was weaker (Table 2). One exception was vitality score, which was significantly lower in ABI categories <1.00 compared to the reference (−1.54 [95% CI −2.25 to −0.83] for ABI <0.9 and −1.38 [95% CI −1.98 to −0.78] for ABI 0.90‐0.99). Similar results were observed in Models 1 and 2 except for the presence of a significant association between lower ABI and lower role emotional score as well as a more pronounced ABI‐vitality association (Tables S3 and S4). High ABI ≥1.30 did not demonstrate significantly lower score in any of the physical and mental domains.
The Other QOL Parameters
We observed dose‐response relationships between ABI and the other physical activity QOL parameters (Table 3). In line with the results for physical components in SF‐12, low ABI <0.90 was significantly associated with these 3 other physical activity parameters (OR 1.34 [95% CI 1.09‐1.64] for “low” leisure‐time exercise, 1.35 [95% CI 1.21‐1.50] for “low” leisure‐time activity, and 1.43 [95% CI 1.25‐1.63] for “low” leisure‐time walk) compared to the reference ABI group. The significantly lower leisure‐time activity level and walking frequency were also observed in borderline low ABI 0.90 to 0.99. Of note, low normal ABI 1.00 to 1.09 was also significantly associated with these 3 parameters of physical activity (OR 1.21 [95% CI 1.09‐1.35] for “low” leisure‐time exercise, 1.15 [95% CI 1.04‐1.26] for “low” leisure‐time activity, and 1.24 [95% CI 1.11‐1.39] for “low” leisure‐time walk). Again, stronger associations were observed in Models 1 and 2 (Tables S5 and S6).
Table 3.
Fully Adjusteda Odds Ratio of Other Quality of Life Aspects According to ABI Categories
| Other QOL Aspects | ABI Categories (n) | |||||
|---|---|---|---|---|---|---|
| <0.90 | 0.90 to 0.99 (426) | 1.00 to 1.09 (1234) | 1.10 to 1.19 (1900) | 1.20 to 1.29 (694) | ≥1.30 (459) | |
| No. Low QOL/Total ORb (95% CI) | No. Low QOL/Total OR (95% CI) | No. Low QOL/Total OR (95% CI) | Reference | No. Low QOL/Total OR (95% CI) | No. Low QOL/Total OR (95% CI) | |
| Physical activity | ||||||
| “Low” leisure‐time exercise |
230/402 1.34 (1.09‐1.64)c |
248/426 1.38 (0.98‐1.93) |
656/1234 1.21 (1.09‐1.35)c |
859/1900 1 |
299/694 1.01 (0.79‐1.30) |
191/459 0.95 (0.68‐1.32) |
| “Low” leisure‐time activity |
101/402 1.35 (1.21‐1.50)c |
103/426 1.26 (1.13‐1.40)c |
253/1234 1.15 (1.04‐1.26)c |
304/1900 1 |
80/694 0.74 (0.62‐0.88)c |
59/459 0.76 (0.60‐0.98)c |
| “Low” leisure‐time walk |
158/402 1.43 (1.25‐1.63)c |
162/426 1.38 (1.08‐1.76)c |
413/1234 1.24 (1.11‐1.39)c |
501/1900 1 |
163/694 0.94 (0.77‐1.14) |
110/459 0.93 (0.80‐1.08) |
| Mental health | ||||||
| Significant depressive symptoms |
46/402 1.51 (0.87‐2.62) |
35/426 1.19 (0.79‐1.81) |
91/1234 1.22 (0.98‐1.52) |
95/1900 1 |
24/694 0.79 (0.46‐1.34) |
19/459 0.91 (0.62‐1.32) |
| Feel hopeless |
55/402 1.13 (0.97‐1.31) |
56/426 1.20 (1.07‐1.33)c |
159/1234 1.32 (0.96‐1.80) |
165/1900 1 |
50/694 0.91 (0.63‐1.32) |
35/459 0.94 (0.53‐1.66) |
ABI indicates ankle‐brachial index; QOL, quality of life.
Adjusted for age, sex, ethnicity, education level, economic status, current smoking/drinking status, body mass index, total cholesterol, diabetes mellitus, hypertension, history of coronary heart disease (CHD), heart failure, and stroke, lung disease, and reduced kidney function.
Logistic regression models were applied to assess the 5 binary other QOL aspects.
Indicates significant results.
The other mental parameters, significant depressive symptoms and hopeless feeling, demonstrated weaker independent associations with lower ABI compared to the other physical activity parameters (Table 3). Although significant depressive symptoms tended to associate with lower ABI, none of the odds ratios was significant. The odds of hopeless feeling were significantly higher only in borderline low ABI 0.90 to 0.99 compared to the reference ABI group. However, these mental parameters were consistently associated with low ABI categories in demographically adjusted Model 1 (Table S5).
Subgroup Analysis
The associations between ABI and physical components of QOL were also observed in most subgroups tested (Table S7 for SF‐12 physical summary score and Table S8 for the other physical QOL parameters). Notably, the associations between lower ABI and poor physical QOL components were consistently stronger in participants without a history of CVD than in those with history of CVD (Tables S7 and S8).
Reflecting overall weak association, we did not observe consistently lower mental QOL components in any subgroups for SF‐12 mental summary score (Table S9). However, of interest, for the other mental QOL parameters, lower ABI (<1.00), compared to ABI ≥1.00, was significantly associated with higher odds of significant depressive symptoms and hopeless feeling in whites, whereas lower ABI was significantly related to lower odds of those mental conditions in blacks (Table S10).
Discussion
This study quantified the association between ABI and several domains of QOL among community‐dwelling older adults from 4 US communities. With ABI 1.10 to 1.19 as reference group, low ABI (<0.90) and borderline low ABI (0.90‐0.99) were associated with poorer QOL status, as defined by a number of subdomains. The association with lower ABI was more evident for physical components of QOL than for mental components in SF‐12. The association of ABI with physical components of QOL was confirmed by analyses of leisure‐time exercise, activity, and walking. A lower ABI was also significantly associated with a mental domain in SF‐12, ie, vitality, which may be a psychological basis to be engaged in physical activity. Poor QOL status for physical health components was observed even among those participants who were without any other clinically recognized CVD (ie, CHD, stroke, and heart failure) or clinical conditions tightly related to PAD (eg, reduced kidney function).
The more evident association of low ABI with physical components of QOL status compared to mental components of QOL is consistent with previous studies performed in clinical populations.11, 16, 17, 42 Of note, our study is the first to test the full spectrum of ABI in this context among community‐dwelling older adults not selected for clinical conditions. It is of importance that even borderline low ABI was associated with reduced physical QOL. There are several potential mechanisms linking PAD to the impairments for physical components of QOL. Individuals with low ABI often have other manifestations of CVD and comorbidities, which may lead to poor physical condition and reduce QOL. However, the association of ABI with physical domains of QOL was independent of major comorbidities. These relationships could certainly be hypothesized to have potential PAD‐specific mechanisms, such as decreased blood flow to the legs, which can induce clinically significant skeletal muscle atrophy and denervation through ongoing ischemia.43 Exertional leg pain or discomfort (whether due to classic claudication or atypical leg pain) may additionally contribute to these lower physical ability QOL scores. Importantly, fear of falling was reported among those with intermittent claudication44 and could further impact and limit their physical activity.
Low ABI was significantly associated with lower vitality, a mental measure of QOL. The data from this ARIC analysis are concordant with those of a previous study that demonstrated a stronger association of low ABI values with diminished vitality than with other mental domains.17 We also observed significant associations with significant depressive symptoms and hopeless feeling in some low‐ABI categories, particularly in Models 1 and 2. Although the exact mechanisms for the association between PAD and mental elements of QOL represented by vitality are not clear, limited physical activity as well as impaired working ability due to PAD may impact mental QOL domains. In addition, resting or exertional leg symptoms or fear of falling due to PAD, mentioned above, may further contribute to reduced mental elements of QOL.
Although a weaker association was seen among individuals with a history of CVD than those without, the contribution of low ABI to physical QOL parameters was qualitatively consistent across all subgroups tested in our study. The weaker association among those with a history of CVD may be due to the fact that many participants with prior CVD had already reduced QOL, especially for physical components, and low ABI may not considerably contribute to further discrimination beyond other CVDs. Indeed, the mean Physical Component Summary score was lower among participants with a history of CVD compared to those without CVD (43.6 versus 47.5). Regarding mental elements, we observed a significant qualitative racial interaction for low ABI and significant depressive symptoms/hopeless feeling, with positive associations in whites but negative associations in blacks. The potential background for this interaction is not clear. Nonetheless, this subgroup analysis should be considered hypothesis generating, as we tested multiple subgroups without an a priori hypothesis of biological interaction.
Our findings of the association of low ABI and impaired QOL among community‐dwelling older adults may have some clinical and public health implications. Staying physically active is an important goal for older adults as a determinant of the ability to sustain independent living. Because several lifestyle (eg, smoking cessation or supervised exercise) or medical (eg, cilostazol or revascularization) interventions have demonstrated improvement in physical function among patients with PAD,45, 46, 47 it seems important to identify the contribution of PAD to the reduction of physical components of QOL in older adults. It is important to keep in mind that reduced QOL was also observed in those with borderline low ABI (<1.00) in our study. Further studies might assess whether PAD‐specific interventions would comprehensively improve physical components of QOL and vitality in community‐dwelling older adults with a low ABI but otherwise not diagnosed as PAD.
Our study has several limitations. First, for any cross‐sectional analysis, it is not possible to establish the temporality or causality of our observed associations of low ABI with poor QOL status.48 Second, the ARIC Study did not collect specific information on leg pain at visit 5. Third, our results may not be entirely generalizable to racial/ethnic groups other than whites and blacks. Fourth, it is possible that those individuals with a very low ABI and/or severely reduced QOL were less likely to attend ARIC visit 5, raising the possibility that our results may underestimate the relationship between low ABI <0.9 and QOL. Finally, as any observational study, we cannot deny the possibility of residual confounding.
In conclusion, among older adults in the community, those with ABI <1.0 were independently associated with reduced QOL status. The association was particularly evident for physical components of QOL but was also observed for a mental domain, vitality. Because QOL is a critical element in addition to survival, especially in older adults, further studies are warranted to assess the causality of ABI in the development of poor QOL and, if so, to explore whether the management of PAD can improve QOL among older adults afflicted by this disease.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This specific study was partly supported by a Professional Developmental Award from the National Kidney Foundation of Maryland to Matsushita. Selvin was supported by NIH/NIDDK grant K24DK106414.
Disclosures
Matsushita received a grant and personal fee from Fukuda Denshi, unrelated to this work. Other authors declare no conflict of interest.
Supporting information
Table S1. Baseline Characteristics in Participants Included and Those Excluded in the Analysis
Table S2. Description of Participant's SF‐12 Quality of Life (QOL) Parameters According to Ankle‐Brachial Index (ABI) Categories
Table S3. Partially Adjusted† Difference in Quality of Life (QOL) Scores According to Ankle‐Brachial Index (ABI) Categories (Model 1)
Table S4. Partially Adjusted† Difference in Quality of Life (QOL) Scores According to Ankle‐Brachial Index (ABI) Categories (Model 2)
Table S5. Partially Adjusted† Odds‐Ratio of Other Quality of Life (QOL) Aspects According to Ankle‐Brachial Index (ABI) Categories (Model 1)
Table S6. Partially Adjusted† Odds‐Ratio of Other Quality of Life (QOL) Aspects According to Ankle‐Brachial Index (ABI) Categories (Model 2)
Table S7. Adjusted† Difference in Physical Component Summary Scores Across Ankle‐Brachial Index (ABI) Categories in Different Subgroups
Table S8. Adjusted† Odds‐Ratio of Other Physical Activity Aspects for Lower Ankle‐Brachial Index (ABI) (<1.00) in Different Subgroups
Table S9. Adjusted† Difference in Mental Component Summary Scores Across Ankle‐Brachial Index (ABI) Categories in Different Subgroups
Table S10. Adjusted† Odds‐Ratio of Other Mental Health Aspects for Lower Ankle‐Brachial Index (ABI) (<1.00) in Different Subgroups
Acknowledgments
The authors thank the staff and participants of The Atherosclerosis Risk in Communities Study for their important contributions.
(J Am Heart Assoc. 2017;6:e004519. DOI: 10.1161/JAHA.116.004519.)
References
- 1. Adesunloye BA, Valadri R, Mbaezue NM, Onwuanyi AE. Impact of peripheral arterial disease on functional limitation in congestive heart failure: results from the National Health and Nutrition Examination Survey (1999‐2004). Cardiol Res Pract. 2012;2012:306852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleg JL, Forman DE, Berra K, Bittner V, Blumenthal JA, Chen MA, Cheng S, Kitzman DW, Maurer MS, Rich MW, Shen WK, Williams MA, Zieman SJ. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation. 2013;128:2422–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999‐2000. Circulation. 2004;110:738–743. [DOI] [PubMed] [Google Scholar]
- 4. Ostchega Y, Paulose‐Ram R, Dillon CF, Gu Q, Hughes JP. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999‐2004. J Am Geriatr Soc. 2007;55:583–589. [DOI] [PubMed] [Google Scholar]
- 5. Murabito JM, Evans JC, Larson MG, Nieto K, Levy D, Wilson PW. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study. Arch Intern Med. 2003;163:1939–1942. [DOI] [PubMed] [Google Scholar]
- 6. Heald CL, Fowkes FG, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle‐brachial index: systematic review. Atherosclerosis. 2006;189:61–69. [DOI] [PubMed] [Google Scholar]
- 7. Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45:S5–S67. [DOI] [PubMed] [Google Scholar]
- 9. McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. [DOI] [PubMed] [Google Scholar]
- 10. Amer MS, Alsadany MA, Tolba MF, Omar OH. Quality of life in elderly diabetic patients with peripheral arterial disease. Geriatr Gerontol Int. 2013;13:443–450. [DOI] [PubMed] [Google Scholar]
- 11. Breek JC, Hamming JF, De Vries J, Aquarius AEAM, van Berge Henegouwen DP. Quality of life in patients with intermittent claudication using the World Health Organisation (WHO) questionnaire. Eur J Vasc Endovasc Surg. 2001;21:118–122. [DOI] [PubMed] [Google Scholar]
- 12. Long J, Modrall JG, Parker BJ, Swann A, Welborn MB III, Anthony T. Correlation between ankle‐brachial index, symptoms, and health‐related quality of life in patients with peripheral vascular disease. J Vasc Surg. 2004;39:723–727. [DOI] [PubMed] [Google Scholar]
- 13. McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. [DOI] [PubMed] [Google Scholar]
- 14. Garg PK, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, Tan J, McDermott MM. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inglis SC, Lewsey JD, Lowe GD, Jhund P, Gillies M, Stewart S, Capewell S, Macintyre K, McMurray JJ. Angina and intermittent claudication in 7403 participants of the 2003 Scottish Health Survey: impact on general and mental health, quality of life and five‐year mortality. Int J Cardiol. 2013;167:2149–2155. [DOI] [PubMed] [Google Scholar]
- 16. Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat‐Jacobson D, McDermott MM, Hirsch AT. The impact of peripheral arterial disease on health‐related quality of life in the peripheral arterial disease awareness, risk, and treatment: new resources for survival (PARTNERS) program. Vasc Med. 2008;13:15–24. [DOI] [PubMed] [Google Scholar]
- 17. Korhonen PE, Seppala T, Kautiainen H, Jarvenpaa S, Aarnio PT, Kivela SL. Ankle‐brachial index and health‐related quality of life. Eur J Prev Cardiol. 2012;19:901–907. [DOI] [PubMed] [Google Scholar]
- 18. Stefanick ML, Brunner RL, Leng X, Limacher MC, Bird CE, Garcia DO, Hogan PE, LaMonte MJ, Mackey RH, Johnson KC, LaCroix A, Robinson JG, Seguin RA, Tindle HA, Wassertheil‐Smoller S. The relationship of cardiovascular disease to physical functioning in women surviving to age 80 and above in the Women's Health Initiative. J Gerontol A Biol Sci Med Sci. 2016;71(suppl 1):S42–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat‐Jacobson D. Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. [DOI] [PubMed] [Google Scholar]
- 20. Guidon M, McGee H. Exercise‐based interventions and health‐related quality of life in intermittent claudication: a 20‐year (1989‐2008) review. Eur J Cardiovasc Prev Rehabil. 2010;17:140–154. [DOI] [PubMed] [Google Scholar]
- 21. Prevost A, Lafitte M, Pucheu Y, Couffinhal T. Education and home based training for intermittent claudication: functional effects and quality of life. Eur J Prev Cardiol. 2015;22:373–379. [DOI] [PubMed] [Google Scholar]
- 22. The ARIC Study . ARIC Visit 5 and NCS Study Protocol, Operations Manual No 2: Home and Field Center Procedures, Version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 2013. [Google Scholar]
- 23. Ankle Brachial Index Collaboration , Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton‐Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta‐analysis. JAMA. 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allison MA, Laughlin GA, Barrett‐Connor E, Langer R. Association between the ankle‐brachial index and future coronary calcium (the Rancho Bernardo study). Am J Cardiol. 2006;97:181–186. [DOI] [PubMed] [Google Scholar]
- 25. Ix JH, Katz R, Peralta CA, de Boer IH, Allison MA, Bluemke DA, Siscovick DS, Lima JAC, Criqui MH. A high ankle brachial index is associated with greater left ventricular mass: the Multi‐Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2010;55:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wattanakit K, Folsom AR, Duprez DA, Weatherley BD, Hirsch AT. Clinical significance of a high ankle‐brachial index: insights from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2007;190:459–464. [DOI] [PubMed] [Google Scholar]
- 27. Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M. Cross‐validation of item selection and scoring for the SF‐12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1171–1178. [DOI] [PubMed] [Google Scholar]
- 28. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 29. Ware JEJ. SF‐36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1996. [Google Scholar]
- 30. Maruish ME. User's Manual for the SF‐12v2 Health Survey. 3rd ed Lincoln, RI: QualityMetric Inc.; 2012. [Google Scholar]
- 31. Kohout FJ, Berkman LF, Evans DA, Cornoni‐Huntley J. Two shorter forms of the CES‐D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. [DOI] [PubMed] [Google Scholar]
- 32. Gellis ZD. Assessment of a brief CES‐D measure for depression in homebound medically ill older adults. J Gerontol Soc Work. 2010;53:289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zauszniewski JA, Bekhet AK. Depressive symptoms in elderly women with chronic conditions: measurement issues. Aging Ment Health. 2009;13:64–72. [DOI] [PubMed] [Google Scholar]
- 34. Giatti L, Camelo LdV, Rodrigues JFdC, Barreto SM. Reliability of the MacArthur scale of subjective social status—Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). BMC Public Health. 2012;12:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh‐Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom Med. 2005;67:855–861. [DOI] [PubMed] [Google Scholar]
- 36. Lutsey PL, Rasmussen‐Torvik LJ, Pankow JS, Alonso A, Smolenski DJ, Tang W, Coresh J, Volcik KA, Ballantyne CM, Boerwinkle E, Folsom AR. Relation of lipid gene scores to longitudinal trends in lipid levels and incidence of abnormal lipid levels among individuals of European ancestry: the Atherosclerosis Risk in Communities (ARIC) study. Circ Cardiovasc Genet. 2012;5:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schroeder EB, Chambless LE, Liao D, Prineas RJ, Evans GW, Rosamond WD, Heiss G. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005;28:668–674. [DOI] [PubMed] [Google Scholar]
- 38. Luft VC, Pereira M, Pankow JS, Ballantyne C, Couper D, Heiss G, Duncan BB. Retinol binding protein 4 and incident diabetes—the Atherosclerosis Risk in Communities Study (ARIC Study). Rev Bras Epidemiol. 2013;16:388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. [DOI] [PubMed] [Google Scholar]
- 41. Korhonen P, Aarnio P. Borderline peripheral arterial disease. Int J Angiol. 2008;17:175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dumville JC, Lee AJ, Smith FB, Fowkes FG. The health‐related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. Br J Gen Pract. 2004;54:826–831. [PMC free article] [PubMed] [Google Scholar]
- 43. Cluff K, Kelly AM, Koutakis P, He XN, Huang X, Lu YF, Pipinos II, Casale GP, Subbiah J. Surface‐enhanced Raman spectral biomarkers correlate with ankle brachial index and characterize leg muscle biochemical composition of patients with peripheral arterial disease. Physiol Rep. 2014;2:e12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lane RA, Mazari F, Mockford KA, Vanicek N, Chetter IC, Coughlin PA. Fear of falling in claudicants and its relationship to physical ability, balance, and quality of life. Vasc Endovascular Surg. 2014;48:297–304. [DOI] [PubMed] [Google Scholar]
- 45. Je HG, Kim BH, Cho KI, Jang JS, Park YH, Spertus J. Correlation between patient‐reported symptoms and ankle‐brachial index after revascularization for peripheral arterial disease. Int J Mol Sci. 2015;16:11355–11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parmenter BJ, Dieberg G, Phipps G, Smart NA. Exercise training for health‐related quality of life in peripheral artery disease: a systematic review and meta‐analysis. Vasc Med. 2015;20:30–40. [DOI] [PubMed] [Google Scholar]
- 47. Kruidenier LM, Nicolai SP, Rouwet EV, Peters RJ, Prins MH, Teijink JA. Additional supervised exercise therapy after a percutaneous vascular intervention for peripheral arterial disease: a randomized clinical trial. J Vasc Interv Radiol. 2011;22:961–968. [DOI] [PubMed] [Google Scholar]
- 48. Wattanakit K, Williams JE, Schreiner PJ, Hirsch AT, Folsom AR. Association of anger proneness, depression and low social support with peripheral arterial disease: the Atherosclerosis Risk in Communities Study. Vasc Med. 2005;10:199–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics in Participants Included and Those Excluded in the Analysis
Table S2. Description of Participant's SF‐12 Quality of Life (QOL) Parameters According to Ankle‐Brachial Index (ABI) Categories
Table S3. Partially Adjusted† Difference in Quality of Life (QOL) Scores According to Ankle‐Brachial Index (ABI) Categories (Model 1)
Table S4. Partially Adjusted† Difference in Quality of Life (QOL) Scores According to Ankle‐Brachial Index (ABI) Categories (Model 2)
Table S5. Partially Adjusted† Odds‐Ratio of Other Quality of Life (QOL) Aspects According to Ankle‐Brachial Index (ABI) Categories (Model 1)
Table S6. Partially Adjusted† Odds‐Ratio of Other Quality of Life (QOL) Aspects According to Ankle‐Brachial Index (ABI) Categories (Model 2)
Table S7. Adjusted† Difference in Physical Component Summary Scores Across Ankle‐Brachial Index (ABI) Categories in Different Subgroups
Table S8. Adjusted† Odds‐Ratio of Other Physical Activity Aspects for Lower Ankle‐Brachial Index (ABI) (<1.00) in Different Subgroups
Table S9. Adjusted† Difference in Mental Component Summary Scores Across Ankle‐Brachial Index (ABI) Categories in Different Subgroups
Table S10. Adjusted† Odds‐Ratio of Other Mental Health Aspects for Lower Ankle‐Brachial Index (ABI) (<1.00) in Different Subgroups


